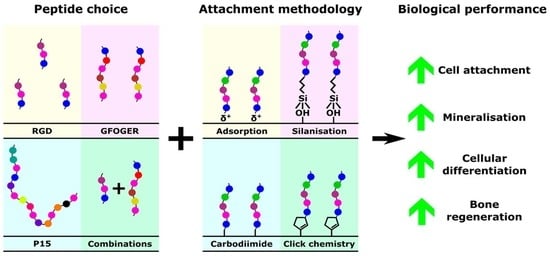
Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes
Abstract
1. Introduction
2. Peptides
3. Attachment Methodology
3.1. Adsorption
3.2. Silanisation
3.3. Carbodiimide Crosslinking
3.4. Click Chemistry
3.5. Hydrogel Incorporation
4. Peptide Selection
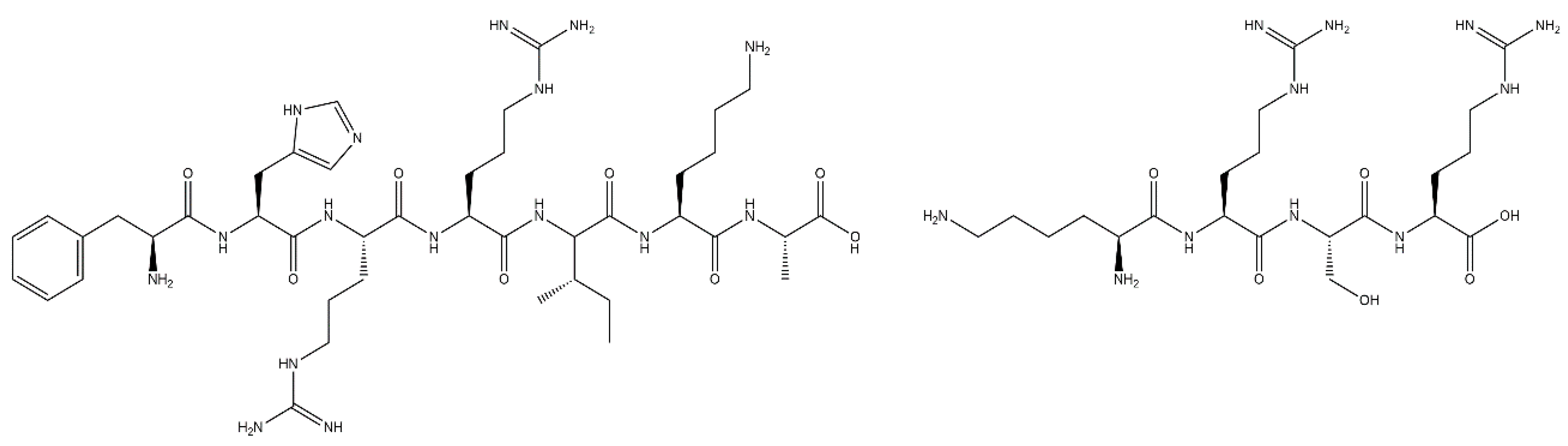
4.1. RGD Peptide
4.2. PHSRN Peptide
4.3. FHRRIKA and KRSR Peptides
4.4. GFOGER Peptide
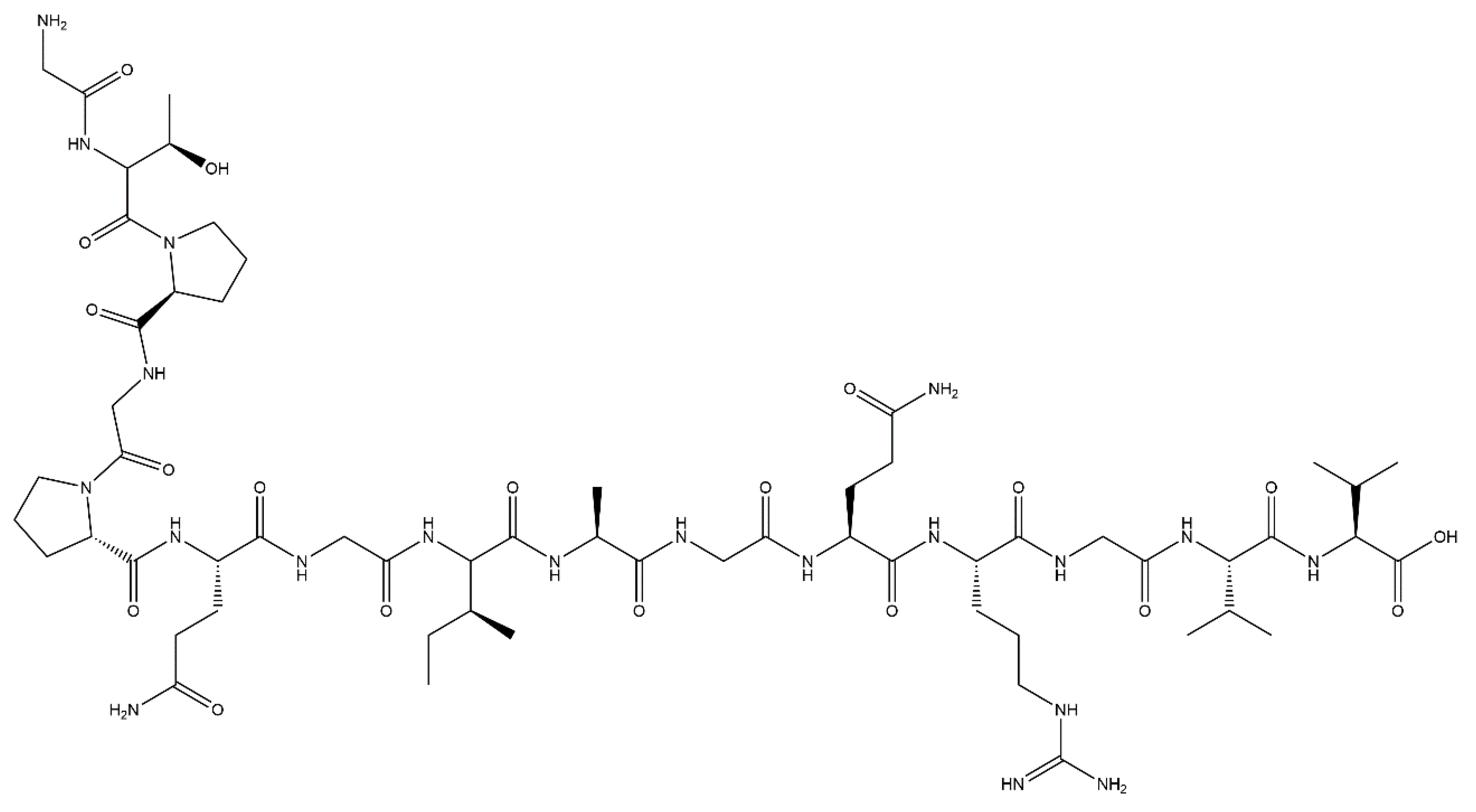
4.5. P15 Peptide
4.6. BMP Mimetic Peptides
4.7. Combinations of Peptides
| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| RGD + FHRIKKA | |||||||||
| GPenGRGDSPCA + (G7 or E7)FHRRIKA | Adsorption | HA | HMSC | ↑ | ≈ | - | - | - | [38] |
| CGGRDGS + CGGFHRRIKA | Silanisation | Titanium | RBMSC | ↑ | ↑ | - | - | - | [56] |
| CGGNGEPRGDTYRAY + CGGFHRRIKA | Silanisation | Quartz | RCO | ↑ | ↑ | - | ↑ | - | [55] |
| CGGNGEPRGDTYRAY + CGGFHRRIKA | Carbodiimide | Hydrogel | RCO | ↑ | - | - | - | - | [129] |
| RGD + KRSR | |||||||||
| GPenGRGDSPCA and (G7 or E7)KRSR | Adsorption | HA | HMSC | ↑ | ≈ | - | - | - | [38] |
| RGDS + KRSRGGG | Silanisation | Glass | RCO | ↑ | - | - | - | - | [87] |
| KRSRG3 | Hydrogel | C2SH48C2 | MG63 | ↑ | - | - | - | - | [79] |
| RGD + KRSR | Silanisation | Titanium | SAOS2 | ↑ | ↑ | - | ↑ | - | [86] |
| RGD + BMP Mimetic | |||||||||
| GGRGDS + KIPKASSVPTELSAISTLYL | Silanisation | Glass | HMSC | ↑ | ↑ | ↑ | - | - | [54] |
| GGRGDS + KIPKASSVPTELSAISMLYL | Carbodiimide | PET | MC3T3-E1 | ↑ | - | ↑ | ↑ | - | [90] |
| RTVPKPSSAPTQLNAISTLYF | Carbodiimide | PET | MC3T3-E1 | ↑ | - | ↑ | ↑ | - | [90] |
| RKVGKASSVPTKLSPISILYK | Carbodiimide | PET | MC3T3-E1 | ↑ | - | ↑ | ↑ | - | [90] |
| GRGDSPC + RKIPKASSVPTELSAISMLYL | Carbodiimide | PET | HMSC | ↑ | ↑ | - | ↑ | - | [91] |
| GGRGDS + KIPKASSVPTELSAISTLYL | Click-chemistry | SAMs | BMSC | ↑ | ↑ | - | ↑ | - | [71] |
| GGRGDS + KIPKASSVPTELSAISTLYL | Click-chemistry | Hydrogel | RBMSC | ↑ | - | ↑ | ↑ | - | [70] |
| FHRIKKA + KRSR + BMP Mimetic | |||||||||
| CFHRRIKA + CKRSR + NSPVNSKIPKACCVPTELSAI | Carbodiimide + Layer-by-Layer | PLGA/HA | RBMSC | ↑ | ↑ | ↑ | ↑ | ↑ | [128] |
| GFHRRIKA + RKIPKASSVPTELSAISMLYL | Carbodiimide | PET | HMSC | ↑ | ↑ | - | ↑ | - | [91] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. Part A 2012, 101, 3349–3364. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Caesar, B. Epidemiology of adult fractures: A review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef]
- Willie, B.M.; Petersen, A.; Schmidt-Bleek, K.; Cipitria, A.; Mehta, M.; Strube, P.; Lienau, J.; Wildemann, B.; Fratzl, P.; Duda, G. Designing biomimetic scaffolds for bone regeneration: Why aim for a copy of mature tissue properties if nature uses a different approach? Soft Matter 2010, 6, 4976–4987. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Sen, M.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38 (Suppl. S1), S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, C.; Bühren, V. General Trauma Care and Related Aspects; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 1–22. [Google Scholar] [CrossRef]
- Dawson, J.I.; Kingham, E.; Evans, N.R.; Tayton, E.; Oreffo, R.O. Skeletal Regeneration: Application of nanotopography and biomaterials for skeletal stem cell based bone repair. Inflamm. Regen. 2012, 32, 72–89. [Google Scholar] [CrossRef][Green Version]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice: The evidence for their use. Bone Jt. J. 2013, 95, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4, S343–S352. [Google Scholar] [CrossRef]
- Scarano, A.; Iezzi, G.; Petrone, G.; Orsini, G.; Degidi, M.; Strocchi, R.; Piattelli, A. Cortical Bone Regeneration With a Synthetic Cell-Binding Peptide: A Histologic and Histomorphometric Pilot Study. Implant Dent. 2003, 12, 318–324. [Google Scholar] [CrossRef]
- Arnold, P.M.; Sasso, R.C.; Janssen, M.E.; Fehlings, M.G.; Smucker, J.D.; Vaccaro, A.R.; Heary, R.F.; Patel, A.I.; Goulet, B.; Kalfas, I.H.; et al. Efficacy of i-FactorTM Bone Graft versus Autograft in Anterior Cervical Discectomy and Fusion: Results of the Prospective Randomized Single-blinded Food and Drug Administration Investiga-tional Device Exemption Study. Spine 2016, 41, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; Longaker, M.T. Concise Review: Adipose-Derived Stromal Cells for Skeletal Regenerative Medicine. Stem Cells 2011, 29, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; Borzabadi-Farahani, A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J. Cranio-Maxillofac. Surg. 2014, 42, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.N. Osteobiologics. Bull. Hosp. Jt. Dis. 2004, 62, 13–17. [Google Scholar] [CrossRef]
- Sammons, R.L. Modifying biomaterial surfaces to optimise interactions with bone. In Surface Modification of Biomaterials: Methods Analysis and Applications; Woodhead Publishing: Cambridge, UK, 2011; pp. 365–400. [Google Scholar] [CrossRef]
- Artzi, Z.; Kozlovsky, A.; Nemcovsky, C.E.; Moses, O.; Tal, H.; Rohrer, M.D.; Prasad, H.S.; Weinreb, M. Histomorphometric evaluation of natural mineral combined with a synthetic cell-binding peptide (P-15) in critical-size defects in the rat calvaria. Int. J. Oral Maxillofac. Implants 2009, 23, 1063–1070. [Google Scholar]
- Liu, Y.; Ming, L.; Luo, H.; Liu, W.; Zhang, Y.; Liu, H.; Jin, Y. Integration of a calcined bovine bone and BMSC-sheet 3D scaffold and the promotion of bone regeneration in large defects. Biomaterials 2013, 34, 9998–10006. [Google Scholar] [CrossRef]
- Jang, J.-W.; Yun, J.-H.; Lee, K.-I.; Jung, U.-W.; Kim, C.-S.; Choi, S.-H.; Cho, K.-S. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 480–487. [Google Scholar] [CrossRef]
- Cha, J.-K.; Lee, J.-S.; Kim, M.-S.; Choi, S.-H.; Cho, K.-S.; Jung, U.-W. Sinus augmentation using BMP-2 in a bovine hydroxyapatite/collagen carrier in dogs. J. Clin. Periodontol. 2014, 41, 86–93. [Google Scholar] [CrossRef]
- Pradhan, B.B.; Bae, H.W.; Dawson, E.G.; Patel, V.V.; Delamarter, R.B. Graft Resorption With the Use of Bone Morphogenetic Protein: Lessons From Anterior Lumbar Interbody Fusion Using Femoral Ring Allografts and Recombinant Human Bone Morphogenetic Protein-2. Spine 2006, 31, E277–E284. [Google Scholar] [CrossRef]
- Perry, C.R.; Cole, J.D.; Calhoun, J.H. Osteogenic Protein-1 (Bone Morphogenetic Protein-7) in the Treatment of Tibial Nonun-ions. J. Bone Jt. Surg. 2001, 83, S151–S158. [Google Scholar]
- Lammens, J.; Nijs, J.; Schepers, E.; Ectors, N.; Lismont, D.; Verduyckt, B. The effect of bone morphogenetic protein-7 (OP-1) and demineralized bone matrix (DBM) in the rabbit tibial distraction model. Acta Orthop. Belg. 2009, 75, 103–109. [Google Scholar] [PubMed]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Misra, G.; Amsden, B. Immobilization of a bone and cartilage stimulating peptide to a synthetic bone graft. J. Mater. Sci. Mater. Electron. 2008, 19, 2145–2155. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef]
- Chollet, C.; Chanseau, C.; Brouillaud, B.; Durrieu, M. RGD peptides grafting onto poly(ethylene terephthalate) with well controlled densities. Biomol. Eng. 2007, 24, 477–482. [Google Scholar] [CrossRef]
- Benoit, D.S.; Anseth, K.S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 2005, 26, 5209–5220. [Google Scholar] [CrossRef]
- Segvich, S.J.; Smith, H.C.; Kohn, D.H. The adsorption of preferential binding peptides to apatite-based materials. Biomaterials 2009, 30, 1287–1298. [Google Scholar] [CrossRef]
- Haycock, J.W. 3D Cell Culture: A Review of Current Approaches and Techniques; Springer Science and Business Media LLC: Amsterdam, The Netherlands, 2010; Volume 695, pp. 1–15. [Google Scholar] [CrossRef]
- LeBaron, R.G.; Athanasiou, K.A. Extracellular Matrix Cell Adhesion Peptides: Functional Applications in Orthopedic Materials. Tissue Eng. 2000, 6, 85–103. [Google Scholar] [CrossRef]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. J. Control. Release 2016, 244, 122–135. [Google Scholar] [CrossRef]
- Wu, P.; Hoying, J.B.; Williams, S.K.; Kozikowski, B.A.; Lauffenburger, D.A. Integrin-binding peptide in solution inhibits or en-hances endothelial cell migration, predictably from cell adhesion. Ann. Biomed. Eng. 1994, 22, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Pilling, D.; Henriquez, N.V.; Parsonage, G.; Threlfall, K.; Scheel-Toellner, D.; Simmons, D.L.; Akbar, A.N.; Lord, J.M.; Salmon, M. RGD peptides induce apoptosis by direct caspase-3 activation. Nat. Cell Biol. 1999, 397, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, K.M.; Pollot, B.E.; Clem, W.C.; Phipps, M.C.; Sawyer, A.A.; Culpepper, B.K.; Bellis, S.L. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials 2009, 30, 1898–1909. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007, 28, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef]
- Fujisawa, R.; Mizuno, M.; Nodasaka, Y.; Yoshinori, K. Attachment of osteoblastic cells to hydroxyapatite crystals by a synthetic peptide (Glu7-Pro-Arg-Gly-Asp-Thr) containing two functional sequences of bone sialoprotein. Matrix Biol. 1997, 16, 21–28. [Google Scholar] [CrossRef]
- Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanograined Ti modified with KRSR. J. Biomed. Mater. Res. Part A 2007, 80, 602–611. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Kitamura, M.; Ogata, S.-I.; Yoshihara, Y.; Masuda, S.; Ohtsuki, C.; Tanihara, M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. J. Biomed. Mater. Res. Part A 2006, 77, 700–706. [Google Scholar] [CrossRef]
- Acharya, B.; Chun, S.-Y.; Kim, S.-Y.; Moon, C.; Shin, H.-I.; Park, E.K. Surface immobilization of MEPE peptide onto HA/β-TCP ceramic particles enhances bone regeneration and remodeling. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 841–849. [Google Scholar] [CrossRef]
- Schuessele, A.; Mayr, H.; Tessmar, J.; Goepferich, A. Enhanced bone morphogenetic protein-2 performance on hydroxyapatite ceramic surfaces. J. Biomed. Mater. Res. Part A 2009, 90, 959–971. [Google Scholar] [CrossRef]
- Sawyer, A.; Hennessy, K.; Bellis, S. Regulation of mesenchymal stem cell attachment and spreading on hydroxyapatite by RGD peptides and adsorbed serum proteins. Biomaterials 2005, 26, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil Mur, F.J. Novel Peptide-Based Platform for the Dual Presentation of Biologically Active Peptide Motifs on Biomaterials. ACS Appl. Mater. Interfaces 2014, 6, 6525–6536. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.L. Targeted Delivery of Osteoinductive Peptides to Bone Graft Utilizing a Calcium Binding Domain to Enhance the Re-generative Potential. Ph.D. Thesis, The University of Alabama at Birmingham, Birmingham, AL, USA, 2014. [Google Scholar]
- Lin, Z.-Y.; Duan, Z.-X.; Guo, X.-D.; Li, J.-F.; Lu, H.-W.; Zheng, Q.-X.; Quan, D.-P.; Yang, S.-H. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J. Control. Release 2010, 144, 190–195. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Q.; Guo, X.; Zou, Z.; Liu, Y.; Lan, S.; Chen, L.; Deng, Y. Bone induction by surface-double-modified true bone ceramics in vitro and in vivo. Biomed. Mater. 2013, 8, 035005. [Google Scholar] [CrossRef]
- Rodríguez-Cano, A.; Cintas, P.; Calderón, M.F.; Pacha-Olivenza, M.-Á.; Crespo, L.; Saldaña, L.; Vilaboa, N.; González-Martín, M.-L.; Babiano, R.; Caballero, R.B. Controlled silanization–amination reactions on the Ti6Al4V surface for biomedical applications. Colloids Surf. B Biointerfaces 2013, 106, 248–257. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Zhang, Y.; Tan, Y.; Zhou, J.; Zhou, D. Immobilization of RGD peptide onto the surface of apatite-wollastonite ceramic for enhanced osteoblast adhesion and bone regeneration. J. Wuhan Univ. Technol. Sci. Ed. 2014, 29, 626–634. [Google Scholar] [CrossRef]
- Liu, Q.; Limthongkul, W.; Sidhu, G.; Zhang, J.; Vaccaro, A.; Shenck, R.; Hickok, N.; Shapiro, I.; Freeman, T. Covalent attachment of P15 peptide to titanium surfaces enhances cell attachment, spreading, and osteogenic gene expression. J. Orthop. Res. 2012, 30, 1626–1633. [Google Scholar] [CrossRef]
- Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E.; Durrieu, M.; Laroche, G. RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater. 2016, 36, 132–142. [Google Scholar] [CrossRef]
- Rezania, A.; Healy, K.E. Biomimetic Peptide Surfaces That Regulate Adhesion, Spreading, Cytoskeletal Organization, and Mineralization of the Matrix Deposited by Osteoblast-like Cells. Biotechnol. Prog. 1999, 15, 19–32. [Google Scholar] [CrossRef]
- Paredes, V.; Salvagni, E.; Rodríguez-Castellon, E.; Gil, F.J.; Manero, J.M. Study on the use of 3-aminopropyltriethoxysilane and 3-chloropropyltriethoxysilane to surface biochemical modification of a novel low elastic modulus Ti–Nb–Hf alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 495–502. [Google Scholar] [CrossRef]
- Szili, E.J.; Short, R.D.; Steele, D.A.; Bradley, J.W. Surface modification of biomaterials by plasma polymerization. In Surface Modification of Biomaterials; Williams, R., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–39. [Google Scholar] [CrossRef]
- Rosengren, A.; Pavlovic, E.; Oscarsson, S.; Krajewski, A.; Ravaglioli, A.; Piancastelli, A. Plasma protein adsorption pattern on characterized ceramic biomaterials. Biomaterials 2002, 23, 1237–1247. [Google Scholar] [CrossRef]
- Williams, R.; Blanch, H. Covalent immobilization of protein monolayers for biosensor applications. Biosens. Bioelectron. 1994, 9, 159–167. [Google Scholar] [CrossRef]
- Wang, C.; Yan, Q.; Liu, H.-B.; Zhou, X.-H.; Xiao, S.-J. Different EDC/NHS Activation Mechanisms between PAA and PMAA Brushes and the Following Amidation Reactions. Langmuir 2011, 27, 12058–12068. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef]
- Strola, S.; Ceccone, G.; Gilliand, D.; Valsesia, A.; Lisboa, P.; Rossi, F. Comparison of surface activation processes for protein immobilization on plasma-polymerized acrylic acid films. Surf. Interface Anal. 2010, 42, 1311–1315. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jung, I.-H.; Kim, Y.-K.; Lim, H.-C.; Lee, J.-S.; Jung, U.-W.; Choi, S.-H. Guided bone regeneration using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-cross-linked type-I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomater. Res. 2015, 19, 1–10. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconjug. Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mojarradi, H. Coupling of Substances Containing a Primary Amine to Hyaluronan via Carbodiimide-Mediated Amidation. Dissertation, Uppsala University, Uppsala, Sweden, 2010. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-149284 (accessed on 29 March 2021).
- Totaro, K.A.; Liao, X.; Bhattacharya, K.; Finneman, J.I.; Sperry, J.B.; Massa, M.A.; Thorn, J.; Ho, S.V.; Pentelute, B.L. Systematic Investigation of EDC/sNHS-Mediated Bioconjugation Reactions for Carboxylated Peptide Substrates. Bioconjug. Chem. 2016, 27, 994–1004. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- ElZahhar, P.; Belal, A.S.F.; Elamrawy, F.; Helal, N.A.; Nounou, M.I. Bioconjugation in Drug Delivery: Practical Perspectives and Future Perceptions; Humana: New York, NY, USA, 2019; pp. 125–182. [Google Scholar] [CrossRef]
- He, X.; Ma, J.; Jabbari, E. Effect of Grafting RGD and BMP-2 Protein-Derived Peptides to a Hydrogel Substrate on Osteogenic Differentiation of Marrow Stromal Cells. Langmuir 2008, 24, 12508–12516. [Google Scholar] [CrossRef]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011, 7, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Piluso, S.; Hiebl, B.; Gorb, S.N.; Kovalev, A.; Lendlein, A.; Neffe, A.T. Hyaluronic Acid-Based Hydrogels Crosslinked by Copper-Catalyzed Azide-Alkyne Cycloaddition with Tailorable Mechanical Properties. Int. J. Artif. Organs 2011, 34, 192–197. [Google Scholar] [CrossRef]
- Nwe, K.; Brechbiel, M.W. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother. Radiopharm. 2009, 24, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Baker, A.E.; Shoichet, M.S. Designing Peptide and Protein Modified Hydrogels: Selecting the Optimal Conjugation Strategy. J. Am. Chem. Soc. 2017, 139, 7416–7427. [Google Scholar] [CrossRef]
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. The use of immobilized osteogenic growth peptide on gradient substrates synthesized via click chemistry to enhance MC3T3-E1 osteoblast proliferation. Biomaterials 2010, 31, 1604–1611. [Google Scholar] [CrossRef]
- Shin, H.; Zygourakis, K.; Farach-Carson, M.C.; Yaszemski, M.J.; Mikos, A.G. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials 2004, 25, 895–906. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Suh, J.-S.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials 2009, 30, 3532–3541. [Google Scholar] [CrossRef]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef]
- Włodarczyk-Biegun, M.K.; Werten, M.W.T.; Posadowska, U.; Storm, I.M.; De Wolf, F.A.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Stuart, M.A.C.; Kamperman, M. Nanofibrillar hydrogel scaffolds from recombinant protein-based polymers with integrin- and proteoglycan-binding domains. J. Biomed. Mater. Res. Part A 2016, 104, 3082–3092. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nat. Cell Biol. 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Durrieu, M.-C.; Pallu, S.; Guillemot, F.; Bareille, R.; Amédée, J.; Baquey, C.; Labrugère, C.; Dard, M. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J. Mater. Sci. Mater. Med. 2004, 15, 779–786. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Dettin, M.; Conconi, M.T.; Gambaretto, R.; Pasquato, A.; Folin, M.; Di Bello, C.; Parnigotto, P.P. Novel osteoblast-adhesive peptides for dental/orthopedic biomaterials. J. Biomed. Mater. Res. 2002, 60, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Hasenbein, M.; Andersen, T.; Bizios, R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002, 23, 3937–3942. [Google Scholar] [CrossRef]
- Hoyos-Nogués, M.; Falgueras-Batlle, E.; Ginebra, M.-P.; Manero, J.M.; Gil, J.; Mas-Moruno, C. A Dual Molecular Biointerface Combining RGD and KRSR Sequences Improves Osteoblastic Functions by Synergizing Integrin and Cell-Membrane Proteoglycan Binding. Int. J. Mol. Sci. 2019, 20, 1429. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.C.; Andersen, T.T.; Bizios, R. Design and function of novel osteoblast-adhesive peptides for chemical modification of biomaterials. J. Biomed. Mater. Res. 1998, 40, 371–377. [Google Scholar] [CrossRef]
- Kim, J.W.; Ki, C.S.; Park, Y.H.; Kim, H.J.; Um, I.C. Effect of RGDS and KRSR peptides immobilized on silk fibroin nanofibrous mats for cell adhesion and proliferation. Macromol. Res. 2010, 18, 442–448. [Google Scholar] [CrossRef]
- Sofia, S.; McCarthy, M.B.; Gronowicz, G.; Kaplan, D.L. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2001, 54, 139–148. [Google Scholar] [CrossRef]
- Zouani, O.F.; Chollet, C.; Guillotin, B.; Durrieu, M.-C. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials 2010, 31, 8245–8253. [Google Scholar] [CrossRef] [PubMed]
- Padiolleau, L.; Chanseau, C.; Durrieu, S.; Chevallier, P.; Laroche, G.; Durrieu, M.-C. Single or Mixed Tethered Peptides To Promote hMSC Differentiation toward Osteoblastic Lineage. ACS Appl. Bio Mater. 2018, 1, 1800–1809. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X.; Wu, B.; Zou, Z.; Duan, Z. A novel peptide-modified and gene-activated biomimetic bone matrix accelerating bone regeneration. J. Biomed. Mater. Res. Part A 2013, 102, 2864–2874. [Google Scholar] [CrossRef]
- Connelly, J.; Petrie, T.; García, A.; Levenston, M. Fibronectin- and collagen-mimetic ligands regulate bone marrow stromal cell chondrogenesis in three-dimensional hydrogels. Eur. Cells Mater. 2011, 22, 168–177. [Google Scholar] [CrossRef]
- Scholz, M.; Schleicher, P.; Sewing, A.; Gelinsky, M.; Kandziora, F. Cyclic-RGD Is as Effective as rhBMP-2 in Anterior Interbody Fusion of the Sheep Cervical Spine. Spine 2013, 38, E59–E65. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Mochizuki, M.; Rothenfluh, D.A.; Rempel, S.A.; Hubbell, J.A.; Barker, T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 2009, 30, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Capadona, J.R.; Reyes, C.D.; García, A.J. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials 2006, 27, 5459–5470. [Google Scholar] [CrossRef]
- Nakamura, H.; Ozawa, H. Immunohistochemical localization of heparan sulfate proteoglycan in rat tibiae. J. Bone Miner. Res. 2009, 9, 1289–1299. [Google Scholar] [CrossRef]
- Gentile, P.; Ghione, C.; Tonda-Turo, C.; Kalaskar, D.M. Peptide functionalisation of nanocomposite polymer for bone tissue engineering using plasma surface polymerisation. RSC Adv. 2015, 5, 80039–80047. [Google Scholar] [CrossRef]
- Healy, K.E.; Rezania, A.; Stile, R.A. Designing Biomaterials to Direct Biological Responses. Ann. N. Y. Acad. Sci. 1999, 875, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Hamilton, D.W.; Kunzler, T.P.; Sprecher, C.M.; De Wild, M.; Brunette, D.M.; Textor, M.; Tosatti, S.G.P. Comparison of the response of cultured osteoblasts and osteoblasts outgrown from rat calvarial bone chips to nonfouling KRSR and FHRRIKA-peptide modified rough titanium surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Balasundaram, G.; Webster, T.J. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite func-tionalized with KRSR. Int. J. Nanomed. 2006, 1, 339–349. [Google Scholar]
- Anderson, J.M.; Kushwaha, M.; Tambralli, A.; Bellis, S.L.; Camata, R.P.; Jun, H.-W. Osteogenic Differentiation of Human Mesenchymal Stem Cells Directed by Extracellular Matrix-Mimicking Ligands in a Biomimetic Self-Assembled Peptide Amphiphile Nanomatrix. Biomacromolecules 2009, 10, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; García, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. Part A 2010, 96, 261–272. [Google Scholar] [CrossRef]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; García, A.J.; Guldberg, R.E. Nanofiber Orientation and Surface Functionalization Modulate Human Mesenchymal Stem Cell Behavior In Vitro. Tissue Eng. Part A 2014, 20, 398–409. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Gough, C.A. The Role in Cell Binding of a β1-bend within the Triple Helical Region in Collagen αl(I) Chain: Structural and Biological Evidence for Conformational Tautomerism on Fiber Surface. J. Biomol. Struct. Dyn. 1997, 14, 547–560. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of Biomimetic Habitats for Tissue Engineering with P-15, a Synthetic Peptide Analogue of Collagen. Tissue Eng. 1999, 5, 53–65. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Smith, N. Construction of Biomimetic Environments with A Synthetic Peptide Analogue of Collagen. MRS Online Proc. Libr. 1998, 530, 43. [Google Scholar] [CrossRef]
- Pedersen, R.H.; Rasmussen, M.; Overgaard, S.; Ding, M. Effects of P-15 Peptide Coated Hydroxyapatite on Tibial Defect Repair In Vivo in Normal and Osteoporotic Rats. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yukna, R.A.; Callan, D.P.; Krauser, J.T.; Evans, G.H.; Aichelmann-Reidy, M.E.; Moore, K.; Cruz, R.; Scott, J.B. Multi-Center Clinical Evaluation of Combination Anorganic Bovine-Derived Hydroxyapatite Matrix (ABM)/Cell Binding Peptide (P-15) as a Bone Replacement Graft Material in Human Periodontal Osseous Defects. 6-Month Results. J. Periodontol. 1998, 69, 655–663. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Maharaj, M.; Rao, P.J. Clinical outcomes and fusion rates following anterior lumbar interbody fusion with bone graft substitute i-FACTOR, an anorganic bone matrix/P-15 composite. J. Neurosurg. Spine 2014, 21, 867–876. [Google Scholar] [CrossRef]
- Novaes, A.B.; Fernandes, P.G.; Suaid, F.A.; De Moraes Grisi, M.F.; De Souza, S.L.S.; Taba, M.; Palioto, D.B.; Muglia, V.A. Ridge preservation with acellular dermal matrix and anorganic bone matrix cell-binding peptide P-15 after tooth extraction in humans. A histologic and morphometric study. J. Osseointegr. 2012, 4, 23–30. [Google Scholar] [CrossRef]
- Nguyen, H.; Qian, J.J.; Bhatnagar, R.S.; Li, S. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem. Biophys. Res. Commun. 2003, 311, 179–186. [Google Scholar] [CrossRef]
- Gomar, F.; Orozco, R.; Villar, J.L.; Arrizabalaga, F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial. Int. Orthop. 2006, 31, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.R.; Novaes, A.B.; Roriz, V.M.; Oliveira, R.R.; Grisi, M.F.; Souza, S.L.; Taba, M.; Palioto, D.B.; Mario, J.T. Anorganic Bovine Matrix/P-15 “Flow” in the Treatment of Periodontal Defects: Case Series With 12 Months of Follow-Up. J. Periodontol. 2006, 77, 1280–1287. [Google Scholar] [CrossRef]
- Yang, X.B.; Bhatnagar, R.S.; Li, S.; Oreffo, R.O. Biomimetic Collagen Scaffolds for Human Bone Cell Growth and Differentiation. Tissue Eng. 2004, 10, 1148–1159. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Koepple, M.; Moest, T.; Neumann, K.; Weisel, T.; Schlegel, K.A. In vivo evaluation of biofunctionalized implant surfaces with a synthetic peptide (P-15) and its impact on osseointegration. A preclinical animal study. Clin. Oral Implants Res. 2015, 27, 1339–1348. [Google Scholar] [CrossRef]
- Kasaj, A.; Röhrig, B.; Reichert, C.; Willershausen, B. Clinical evaluation of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide (P-15) in the treatment of human infrabony defects. Clin. Oral Investig. 2008, 12, 241–247. [Google Scholar] [CrossRef]
- Duan, Z.; Zheng, Q.; Guo, X.; Yuan, Q.; Chen, S. Experimental research on ectopic osteogenesis of BMP2-derived peptide P24 combined with PLGA copolymers. Acta Acad. Med. Wuhan 2007, 27, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Suzuki, Y.; Ogata, S.-I.; Ohtsuki, C.; Tanihara, M. Prolonged ectopic calcification induced by BMP-2-derived synthetic peptide. J. Biomed. Mater. Res. 2004, 70, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zheng, Q.; Guo, X.; Wu, Y.; Wang, Y.; Cui, F. Preparation and ectopic osteogenesis in vivo of scaffold based on mineralized recombinant human-like collagen loaded with synthetic BMP-2-derived peptide. Biomed. Mater. 2008, 3, 044111. [Google Scholar] [CrossRef]
- Kumar Boda, S.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J.; Author, A.B. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration HHS Public Access Author manuscript. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Ogata, S.-I.; Ohtsuki, C.; Tanihara, M. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J. Biomed. Mater. Res. 2004, 72, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tanihara, M.; Suzuki, K.; Saitou, A.; Sufan, W.; Nishimura, Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J. Biomed. Mater. Res. 2000, 50, 405–409. [Google Scholar] [CrossRef]
- Zouani, O.F.; Kalisky, J.; Ibarboure, E.; Durrieu, M.-C. Effect of BMP-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials 2013, 34, 2157–2166. [Google Scholar] [CrossRef]
- He, X.; Yang, X.; Jabbari, E. Combined Effect of Osteopontin and BMP-2 Derived Peptides Grafted to an Adhesive Hydrogel on Osteogenic and Vasculogenic Differentiation of Marrow Stromal Cells. Langmuir 2012, 28, 5387–5397. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer Nanoscale Encapsulation of Biofunctional Peptides to Enhance Bone Tissue Regeneration In Vivo. Adv. Health Mater. 2017, 6, 1601182. [Google Scholar] [CrossRef] [PubMed]
- Stile, R.A.; Healy, K.E. Thermo-Responsive Peptide-Modified Hydrogels for Tissue Regeneration. Biomacromolecules 2001, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]


| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| GPenGRGDSPCA | Adsorption | HA | HMSC | ↑ | ↓ | - | - | - | [37] |
| RGD | Adsorption | Titanium | SAOS-2 | ↑ | ↑ | ↑ | - | - | [47] |
| GPenGRGDSPCA | Adsorption | HA | HMSC | ↑ | ↓ | - | - | - | [38] |
| RGD | Adsorption | PS | RCO | ↑ | - | - | - | - | [84] |
| GRGDSPK | Adsorption | PS | RCO | ↑ | - | - | - | - | [84] |
| MAP(RGDSP) | Adsorption | PS | RCO | ↑ | - | - | - | - | [84] |
| (GRGDSP)4K | Adsorption | ABM | RCO | ↑ | - | - | - | - | [84] |
| EEEEEEEPRGDT | Adsorption | HA | MC3T3-E1 | ↑ | ↑ | - | - | - | [40] |
| RGDS | Adsorption | Glass | RCO | ↑ | - | - | - | - | [85] |
| YRGDSPC | Silanisation | HA | HO | ↑ | - | - | - | - | [39] |
| CGGRGDS | Silanisation | Titanium | RBMSC | ↑ | ↑ | - | - | - | [56] |
| RGD | Silanisation | Titanium | SAOS2 | ↑ | ↑ | - | ↑ | - | [86] |
| CGGNGEPRGDTYRAY | Silanisation | Quartz | RCO | ↑ | ↑ | - | ↑ | - | [55] |
| RGD | Silanisation | HA | MG63 | ↑ | ↑ | - | - | ↑ | [52] |
| GGRGDS | Silanisation | Glass | HMSC | ↑ | ↑ | ≈ | - | - | [54] |
| RGDS | Silanisation | Glass | RCO | ≈ | - | - | - | - | [87] |
| RGDS | Carbodiimide | Silk | HO | ↑ | ↑ | - | - | - | [88] |
| GRGDS | Carbodiimide | Silk | SAOS-2 | ↑ | ↑ | ↑ | - | - | [89] |
| RGDSPC | Carbodiimide | PET | MC3T3-E1 | ↑ | - | - | - | - | [90] |
| GRDSPC | Carbodiimide | PET | HMSC | ↑ | ↑ | - | ↑ | - | [91] |
| K16(GRGDSPC) + BMSCs | Carbodiimide | PLGA | BMSC | - | - | ≈ | ≈ | ≈ | [92] |
| K16(GRGDSPC) + TGF-β + BMSCs | Carbodiimide | PLGA | BMSC | - | - | ↑ | ↑ | ↑ | [92] |
| GRGDSP | Carbodiimide | Hydrogel | BMSC | ↑ | ≈ | ↑ | - | - | [93] |
| RGDS | Click-chemistry | SAMs | BMSC | ↑ | ≈ | ≈ | ≈ | - | [71] |
| GRGD | Click-chemistry | Hydrogel | RBMSC | ↑ | - | ↑ | ↑ | - | [70] |
| GRGDS | Hydrogel | PEG | RBMSC | ↑ | ↑ | - | - | - | [76] |
| RGDG | Hydrogel | PEG | RCO | ↑ | ↑ | ≈ | ↓ | - | [29] |
| GRGDSPGGSGGGSGGGSGGRGDSP | Hydrogel | C2SH48C2 | MG63 | ↑ | - | - | - | - | [79] |
| GRGDS + BMP-2 | Hydrogel | PEG | HMSC | ↑ | ↑ | ↑ | ↑ | ≈ | [78] |
| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| PHSRN | Adsorption | Titanium | SAOS-2 | ≈ | ≈ | ≈ | - | - | [47] |
| CGGPHRSN | Silanisation | Titanium | RBMSC | ↑ | ↑ | - | - | - | [56] |
| RGD + PHSRN | Adsorption | Titanium | SAOS-2 | ↑ | ↑ | ≈ | - | - | [47] |
| RGD + PHSRN | Adsorption | Titanium | SAOS-2 | ↑ | ↑ | ≈ | - | - | [47] |
| GRGD + G13 + PHSRN | Adsorption | PS | BMSC | ↑ | - | ↑ | - | - | [95] |
| CGGRDGS + CGGPHSRN | Silanisation | Titanium | RBMSC | ↑ | ≈ | - | - | - | [56] |
| GRGD + G13 + PHSRN | Carbodiimide | SAMs | MC3T3-E1 | ↑ | - | - | - | - | [96] |
| GRGD + G13 + PHSRN | Hydrogel | PEG | RCO | ↑ | ↑ | ↑ | ↓ | - | [29] |
| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| (G7 or E7)FHRRIKA | Adsorption | HA | HMSC | ↑ | ↑ | - | - | - | [38] |
| CGGFHRRIKA | Silanisation | Quartz | RCO | ↑ | ↑ | - | ≈ | - | [55] |
| CGGFHRRIKA | Silanisation | Titanium | RBMSC | ↑ | ↑ | - | - | - | [56] |
| FHRRIKA | Carbodiimide | POSS-PCU | BMSC | ↑ | ↑ | ↑ | - | - | [98] |
| GFHRRIKA | Carbodiimide | PET | HMSC | ↑ | ↑ | - | ↑ | - | [91] |
| (G7 or E7)KRSR | Adsorption | HA | HMSC | ↑ | ↑ | - | - | - | [38] |
| KRSR | Adsorption | PS | RCO | ↑ | - | - | - | - | [84] |
| KRSR | Adsorption | PS | RCO | ↑ | - | - | - | - | [84] |
| MAP(KRSR) | Adsorption | PS | RCO | ↑ | - | - | - | - | [84] |
| KRSR | Adsorption | Glass | RCO | ↑ | - | - | - | - | [85] |
| KRSRGGG | Silanisation | Glass | RCO | ↑ | - | - | - | - | [87] |
| KRSRGYC | Silanisation | Titanium | HO | ↑ | - | - | - | - | [41] |
| KRSR | Silanisation | Titanium | SAOS2 | ↑ | ↑ | - | ↑ | - | [86] |
| KRSR | Carbodiimide | Silk | HO | ↑ | ↑ | - | - | - | [88] |
| KRSR | Carbodiimide | POSS-PCU | BMSC | ↑ | ↑ | ≈ | - | - | [98] |
| FHRIKKA + KRSR | Carbodiimide | POSS-PCU | BMSC | ↑ | ↑ | ↑ | - | - | [98] |
| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| GPC(GPP)5GFOGER(GPP)5GPC | Adsorption | HA | HMSC | ≈ | ↓ | - | - | - | [37] |
| GGYGGGPC(GPP)5 GFOGER | Adsorption | PCL | HMSC | ↑ | ↑ | ↑ | - | - | [106] |
| GGYGGGPC(GPP)5 GFOGER(GPP)5GPC | Adsorption | Titanium | BMSC | ↑ | ↑ | ↑ | ↑ | ↑ | [104] |
| GGYGGGPC(GPP)5 GFOGER(GPP)5GPC | Adsorption | PCL | BMSC | - | - | - | - | ↑ | [105] |
| GGYGGGPC(GPP)5 GFOGER(GPP)5GPC | Carbodiimide | Hydrogel | BMSC | ↑ | ↑ | ↑ | - | - | [93] |
| GGYGGGPC(GPP)5 GFOGER(GPP)5GPC | Hydrogel | PEG | HMSC | - | - | - | - | ↑ | [78] |
| GGYGGGPC(GPP)5 GFOGER(GPP)5GPC + BMP-2 | Hydrogel | PEG | HMSC | ↑ | ↑ | ↑ | ↑ | ↑ | [78] |
| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| GTPGPQGIAGQRGVV | Adsorption | HA | HMSC | ↑ | ↑ | ↑ | ↑ | ↑ | [37] |
| GTPGPQGIAGQRGVV | Adsorption | HA | HMSC | ↑ | ↑ | ↑ | ↑ | - | [117] |
| GTPGPQGIAGQRGVV | Silanisation | Titanium | C3H10T1 | ↑ | ↑ | ↑ | ↑ | - | [53] |
| Peptide Sequence + Additional Bioactive Components | Attachment Methodology | Substrate | Cell Type | In Vitro Attachment | In Vitro Spreading | In Vitro Differentiation | In Vitro Mineralisation | In Vivo Efficacy | Publication |
|---|---|---|---|---|---|---|---|---|---|
| KIPKASSVPTELSAISTLYLDDD | Adsorption | TBC-Collagen | HO | ↑ | ↑ | ↑ | ↑ | ↑ | [50] |
| KIPKASSVPTELSAISTLYL | Adsorption | αTCP | - | - | - | - | - | ↑ | [43] |
| KIPKASSVPTELSAISTLYLDDD | Adsorption | HA/Collagen/PLA | - | - | - | - | - | ↑ | [122] |
| KIPKASSVPTELSAISTLYLDDD | Adsorption + encapsulation | HA/Collagen/PLLA | HMSC | ↑ | ↑ | ↑ | - | - | [42] |
| EEEEEEEKIPKASSVPTELSAISTLYL | Adsorption | PLGA | - | - | - | - | - | ↑ | [123] |
| KIPKASSVPTELSAISTLYL | Silanisation | Glass | HMSC | ↑ | ↑ | ↑ | - | - | [54] |
| KIPKASSVPTELSAISTLYLDDD | Carbodiimide | PLGA | - | - | - | - | - | ↑ | [120] |
| KIPKASSVPTELSAISTLYLDDD | Carbodiimide | PLGA | HO | ↑ | ↑ | ↑ | ↑ | ↑ | [49] |
| KIPKASSVPTELSAISTLYLDDD | Carbodiimide | Alginate | C3H10T | ↑ | - | ↑ | ↑ | - | [121] |
| KIPKASSVPTELSAISTLYLDDD | Carbodiimide | Alginate | - | - | - | - | - | ↑ | [121] |
| KIPKASSVPTELSAISTLYLDDD | Carbodiimide | Alginate | - | - | - | - | - | ↑ | [124] |
| NSVNSKIPKACCVPTELSAI | Carbodiimide | Alginate | - | - | - | - | - | ↑ | [125] |
| KIPKASSVPTELSAISMLYL | Carbodiimide | PAA | HMSC | ↑ | ↑ | ↑ | - | - | [126] |
| KIPKASSVPTELSAISTLYL | Click-chemistry | SAMs | BMSC | ↑ | ↑ | ↑ | ≈ | - | [71] |
| KIPKASSVPTELSAISTLYL | Click-chemistry | Hydrogel | RBMSC | ↑ | - | ↑ | ↑ | - | [70] |
| KIPKASSVPTELSAISTLYL + SVVYGLR | Click-chemistry | Hydrogel | RBMSC | ↑ | ↑ | ↑ | ↑ | - | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes. J. Funct. Biomater. 2021, 12, 22. https://doi.org/10.3390/jfb12020022
Bullock G, Atkinson J, Gentile P, Hatton P, Miller C. Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes. Journal of Functional Biomaterials. 2021; 12(2):22. https://doi.org/10.3390/jfb12020022
Chicago/Turabian StyleBullock, George, Joss Atkinson, Piergiorgio Gentile, Paul Hatton, and Cheryl Miller. 2021. "Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes" Journal of Functional Biomaterials 12, no. 2: 22. https://doi.org/10.3390/jfb12020022
APA StyleBullock, G., Atkinson, J., Gentile, P., Hatton, P., & Miller, C. (2021). Osteogenic Peptides and Attachment Methods Determine Tissue Regeneration in Modified Bone Graft Substitutes. Journal of Functional Biomaterials, 12(2), 22. https://doi.org/10.3390/jfb12020022