The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review
Abstract
1. Introduction
- What are the most common complications, outside of sepsis, regarding the use of PMMA in rTKA?
- What are the current applications and challenges using PMMA to manage bone loss in rTKA?
- How is PMMA used to address infection in first stage rTKA and what are the subsequent complications?
2. PMMA
Chemistry of PMMA
3. Complications after rTKA
3.1. Aseptic Loosening
3.2. Third-Body Wear
3.3. Heat Generation
3.4. Volumetric Shrinkage
4. Management of Bone Defects in rTKA
- The terminology used for femoral and tibial defects is the same because the metaphyseal segment of both femur and tibia are similar;
- As there is no cortical bone in the metaphyseal segment of the proximal tibia and distal femur, the common definitions which were used in most classifications (cortical /cancellous, contained/uncontained, central/peripheral) were eliminated;
- The definitions in AORI are precise. This reduces the ambiguity when characterizing the bone defect;
- The number of defect types in this classification is minimal to allow researchers to have sufficient cases for statistical analysis;
- The classification provides intraoperative and postoperative radiographic data, therefore; researchers would have access to the retrospective categorization of cases.
4.1. Management of F1/T1 Defect
4.2. Management of F2/T2 Defect
4.2.1. Management of F2A
4.2.2. Management of F2B
Management of T2A
Management of T2B
Stems
4.3. Management of F3/T3 Defect
4.3.1. Management of F3 Defect
4.3.2. Management of T3 Defect
5. Management of Infection in rTKA
5.1. Single-Stage rTKA
5.2. Two-Stage rTKA
5.3. Comparison between Single-Stage and Two-Stage rTKA
5.4. Antibiotic-Loaded PMMA
5.4.1. Static-PMMA Based Spacer
5.4.2. Static Versus Dynamic Spacers
5.4.3. Dynamic-PMMA Based Spacer
Cement-on-Cement Dynamic Spacers
Metal-on-Polyethylene Spacer (PROSTALAC™)
5.4.4. Concerns Regarding Use of Antibiotic-Impregnated PMMA Spacers
6. Conclusions
- Regarding the most common complications, outside of sepsis, involving the use of PMMA in rTKA. Most literature reported infection as the major cause of failure in rTKA [96,98,119]. Using PMMA also resulted in wear debris, bone necrosis and volumetric shrinkage. These might lead to complications such as tissue necrosis, instability of the implant, increased bone loss and subsequent loosening;
- Investigating the current applications and drawbacks of using PMMA in addressing bone loss in rTKA. Literature since 1984 has mentioned limitations in using PMMA for large defects and recent reports showed no deviation on this issue [6,8,54,59,60,61,62,64]. According to the AORI classification, PMMA alone can only be used in F1/T1 defects having a depth of less than 5 mm and covering less than 50% of the bone surface [9,60,61,62,63]. We conclude that drawbacks such as crack propagation and loosening [18] restrict the use of PMMA in large bone defects.
- Reviewing how PMMA is used to address bone infection in rTKA and what subsequent complications might result; PMMA is the standard for delivering antibiotics in infected rTKA. Antibiotic PMMA spacers are used as a treatment for patients with late chronic infection in two-stage rTKA. Although dynamic-PMMA spacers facilitate some ROM, care should be taken in using them in type-F3 bone defects. Moreover, the optimal procedure for infection eradication (single or two-stage rTKA) is still controversial in the literature [88,93,94,95,96]. Therefore, further research for guidance on single-stage vs. two-stage rTKA in managing infection is warranted. Our review demonstrated that issues such as the initial burst of antibiotic release from PMMA, with poor subsequent sustained elution, bacterial colonization on the surface of the PMMA, roughness, heat generation during polymerization and lack of porosity influence the long-term effect of PMMA-loaded antibiotics.
Author Contributions
Funding
Conflicts of Interest
References
- Fehring, T.K.; Christie, M.J.; Lavernia, C.; Mason, J.B.; McAuley, J.P.; MacDonald, S.J.; Springer, B.D. Revision total knee arthroplasty: Planning, management, and controversies. Instr. Course Lect. 2008, 57, 341–363. [Google Scholar]
- Parratte, S.; Abdel, M.P.; Lunebourg, A.; Budhiparama, N.; Lewallen, D.G.; Hanssen, A.D.; Argenson, J.N. Revision total knee arthroplasty: The end of the allograft era? Eur. J. Orthop. Surg. Traumatol. 2015, 25, 621–622. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information. Hip and Knee Replacements in Canada, 2016–2017: Canadian Jt. Replacement Registry Annual Report; CIHI: Ottawa, ON, USA, 2018. [Google Scholar]
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why are total knee arthroplasties failing today--has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef]
- Whittaker, J.P.; Dharmarajan, R.; Toms, A.D. The management of bone loss in revision total knee replacement. J. Bone Jt. Surg. Br. 2008, 90, 981–987. [Google Scholar] [CrossRef]
- Engh, G.A.; Ammeen, D.J. Bone loss with revision total knee arthroplasty: Defect classification and alternatives for reconstruction. Instr. Course Lect. 1999, 48, 167–175. [Google Scholar] [PubMed]
- Qiu, Y.Y.; Yan, C.H.; Chiu, K.Y.; Ng, F.Y. Review article: Treatments for bone loss in revision total knee arthroplasty. J. Orthop. Surg. Hong Kong 2012, 20, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Panegrossi, G.; Ceretti, M.; Papalia, M.; Casella, F.; Favetti, F.; Falez, F. Bone loss management in total knee revision surgery. Int. Orthop. 2014, 38, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Molloy, R.M.; Ting, N.T. Revision Total Knee Arthroplasty: Management of Bone Loss. In Complex Primary and Revision Total Knee Arthroplasty; Springer, B.D., Curtin, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 143–154. [Google Scholar] [CrossRef]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Stevens, C.M.; Tetsworth, K.D.; Calhoun, J.H.; Mader, J.T. An articulated antibiotic spacer used for infected total knee arthroplasty: A comparative in vitro elution study of Simplex® and Palacos® bone cements. J. Orthop. Res. 2005, 23, 27–33. [Google Scholar] [CrossRef]
- Magnan, B.; Bondi, M.; Maluta, T.; Samaila, E.; Schirru, L.; Dall’Oca, C. Acrylic bone cement: Current concept review. Musculoskelet. Surg. 2013, 97, 93–100. [Google Scholar] [CrossRef]
- Kohl, S.; Evangelopoulos, D.S.; Kohlhof, H.; Krueger, A.; Hartel, M.; Roeder, C.; Eggli, S. An intraoperatively moulded PMMA prostheses like spacer for two-stage revision of infected total knee arthroplasty. Knee 2011, 18, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.A.; Duncan, C.P.; Beauchamp, C.P. Long-term elution of antibiotics from bone-cement: An in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J. Arthroplast. 1998, 13, 331–338. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Jt. Surg. Am. 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Arora, M.; Chan, E.K.; Gupta, S.; Diwan, A.D. Polymethylmethacrylate bone cements and additives: A review of the literature. World J. Orthop. 2013, 4, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Lee, R.; Coote, M.L.; Yamago, S. Termination mechanism of the radical polymerization of acrylates. Macromol. Rapid Commun. 2016, 36, 506–513. [Google Scholar] [CrossRef]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Deramond, H.; Wright, N.T.; Belkoff, S.M. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone 1999, 25, 17S–21S. [Google Scholar] [CrossRef]
- Yang, J.M. Polymerization of acrylic bone cement using differential scanning calorimetry. Biomaterials 1997, 18, 1293–1298. [Google Scholar] [CrossRef]
- Hvid, I. Mechanical strength of trabecular bone at the knee. Dan Med. Bull. 1988, 35, 345–365. [Google Scholar]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng. 2015, 137, 010802. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C.; Oussedik, S. Total Knee Arthroplasty: A Comprehensive Guide; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lee, D.-H.; Lee, S.-H.; Song, E.-K.; Seon, J.-K.; Lim, H.-A.; Yang, H.-Y. Causes and Clinical Outcomes of Revision Total Knee Arthroplasty. Knee Surg. Relat. Res. 2017, 29, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.; Griffin, W.; Springer, B.; Fehring, T.; Mason, J.B.; Odum, S. Why do revision knee arthroplasties fail? J. Arthroplast. 2008, 23, 99–103. [Google Scholar] [CrossRef]
- Postler, A.; Lützner, C.; Beyer, F.; Tille, E.; Lützner, J. Analysis of Total Knee Arthroplasty revision causes. BMC Musculoskelet. Disord. 2018, 19, 55. [Google Scholar] [CrossRef]
- Kasahara, Y.; Majima, T.; Kimura, S.; Nishiike, O.; Uchida, J. What Are the Causes of Revision Total Knee Arthroplasty in Japan? Clin. Orthop. 2013, 471, 1533–1538. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Molligan, J.; Austin, M.S.; Purtill, J.J.; Hozack, W.J.; Parvizi, J. Failure following revision total knee arthroplasty: Infection is the major cause. Int. Orthop. 2011, 35, 1157–1164. [Google Scholar] [CrossRef]
- Kang, S.G.; Park, C.H.; Song, S.J. Stem Fixation in Revision Total Knee Arthroplasty: Indications, Stem Dimensions, and Fixation Methods. Knee Surg. Relat. Res. 2018, 30, 187–192. [Google Scholar] [CrossRef][Green Version]
- Radnay, C.S.; Scuderi, G.R. Management of bone loss: Augments, cones, offset stems. Clin. Orthop. 2006, 446, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kinaci, A.; Neuhaus, V.; Ring, D.C. Trends in bone graft use in the United States. Orthopedics 2014, 37, e783–e788. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Clark, C.R.; Wright, T.M. The problem in total joint arthroplasty: Aseptic loosening. J. Bone Jt. Surg. Am. 1993, 75, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Mjöberg, B. Fixation and loosening of hip prostheses. Acta Orthop. Scand. 1991, 62, 500–508. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [PubMed]
- Wooley, P.H.; Schwarz, E.M. Aseptic loosening. Gene Ther. 2004, 11, 402–407. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gibon, E.; Yao, Z. The basic science of periprosthetic osteolysis. Instr. Course Lect. 2013, 62, 201–206. [Google Scholar] [PubMed]
- Santavirta, S.S.; Lappalainen, R.; Pekko, P.; Anttila, A.; Konttinen, Y.T. The counterface, surface smoothness, tolerances, and coatings in total joint prostheses. Clin. Orthop. 1999, 369, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef]
- De Baets, T.; Waelput, W.; Bellemans, J. Analysis of third body particles generated during total knee arthroplasty: Is metal debris an issue? Knee 2008, 15, 95–97. [Google Scholar] [CrossRef]
- Gibon, E.; Córdova, L.A.; Lu, L.; Lin, T.-H.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1685–1691. [Google Scholar] [CrossRef]
- Niki, Y.; Matsumoto, H.; Otani, T.; Tomatsu, T.; Toyama, Y. How Much Sterile Saline Should be Used for Efficient Lavage During Total Knee Arthroplasty? Effects of Pulse Lavage Irrigation on Removal of Bone and Cement Debris. J. Arthroplast. 2007, 22, 95–99. [Google Scholar] [CrossRef]
- Chiu, R.; Ma, T.; Smith, R.L.; Goodman, S.B. Polymethylmethacrylate particles inhibit osteoblastic differentiation of MC3T3-E1 osteoprogenitor cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 932–936. [Google Scholar] [CrossRef]
- Gundapaneni, D.; Goswami, T. Thermal isotherms in PMMA and cell necrosis during total hip arthroplasty. J. Appl. Biomater. Funct. Mater. 2014, 12, 193–202. [Google Scholar] [CrossRef]
- Revie, I.; Wallace, M.E.; Orr, J.F. The Effect of PMMA Thickness on Thermal Bone Necrosis around Acetabular Sockets. Proc. Inst. Mech. Eng. Part H 1994, 208, 45–51. [Google Scholar] [CrossRef]
- Berman, A.T.; Reid, J.S.; Yanicko, J.D.; Sih, G.C.; Zimmerman, M.R. Thermally induced bone necrosis in rabbits. Relation to implant failure in humans. Clin. Orthop. 1984, 186, 284–922. [Google Scholar]
- Webb, J.C.J.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. 2007, 89, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Hashimoto, Y.; Yoshiya, S.; Kurosaka, M.; Matsuda, M.; Kawamura, S.; Iwatsubo, T. Conduction analysis of cement interface temperature in total knee arthroplasty. Kobe J. Med. Sci. 2002, 48, 63–72. [Google Scholar]
- Orr, J.F.; Dunne, N.J.; Quinn, J.C. Shrinkage stresses in bone cement. Biomaterials 2003, 24, 2933–2940. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Hasenwinkel, J.M.; Wixson, R.L.; Lautenschlager, E.P. A theoretical and experimental analysis of polymerization shrinkage of bone cement: A potential major source of porosity. J. Biomed. Mater. Res. 2000, 52, 210–218. [Google Scholar] [CrossRef]
- de Wijn, J.R.; Driessens, F.C.M.; Slooff, T.J.J.H. Dimensional behavior of curing bone cement masses. J. Biomed. Mater. Res. 2004, 9, 99–103. [Google Scholar] [CrossRef]
- Muller, S.D.; Green, S.M.; McCaskie, A.W. The dynamic volume changes of polymerising polymethyl methacrylate bone cement. Acta Orthop. Scand. 2002, 73, 684–687. [Google Scholar] [CrossRef]
- Orr, J.F.; Dunne, N. Measurement of Shrinkage Stresses in PMMA Bone Cement. Appl. Mech. Mater. 2004, 1–2. [Google Scholar] [CrossRef]
- Lennon, A.B.; Prendergast, P.J. Residual stress due to curing can initiate damage in porous bone cement: Experimental and theoretical evidence. J. Biomech. 2002, 35, 311–321. [Google Scholar] [CrossRef]
- Daines, B.K.; Dennis, D.A. Management of bone defects in revision total knee arthroplasty. J. Bone Jt. Surg. Am. 2012, 94, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Yan, C.H.; Chiu, K.Y.; Ng, F.Y. Review Article: Bone Defect Classifications in Revision Total Knee Arthroplasty. J. Orthop. Surg. 2011, 19, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Engh, G.A.; Ammeen, D.J. Classification and Preoperative Radiographic Evaluation: Knee. Orthop. Clin. 1998, 29, 205–217. [Google Scholar] [CrossRef]
- Mancuso, F.; Beltrame, A.; Colombo, E.; Miani, E.; Bassini, F. Management of metaphyseal bone loss in revision knee arthroplasty. Acta Bio-Med. Atenei Parm. 2017, 88, 98–111. [Google Scholar] [CrossRef]
- Iamaguchi, M.M.; Helito, C.P.; Gobbi, R.G.; Demange, M.K.; Tirico, L.E.P.; Pecora, J.R.; Camanho, G.L. Value of preoperative radiographic evaluations on knee bone defects for revision arthroplasty. Rev. Bras. Ortop. 2012, 47, 714–718. [Google Scholar] [CrossRef][Green Version]
- Brooks, P.J.; Walker, P.S.; Scott, R.D. Tibial component fixation in deficient tibial bone stock. Clin. Orthop. 1984, 184, 302–308. [Google Scholar] [CrossRef]
- Sheth, N.P.; Bonadio, M.B.; Demange, M.K. Bone Loss in Revision Total Knee Arthroplasty: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2017, 25, 348–357. [Google Scholar] [CrossRef]
- Berend, M.E.; Ritter, M.A.; Keating, E.M.; Jackson, M.D.; Davis, K.E.; Malinzak, R.A. Use of screws and cement in revision TKA with primary or revision specific prosthesis with up to 17 years followup. J. Arthroplast. 2015, 30, 86–89. [Google Scholar] [CrossRef]
- Scuderi, G.R.; Parisi, T.J.; Dennis, D.A.; Lewallen, D.G.; Windsor, R.E.; Ponzio, D.Y. Management of Tibial Bone Loss. In Complex Cases Total Knee Arthroplasty Compend; Tria, A.J., Scuderi, G.R., Cushner, F.D., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 245–267. [Google Scholar] [CrossRef]
- Toms, A.D.; Barker, R.L.; McClelland, D.; Chua, L.; Spencer-Jones, R.; Kuiper, J.-H. Repair of defects and containment in revision total knee replacement: A comparative biomechanical analysis. J. Bone Jt. Surg. Br. 2009, 91, 271–277. [Google Scholar] [CrossRef][Green Version]
- Schemitsch, E.H. Size matters: Defining critical in bone defect size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Lombardi, A.V.; Berend, K.R.; Adams, J.B. Management of Bone Loss in Revision TKA: It’s a Changing World. Orthopedics 2010, 33. [Google Scholar] [CrossRef]
- Ritter, M.A. Screw and cement fixation of large defects in total knee arthroplasty. J. Arthroplast. 1986, 1, 125–129. [Google Scholar] [CrossRef]
- Scuderi, G.R.; Tria, A.J., Jr. Surgical Techniques in Total Knee Arthroplasty; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Rawlinson, J.J.; Closkey, R.F.; Davis, N.; Wright, T.M.; Windsor, R. Stemmed Implants Improve Stability in Augmented Constrained Condylar Knees. Clin. Orthop. 2008, 466, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Nadorf, J.; Gantz, S.; Kohl, K.; Kretzer, J.P. Tibial revision knee arthroplasty: Influence of modular stems on implant fixation and bone flexibility in AORI Type T2a defects. Int. J. Artif. Organs 2016, 39, 534–540. [Google Scholar] [CrossRef]
- Cuckler, J.M. Bone loss in total knee arthroplasty: Graft augment and options11No benefits or funds were received in support of this study. J. Arthroplast. 2004, 19, 56–58. [Google Scholar] [CrossRef]
- Mabry, T.M.; Hanssen, A.D. The Role of Stems and Augments for Bone Loss in Revision Knee Arthroplasty. J. Arthroplast. 2007, 22, 56–60. [Google Scholar] [CrossRef]
- Conlisk, N.; Gray, H.; Pankaj, P.; Howie, C.R. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Jt. Res. 2012, 1, 281–288. [Google Scholar] [CrossRef]
- Edwards, P.K.; Fehring, T.K.; Hamilton, W.G.; Perricelli, B.; Beaver, W.B.; Odum, S.M. Are Cementless Stems More Durable Than Cemented Stems in Two-stage Revisions of Infected Total Knee Arthroplasties? Clin. Orthop. 2014, 472, 206–211. [Google Scholar] [CrossRef]
- Winemaker, M.J.; Beingessner, D.M.; Rorabeck, C.H. Revision total knee arthroplasty: Should tibial stems be cemented or uncemented? Knee 1998, 5, 175–181. [Google Scholar] [CrossRef]
- Barrack, R.L.; Rorabeck, C.; Burt, M.; Sawhney, J. Pain at the end of the stem after revision total knee arthroplasty. Clin. Orthop. 1999, 367, 216–225. [Google Scholar] [CrossRef]
- Fehring, T.K.; Odum, S.; Olekson, C.; Griffin, W.L.; Mason, J.B.; McCoy, T.H. Stem Fixation in Revision Total Knee Arthroplasty: A Comparative Analysis. Clin. Orthop. Relat. Res. 2003, 416, 217. [Google Scholar] [CrossRef] [PubMed]
- Kosse, N.M.; van Hellemondt, G.G.; Wymenga, A.B.; Heesterbeek, P.J.C. Comparable Stability of Cemented vs Press-Fit Placed Stems in Revision Total Knee Arthroplasty with Mild to Moderate Bone Loss: 6.5-Year Results From a Randomized Controlled Trial with Radiostereometric Analysis. J. Arthroplast. 2017, 32, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, A.N.; Azboy, I.; Fuery, M.; Restrepo, C.; Shao, H.; Parvizi, J. Effect of Stem Size and Fixation Method on Mechanical Failure After Revision Total Knee Arthroplasty. J. Arthroplast. 2017, 32, S202–S208. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Barlow, B.; Ranawat, A.S. Stem length in revision total knee arthroplasty. Curr. Rev. Musculoskelet Med. 2015, 8, 407–412. [Google Scholar] [CrossRef]
- Shannon, B.D.; Klassen, J.F.; Rand, J.A.; Berry, D.J.; Trousdale, R.T. Revision total knee arthroplasty with cemented components and uncemented intramedullary stems. J. Arthroplast. 2003, 18, 27–32. [Google Scholar] [CrossRef]
- Lee, Y.S.; Chen, A.F. Managing bone loss in revision total knee arthroplasty. Ann. Jt. 2016, 22, 32–36. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A. Healing potentials of polymethylmethacrylate bone cement combined with platelet gel in the critical-sized radial bone defect of rats. PLoS ONE 2018, 13, e0194751. [Google Scholar] [CrossRef]
- Tsukayama, D.T.; Goldberg, V.M.; Kyle, R. Diagnosis and management of infection after total knee arthroplasty. J. Bone Jt. Surg. Am. 2013, 85-A (Suppl. S1), S75–S80. [Google Scholar] [CrossRef]
- Jämsen, E.; Huhtala, H.; Puolakka, T.; Moilanen, T. Risk Factors for Infection After Knee Arthroplasty: A Register-Based Analysis of 43,149 Cases. JBJS 2009, 91, 38. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Schwartzenberger, J.; Austin, M.S.; Purtill, J.J.; Parvizi, J. Revision Total Knee Arthroplasty Infection: Incidence and Predictors. Clin. Orthop. Relat. Res. 2010, 468, 2052–2059. [Google Scholar] [CrossRef]
- Rozkydal, Z.; Janík, P.; Janícek, P.; Kunovský, R. [Revision knee arthroplasty due to aseptic loosening]. Acta Chir. Orthop. Traumatol. Cech. 2007, 74, 5–13. [Google Scholar] [PubMed]
- Silvestre, A.; Almeida, F.; Renovell, P.; Morante, E.; López, R. Revision of Infected Total Knee Arthroplasty: Two-Stage Reimplantation Using an Antibiotic-Impregnated Static Spacer. Clin. Orthop. Surg. 2013, 5, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Nagra, N.; Hamilton, T.; Ganatra, S.; W Murray, D.; Pandit, H. One-stage versus two-stage exchange arthroplasty for infected total knee arthroplasty: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 24. [Google Scholar] [CrossRef]
- Vanhegan, I.S.; Morgan-Jones, R.; Barrett, D.S.; Haddad, F.S. Developing a strategy to treat established infection in total knee replacement. J. Bone Jt. Surg. Br. 2012, 94-B, 875–881. [Google Scholar] [CrossRef]
- Aguas, M.; Dannhauser, W.; Fox, R.; Scalzi, M.; Verdi, S. A Novel Technique to Remove Bone Cement in Reoperative Revision Knee Arthroplasty. In Proceedings of the 2013 39th Annual Northeast Bioengineering Conference, Syracuse, NY, USA, 5–7 April 2013; pp. 241–242. [Google Scholar] [CrossRef]
- George, D.A.; Konan, S.; Haddad, F.S. Single-Stage Hip and Knee Exchange for Periprosthetic Joint Infection. J. Arthroplast. 2015, 30, 2264–2270. [Google Scholar] [CrossRef]
- Vaishya, R.; Agarwal, A.K.; Rawat, S.K.; Singh, H.; Vijay, V. Is Single-stage Revision Safe Following Infected Total Knee Arthroplasty? A Critical Review. Cureus 2017, 9, e1629. [Google Scholar] [CrossRef]
- Masters, J.P.; Smith, N.A.; Foguet, P.; Reed, M.; Parsons, H.; Sprowson, A.P. A systematic review of the evidence for single stage and two stage revision of infected knee replacement. BMC Musculoskelet. Disord. 2013, 14, 222. [Google Scholar] [CrossRef]
- Chew, E.; Khan, W.S.; Agarwal, S.; Morgan-Jones, R. Single Stage Knee Arthroplasty Revision Surgery: A Systematic Review of the Literature. Open Orthop. J. 2015, 9, 504–510. [Google Scholar] [CrossRef][Green Version]
- Baker, P.; Petheram, T.G.; Kurtz, S.; Konttinen, Y.T.; Gregg, P.; Deehan, D. Patient reported outcome measures after revision of the infected TKR: Comparison of single versus two-stage revision. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2013, 21, 2713–2720. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Whitehouse, M.R.; Lenguerrand, E.; Blom, A.W.; Beswick, A.D.; Team, I. Re-Infection Outcomes Following One- And Two-Stage Surgical Revision of Infected Knee Prosthesis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0151537. [Google Scholar] [CrossRef]
- Yan, C.H.; Arciola, C.R.; Soriano, A.; Levin, L.S.; Bauer, T.W.; Parvizi, J. Team Approach: The Management of Infection After Total Knee Replacement. JBJS Rev. 2018, 6, e9. [Google Scholar] [CrossRef]
- Mazzucchelli, L.; Rosso, F.; Marmotti, A.; Bonasia, D.E.; Bruzzone, M.; Rossi, R. The use of spacers (static and mobile) in infection knee arthroplasty. Curr. Rev. Musculoskelet. Med. 2015, 8, 373–382. [Google Scholar] [CrossRef]
- Citak, M.; Masri, B.A.; Springer, B.; Argenson, J.-N.; Kendoff, D.O. Are Preformed Articulating Spacers Superior To Surgeon-Made Articulating Spacers in the Treatment Of PJI in THA? A Literature Review. Open Orthop. J. 2015, 9, 255–261. [Google Scholar] [CrossRef]
- Fehring, T.K.; Odum, S.; Calton, T.F.; Mason, J.B. Articulating Versus Static Spacers in Revision Total Knee Arthroplasty for Sepsis. Clin. Orthop. 2000, 380, 9–16. [Google Scholar] [CrossRef]
- Jaekel, D.J.; Day, J.S.; Klein, G.R.; Levine, H.; Parvizi, J.; Kurtz, S.M. Do Dynamic Cement-on-Cement Knee Spacers Provide Better Function and Activity During Two-stage Exchange? Clin. Orthop. 2012, 470, 2599–2604. [Google Scholar] [CrossRef]
- Emerson, R.H.; Muncie, M.; Tarbox, T.R.; Higgins, L.L. Comparison of a static with a mobile spacer in total knee infection. Clin. Orthop. 2002, 404, 132–138. [Google Scholar] [CrossRef]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Calton, T.F.; Fehring, T.K.; Griffin, W.L. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin. Orthop. 1997, 345, 148–154. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wluka, A.E.; Wijethilake, P.; Wang, Y.; Ghasem-Zadeh, A.; Cicuttini, F.M. Wolff’s law in action: A mechanism for early knee osteoarthritis. Arthritis Res. Ther. 2015, 17. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar]
- Classen, T.; von Knoch, M.; Wernsmann, M.; Landgraeber, S.; Löer, F.; Jäger, M. Functional interest of an articulating spacer in two-stage infected total knee arthroplasty revision. Orthop. Traumatol. Surg. Res. 2014, 100, 409–412. [Google Scholar] [CrossRef][Green Version]
- Hsu, Y.C.; Cheng, H.C.; Ng, T.P.; Chiu, K.Y. Antibiotic-Loaded Cement Articulating Spacer for 2-Stage Reimplantation in Infected Total Knee Arthroplasty: A Simple and Economic Method. J. Arthroplast. 2007, 22, 1060–1066. [Google Scholar] [CrossRef]
- Ding, H.; Yao, J.; Chang, W.; Liu, F. Comparison of the efficacy of static versus articular spacers in two-stage revision surgery for the treatment of infection following total knee arthroplasty: A meta-analysis. J. Orthop. Surg. 2017, 12, 151. [Google Scholar] [CrossRef]
- Tria, A.J.; Scuderi, G.R.; Cushner, F.D. (Eds.) Complex Cases in Total Knee Arthroplasty: A Compendium of Current Techniques; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Guild, G.N.; Wu, B.; Scuderi, G.R. Articulating vs. Static antibiotic impregnated spacers in revision total knee arthroplasty for sepsis. A systematic review. J. Arthroplast. 2014, 29, 558–563. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, X.; Jiang, Y.; Wang, Q.; Chen, Y.; Wang, Q.; Shao, J. Intraoperatively-made cement-on-cement antibiotic-loaded articulating spacer for infected total knee arthroplasty. Knee 2010, 17, 407–411. [Google Scholar] [CrossRef]
- Hofmann, A.A.; Kane, K.R.; Tkach, T.K.; Plaster, R.L.; Camargo, M.P. Treatment of infected total knee arthroplasty using an articulating spacer. Clin. Orthop. 1995, 430, 45–54. [Google Scholar] [CrossRef]
- Pitto, R.P.; Castelli, C.C.; Ferrari, R.; Munro, J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int. Orthop. 2005, 29, 305–308. [Google Scholar] [CrossRef][Green Version]
- Corona, P.S.; Barro, V.; Mendez, M.; Cáceres, E.; Flores, X. Industrially Prefabricated Cement Spacers: Do Vancomycin- and Gentamicin-impregnated Spacers Offer Any Advantage? Clin. Orthop. 2014, 472, 923–932. [Google Scholar] [CrossRef]
- Gee, R.; Munk, P.L.; Keogh, C.; Nicolaou, S.; Masri, B.; Marchinkow, L.O.; Ellis, J.; Chan, L.P. Radiography of the PROSTALAC (Prosthesis with Antibiotic-Loaded Acrylic Cement) Orthopedic Implant. Am. J. Roentgenol. 2003, 180, 1701–1706. [Google Scholar] [CrossRef]
- Haddad, F.S.; Masri, B.A.; Campbell, D.; McGraw, R.W.; Beauchamp, C.P.; Duncan, C.P. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J. Bone Jt. Surg. Br. 2000, 82, 807–812. [Google Scholar] [CrossRef]
- Nodzo, S.R.; Boyle, K.K.; Spiro, S.; Nocon, A.A.; Miller, A.O.; Westrich, G.H. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee 2017, 24, 1175–1181. [Google Scholar] [CrossRef]
- Gooding, C.R.; Masri, B.A.; Duncan, C.P.; Greidanus, N.V.; Garbuz, D.S. Durable Infection Control and Function With the PROSTALAC Spacer in Two-stage Revision for Infected Knee Arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 985–993. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Negri, L.; Zatti, G.; Grassi, F.A. Two-stage revision surgery to treat an infected hip implant. A comparison between a custom-made spacer and a pre-formed one. Chir. Organi Mov. 2005, 90, 271–279. [Google Scholar] [PubMed]
- Anagnostakos, K.; Fink, B. Antibiotic-loaded cement spacers—Lessons learned from the past 20 years. Expert Rev. Med. Devices 2018, 15, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakos, K. Therapeutic Use of Antibiotic-loaded Bone Cement in the Treatment of Hip and Knee Joint Infections. J. Bone Jt. Infect. 2017, 2, 29–37. [Google Scholar] [CrossRef]
- Aiken, S.S.; Cooper, J.J.; Florance, H.; Robinson, M.T.; Michell, S. Local Release of Antibiotics for Surgical Site Infection Management Using High-Purity Calcium Sulfate: An In Vitro Elution Study. Surg. Infect. 2014, 16, 54–61. [Google Scholar] [CrossRef]
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J.; Draughn, R.A.; Smith, E.A.; John, J.F. Bacterial Adhesion to Biomaterial Surfaces. In Comprehensive Biomaterials; Wise, D.L., Trantolo, D.J., Altobelli, D.E., Yaszemski, M.J., Gresser, J.D., Eds.; Humana Press: Totowa, NJ, USA, 1996; pp. 19–57. [Google Scholar] [CrossRef]
- Kuechle, D.K.; Landon, G.C.; Musher, D.M.; Noble, P.C. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin. Orthop. 1991, 264, 302–308. [Google Scholar] [CrossRef]
- Lewis, G. Properties of antibiotic-loaded acrylic bone cements for use in cemented arthroplasties: A state-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 558–574. [Google Scholar] [CrossRef]
- Hanssen, A.D.; Spangehl, M.J. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin. Orthop. 2004, 427, 79–85. [Google Scholar] [CrossRef]
- Feng, M.; Li, P. Amine-containing core-shell nanoparticles as potential drug carriers for intracellular delivery. J. Biomed. Mater. Res. A 2007, 80, 184–193. [Google Scholar] [CrossRef]
- Kinnari, T.J.; Esteban, J.; Zamora, N.; Fernandez, R.; López-Santos, C.; Yubero, F.; Mariscal, D.; Puertolas, J.A.; Gomez-Barrena, E. Effect of surface roughness and sterilization on bacterial adherence to ultra-high molecular weight polyethylene. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2010, 16, 1036–1041. [Google Scholar] [CrossRef]
- de Dantas, L.C.M.; da Silva-Neto, J.P.; Dantas, T.S.; Naves, L.Z.; Neves, D.; Domingues, F. Bacterial Adhesion and Surface Roughness for Different Clinical Techniques for Acrylic Polymethyl Methacrylate. Int. J. Dent. 2016. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.L.; Verran, J.; Lees, G.C.; Ward, A.J.P. The influence of substratum topography on bacterial adhesion to polymethyl methacrylate. J. Mater. Sci. Mater. Med. 1998, 9, 17–22. [Google Scholar] [CrossRef] [PubMed]
- van de Belt, H.; Neut, D.; Uges, D.R.A.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Surface roughness, porosity and wettability of gentamicin-loaded bone cements and their antibiotic release. Biomaterials 2000, 21, 1981–1987. [Google Scholar] [CrossRef]

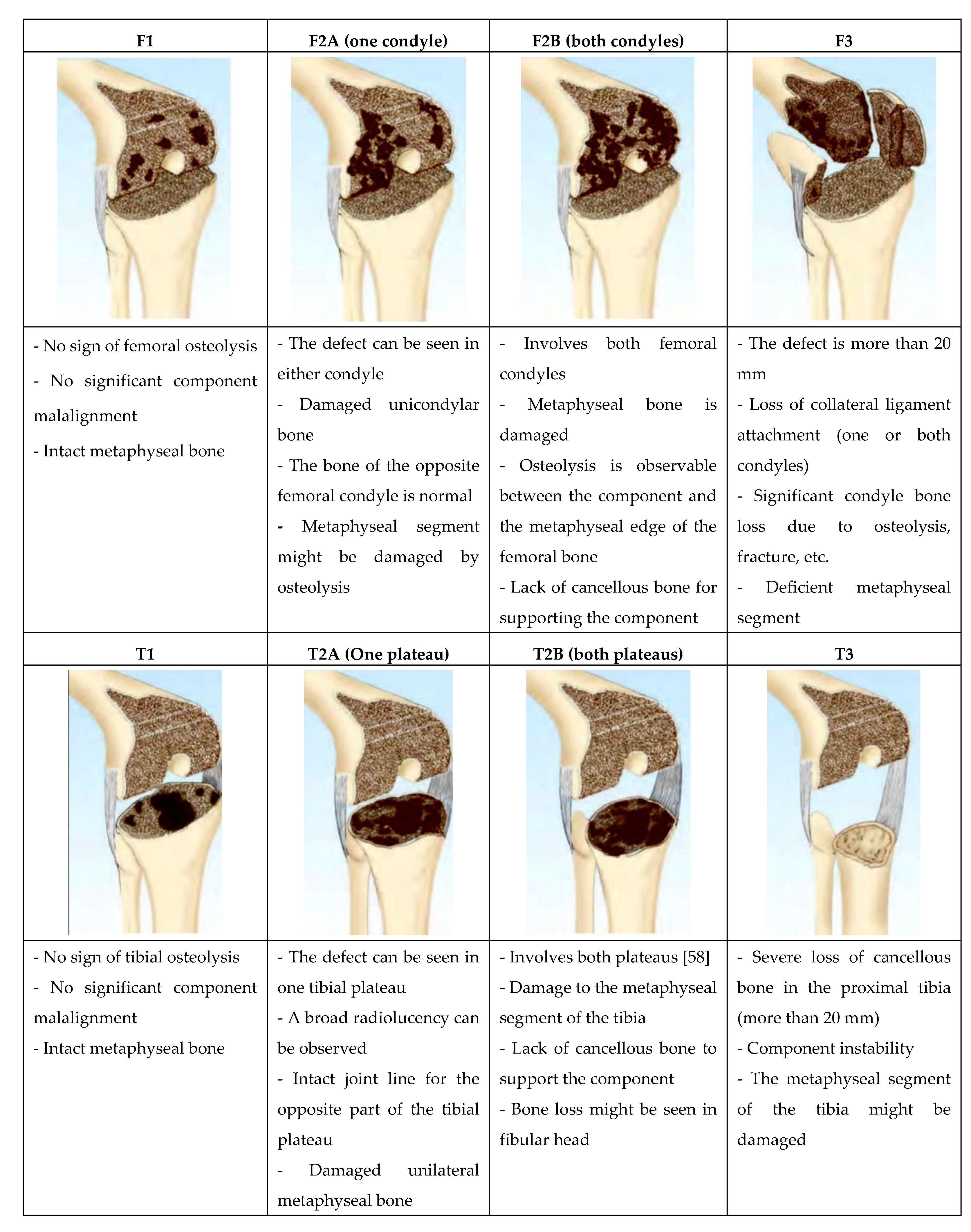
| Type | Description | Characteristics | Treatment Option |
|---|---|---|---|
| 1 | Minor and contained cancellous bony defects | <5 mm depth | PMMA fill, morselized allograft or autograft |
| 2A | Defects in one femoral condyle or one tibial plateau | 5–10 mm depth | Morselized allograft or metal augments |
| 10–20 mm depth | Metal augments, metaphyseal sleeves, structural allografts | ||
| 2B | Both femoral condyles or tibial plateaus are damaged | <20 mm depth | Metal augments, metaphyseal sleeves, structural allografts, custom-made prostheses, cones |
| 3 | Deficient metaphyseal segment; a bone loss that comprises a major portion of the condyle or plateau | >20 mm depth | Structural allografts, custom-made component, cones |
| Author | Year of Study | Number of rTKA | Remarks |
|---|---|---|---|
| Winemaker et al. [74] | 1996–2003 | 17 cemented 15 cementless | Better early stability of cemented stems Short-term radiographic results were not affected by cementing technique |
| Barrack et al. [75] | Not mentioned | 66 cemented 50 cementless | Higher localized pain at the end of the cementless stem (14%) in comparison with cemented stem (11%) |
| Fehring et al. [76] | 1986–2003 | 107 cemented 95 press-fit | Modified Knee Society radiographic scoring system used Higher stability rate for the cemented stem (93%) in comparison with cementless (71%) |
| Edwards et al. [73] | 1990–2010 | 102 cemented 126 cementless | Lower rate of radiographic failure for cementless stem. Similar reinfection rate in both stems. |
| Kosse et al. [77] | 2008–2010 | 12 cemented 11 cementless | No difference in clinical outcome and micro-motion for both cemented and cementless stems |
| Fleischman et al. [78] | 2003–2013 | 108 cemented 316 cementless | Similar risk of mechanical failure for both cemented and cementless stems Higher risk of failure for patients <65 years when cemented stem is used |
| Author | Number of Studies Reviewed | Number of Single-stage and Two-stage rTKA | Outcome |
|---|---|---|---|
| Masters et al. [93] | 63 studies | 58 studies two-stage 4 studies single-stage 1 study mix of two rTKA | Not enough evidence to support a technique |
| Chew et al. [94] | 12 studies (433 revision) | Not mentioned | Lack of evidence to address if single-stage is thorough enough to treat deep infection |
| Baker et al. [95] | 122 cases | 33 single-stage 162 two-stage | No statistical differences on knee function between single-stage and two-stage rTKA, |
| Nagra et al. [88] | 796 studies | 46 single-stage 185 two-stage | No significant differences in the risk of reinfection after single-stage rTKA |
| Kunutsor et al. [96] | 118 studies | 10 single-stage 108 two-stage | Single-stage revision strategy is as effective as the two-stage revision among unselected patients in general |
| Reason for rTKA | Number of Failed Procedures |
|---|---|
| Infection: single-stage rTKA | 288 |
| Infection: stage one of two-stage rTKA | 216 |
| Infection: stage two of two-stage rTKA | 268 |
| Total | 2468 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasandoost, L.; Rodriguez, O.; Alhalawani, A.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review. J. Funct. Biomater. 2020, 11, 25. https://doi.org/10.3390/jfb11020025
Hasandoost L, Rodriguez O, Alhalawani A, Zalzal P, Schemitsch EH, Waldman SD, Papini M, Towler MR. The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review. Journal of Functional Biomaterials. 2020; 11(2):25. https://doi.org/10.3390/jfb11020025
Chicago/Turabian StyleHasandoost, Leyla, Omar Rodriguez, Adel Alhalawani, Paul Zalzal, Emil H. Schemitsch, Stephen D. Waldman, Marcello Papini, and Mark R. Towler. 2020. "The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review" Journal of Functional Biomaterials 11, no. 2: 25. https://doi.org/10.3390/jfb11020025
APA StyleHasandoost, L., Rodriguez, O., Alhalawani, A., Zalzal, P., Schemitsch, E. H., Waldman, S. D., Papini, M., & Towler, M. R. (2020). The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review. Journal of Functional Biomaterials, 11(2), 25. https://doi.org/10.3390/jfb11020025




