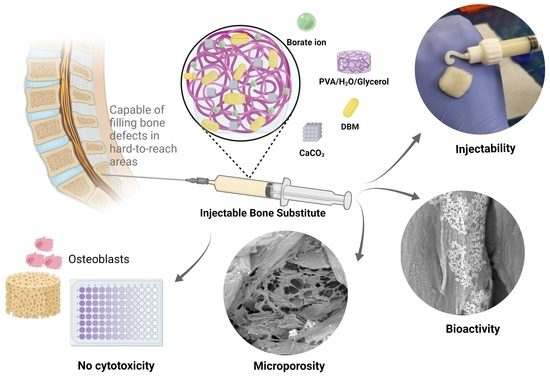
Formulation and Characterization of a New Injectable Bone Substitute Composed PVA/Borax/CaCO3 and Demineralized Bone Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
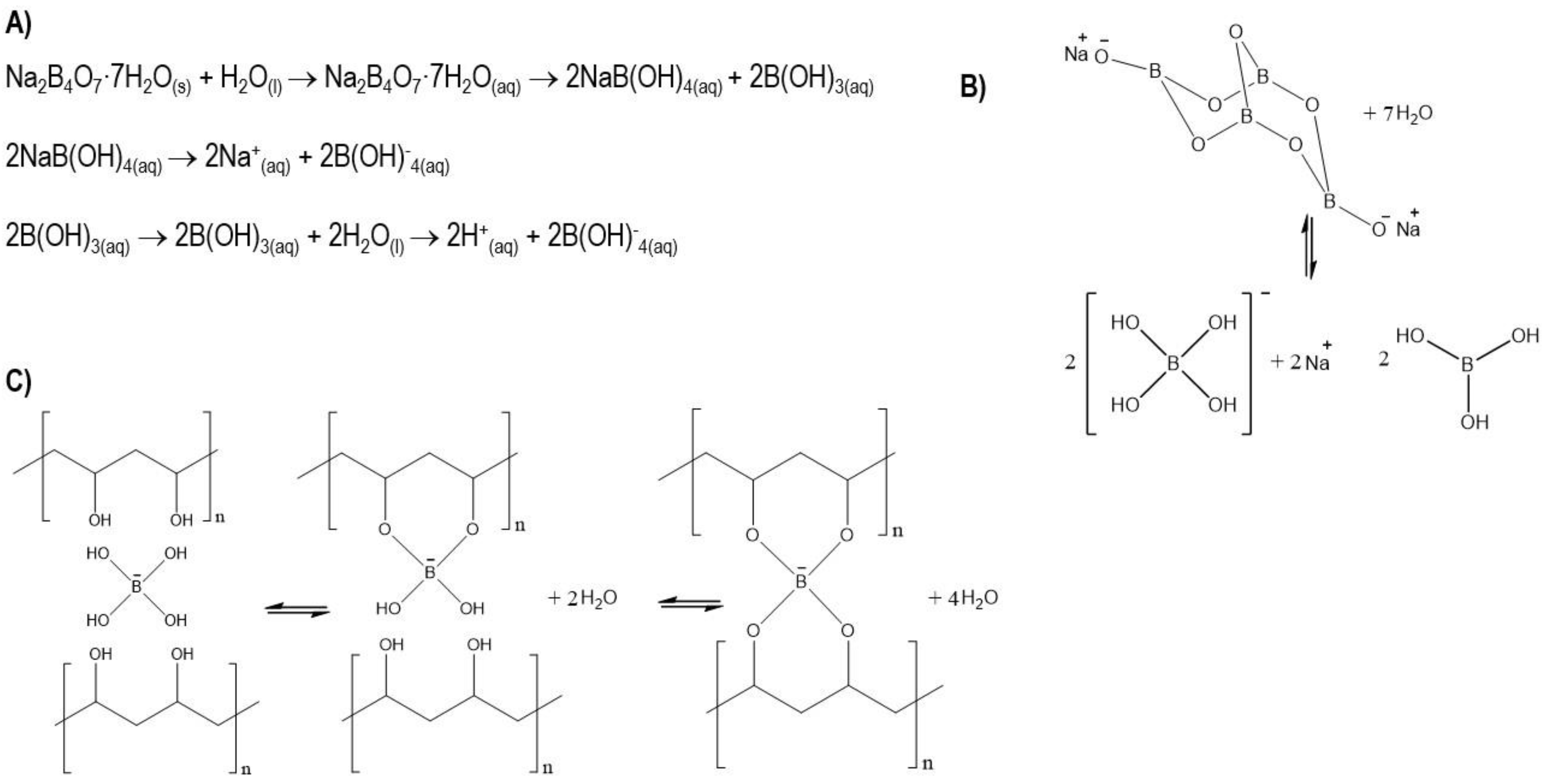
2.2.1. Injectable Bone Substitute Preparation
2.2.2. Injectability
2.2.3. Physicochemical Characterization
2.2.4. Degradation
2.2.5. Bioactivity Evaluation
2.2.6. Cell Culture
2.2.7. MTT Assay
2.2.8. Cell Proliferation
2.2.9. Statistical Analysis
3. Results
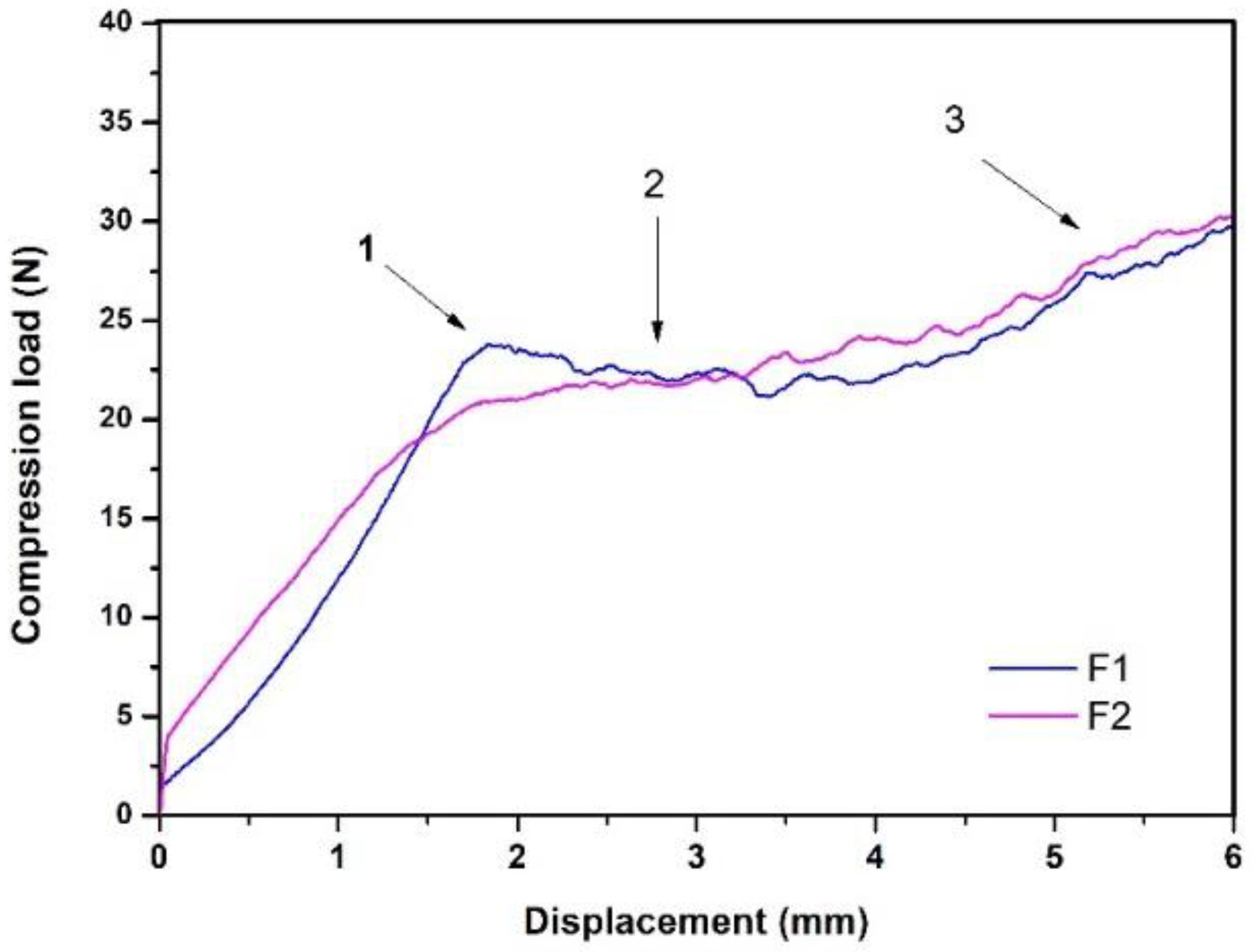
3.1. Injectability
- “Overexertion” or “overshoot”: initial overstrain is required to overcome hydraulic pressure inside the syringe.
- Platea or plateau: this area indicates a greater presence of solids; in this case, the plateau in this case.
- The maximum effort at the end of the injection: this indicates the point of mechanical resistance exerted by the plunger against the end of the syringe.
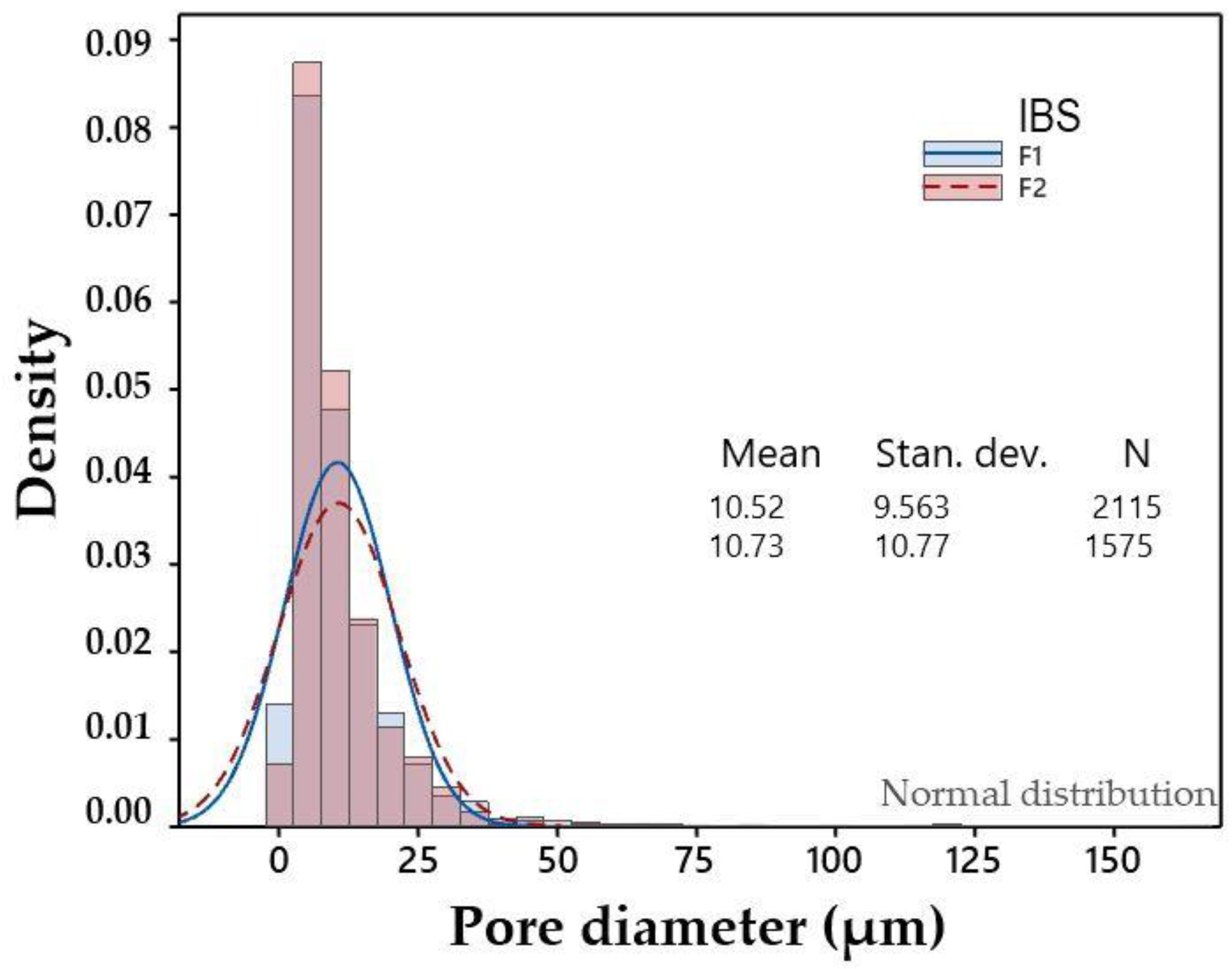
3.2. Physicochemical Characterization
3.3. Degradation
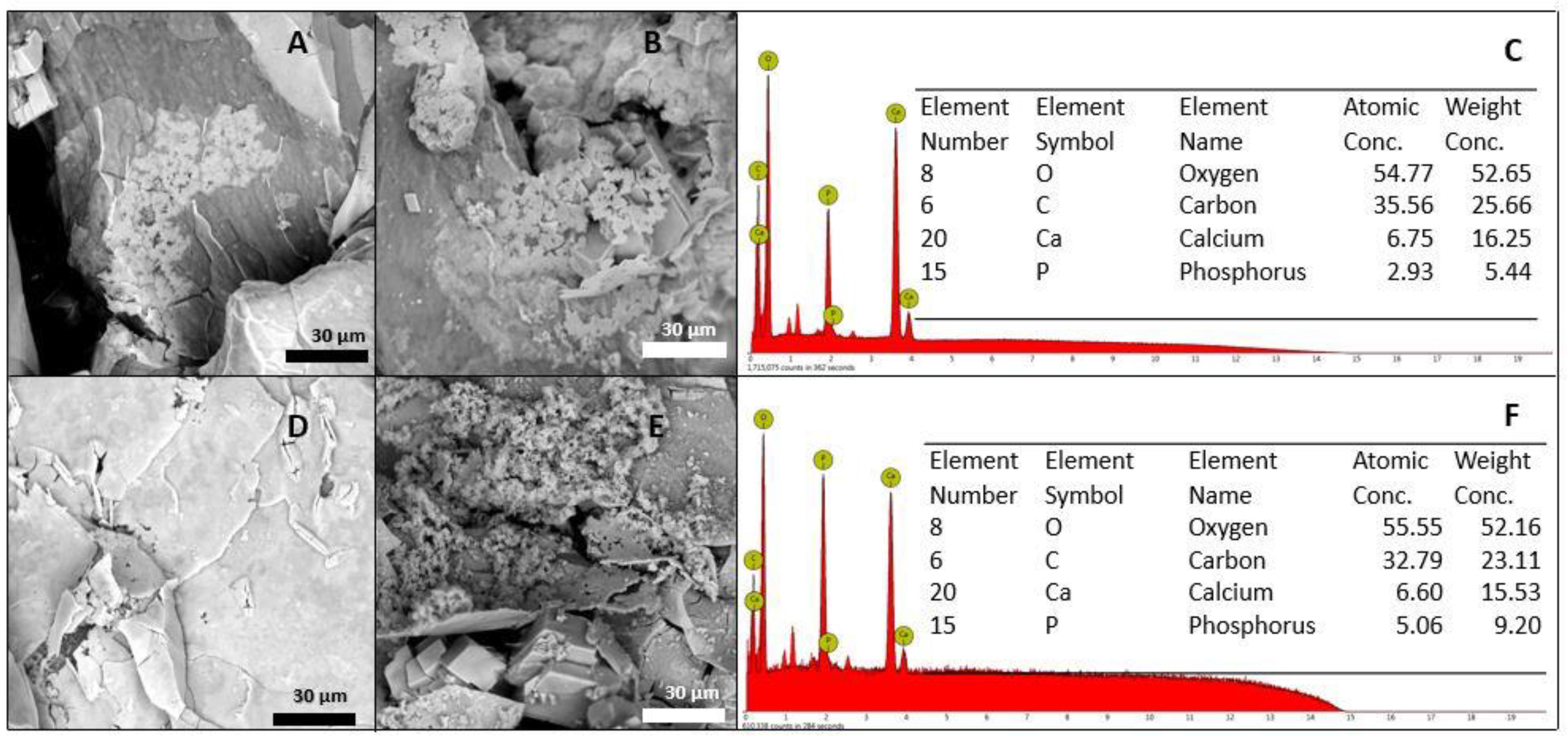
3.4. Bioactivity Evaluation
3.5. MTT Assay
3.6. Cell Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González Ocampo, J.I.; Machado de Paula, M.M.; Bassous, N.J.; Lobo, A.O.; Ossa Orozco, C.P.; Webster, T.J. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Acta Biomater. 2019, 83, 425–434. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, R.; Hou, J.; Nan, X.; Xia, Y.; Guo, Y.; Meng, K.; Xu, C.; Wang, X.; Zhao, B. Application of injectable silk fibroin/graphene oxide hydrogel combined with bone marrow mesenchymal stem cells in bone tissue engineering. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 604, 125318. [Google Scholar] [CrossRef]
- Ren, K.; Cui, H.; Xu, Q.; He, C.; Li, G.; Chen, X. Injectable Polypeptide Hydrogels with Tunable Microenvironment for 3D Spreading and Chondrogenic Differentiation of Bone-Marrow-Derived Mesenchymal Stem Cells. Biomacromolecules 2016, 17, 3862–3871. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Z.; Gu, Z.; Xu, J.; Zhao, M.; Liu, G.; Wu, J. Acid-responsive composite hydrogel platform with space-controllable stiffness and calcium supply for enhanced bone regeneration. Chem. Eng. J. 2020, 396, 125353. [Google Scholar] [CrossRef]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C 2018, 96, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.N.; Amirthalingam, S.; Mony, U.; Varma, P.K.; Jayakumar, R. Injectable chitosan-nano bioglass composite hemostatic hydrogel for effective bleeding control. Int. J. Biol. Macromol. 2019, 129, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, A.; Abdouss, M.; Afshar Taromi, F. Fabrication of biocompatible antibacterial nanowafers based on HNT/PVA nanocomposites loaded with minocycline for burn wound dressing. Mater. Sci. Eng. C 2020, 110, 110685. [Google Scholar] [CrossRef]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Das, P.; Ojah, N.; Kandimalla, R.; Mohan, K.; Gogoi, D.; Dolui, S.K.; Choudhury, A.J. Surface modification of electrospun PVA/chitosan nanofibers by dielectric barrier discharge plasma at atmospheric pressure and studies of their mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2018, 114, 1026–1032. [Google Scholar] [CrossRef]
- Ai, J.; Li, K.; Li, J.; Yu, F.; Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 2021, 172, 66–73. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104837. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Takada, A.; Nemoto, N. Dynamic light scattering and dynamic viscoelasticity of poly(vinyl alcohol) in aqueous borax solutions. 5. Temperature effects. Macromolecules 1999, 32, 8872–8879. [Google Scholar] [CrossRef]
- Mahjoub, H.F.; Zammali, M.; Abbes, C.; Othman, T. Microrheological study of PVA/borax physical gels: Effect of chain length and elastic reinforcement by sodium hydroxide addition. J. Mol. Liq. 2019, 291, 111272. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Chen, Q. Conformation of dilute poly(vinyl alcohol)-borax complex by asymmetric flow field-flow fractionation. J. Chromatogr. A 2020, 1624, 461260. [Google Scholar] [CrossRef]
- Lin, H.-L.; Liu, Y.-F.; Yu, T.L.; Liu, W.-H.; Rwei, S.-P. Light scattering and viscoelasticity study of poly(vinyl alcohol)-borax aqueous solutions and gels. Polymer 2005, 46, 5541–5549. [Google Scholar] [CrossRef]
- Zhao, C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-Triggered, Self-Assembled, and Bioprintable Hybrid Hydrogel Scaffold for Mesenchymal Stem Cell Based Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 51. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.D.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kato, M.; Taguchi, T.; Ikoma, T.; Miyashita, H.; Shimmura, S.; Tsubota, K.; Tanaka, J. Collagen immobilized PVA hydrogel-hydroxyapatite composites prepared by kneading methods as a material for peripheral cuff of artificial cornea. Mater. Sci. Eng. C 2004, 24, 729–735. [Google Scholar] [CrossRef]
- Hameed, N.; Glattauer, V.; Ramshaw, J.A.M. Evaluation of polyvinyl alcohol composite membranes containing collagen and bone particles. J. Mech. Behav. Biomed. Mater. 2015, 48, 38–45. [Google Scholar] [CrossRef]
- Kinard, L.A.; Dahlin, R.L.; Lam, J.; Lu, S.; Lee, E.J.; Kasper, F.K.; Mikos, A.G. Synthetic biodegradable hydrogel delivery of demineralized bone matrix for bone augmentation in a rat model. Acta Biomater. 2014, 10, 4574–4582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Yang, Z.; Kuwahara, K.; Nimni, M.E.; Wan, C.; Han, B. Delivery of demineralized bone matrix powder using a thermogelling chitosan carrier. Acta Biomater. 2012, 8, 753–762. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium Carbonate Nanoparticles; Potential in Bone and Tooth Disorders. Pharm. Sci. 2015, 20, 175–182. [Google Scholar]
- He, F.; Zhang, J.; Yang, F.; Zhu, J.; Tian, X.; Chen, X. In vitro degradation and cell response of calcium carbonate composite ceramic in comparison with other synthetic bone substitute materials. Mater. Sci. Eng. C 2015, 50, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Eglin, D.; Mortisen, D.; Alini, M. Degradation of synthetic polymeric scaffolds for bone and cartilage tissue repairs. Soft Matter 2009, 5, 938–947. [Google Scholar] [CrossRef]
- Hikmawati, D.; Maulida, H.N.; Putra, A.P.; Budiatin, A.S.; Syahrom, A. Synthesis and Characterization of Nanohydroxyapatite-Gelatin Composite with Streptomycin as Antituberculosis Injectable Bone Substitute. Int. J. Biomater. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J. 2019, 119, 155–168. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, F.; Zhang, W.; Yang, Z.; Luo, M.; Liu, L.; Cao, X.; Chen, D.; Chen, X. Degradable photothermal bioactive glass composite hydrogel for the sequential treatment of tumor-related bone defects: From anti-tumor to repairing bone defects. Chem. Eng. J. 2021, 419, 129520. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Salick, M.R.; Cordie, T.; Crone, W.C.; Peng, X.F.; Turng, L.S. Morphology, mechanical properties, and shape memory effects of poly(lactic acid)/thermoplastic polyurethane blend scaffolds prepared by thermally induced phase separation. J. Cell. Plast. 2014, 50, 361–379. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Han, J.; Mo, J.; Dong, P.; Zhuo, Y.; Feng, Y. Biocompatiable silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose nanocrystal composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 136, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. SaOS2 osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef]
- Pal, A.; Vernon, B.L.; Nikkhah, M. Therapeutic neovascularization promoted by injectable hydrogels. Bioact. Mater. 2018, 3, 389–400. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.M.; Tancret, F.; Weiss, P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: Syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef]
- Bohner, M.; Baroud, G. Injectability of calcium phosphate pastes. Biomaterials 2005, 26, 1553–1563. [Google Scholar] [CrossRef]
- Shaw, D.H. Drugs Acting on the Gastrointestinal Tract. In Pharmacology and Therapeutics for Dentistry: Seventh Edition; Elsevier: Amsterdam, The Netherlands, 2017; pp. 404–416. ISBN 9780323393072. [Google Scholar]
- Oyeneyin, B. Introduction to the Hydrocarbon Composite Production System; Elsevier: Amsterdam, The Netherlands, 2015; Volume 63, ISBN 9780444626370. [Google Scholar]
- Vu, A.A.; Burke, D.A.; Bandyopadhyay, A.; Bose, S. Effects of surface area and topography on 3D printed tricalcium phosphate scaffolds for bone grafting applications. Addit. Manuf. 2021, 39, 101870. [Google Scholar] [CrossRef]
- El-Bassyouni, G.T.; Guirguis, O.W.; Abdel-Fattah, W.I. Morphological and macrostructural studies of dog cranial bone demineralized with different acids. Curr. Appl. Phys. 2013, 13, 864–874. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S.; Panduranga Rao, K. Nanoporous hydroxy-carbonate apatite scaffold made of natural bone. Mater. Lett. 2006, 60, 2844–2847. [Google Scholar] [CrossRef]
- Qashou, S.I.; El-Zaidia, E.F.M.; Darwish, A.A.A.; Hanafy, T.A. Methylsilicon phthalocyanine hydroxide doped PVA films for optoelectronic applications: FTIR spectroscopy, electrical conductivity, linear and nonlinear optical studies. Phys. B Condens. Matter 2019, 571, 93–100. [Google Scholar] [CrossRef]
- Peppas, N.A. Tear propagation resistance of semicrystalline polymeric networks. Polymer (Guildf) 1977, 18, 403–407. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Thomas, J.; Lowman, A.; Marcolongo, M. Novel associated hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. Part A 2003, 67, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, T.; Wang, F. The physical and chemical properties of the polyvinylalcohol/polyvinylpyrrolidone/hydroxyapatite composite hydrogel. Mater. Sci. Eng. C 2016, 59, 948–957. [Google Scholar] [CrossRef]
- Harrison, J.P.; Berry, D. Vibrational spectroscopy for imaging single microbial cells in complex biological samples. Front. Microbiol. 2017, 8, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Kitaoka, Y.; Kobayashi, M. Complex formation of boric acids with DI- and TRI- carboxylic acids and poly(vinyl alcohol) in aqueous solutions. Macromol. Symp. 1997, 114, 303–308. [Google Scholar] [CrossRef]
- Huang, M.; Hou, Y.; Li, Y.; Wang, D.; Zhang, L. High performances of dual network PVA hydrogel modified by PVP using borax as the structure-forming accelerator. Des. Monomers Polym. 2017, 20, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera, J.; Rodríguez, J.; Perilla, J.; Algecira, N. Estudio de la degradación térmica de poli(alcohol vinílico) mediante termogravimetría y termogravimetría diferencial. Ing. Investig. 2007, 27, 100–105. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-84887494116&origin=inward&txGid=4a7491656262c8f2ee6a5b7748c813a6 (accessed on 22 November 2020).
- Premalatha, M.; Vijaya, N.; Selvasekarapandian, S.; Selvalakshmi, S. Characterization of blend polymer PVA-PVP complexed with ammonium thiocyanate. Ionics (Kiel) 2016, 22, 1299–1310. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Zhang, Y.; Wan, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. C 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Gupta, A.; Kumari, M. Dielectric and Modulus spectra (Bode)—Studies on electrical conductivity and dielectric behaviour of PVdF–HFP–PMMA–NaI polymer blend electrolyte. Bull. Mater. Sci. 2012, 35, 969–975. [Google Scholar] [CrossRef]
- Yang, C.C.; Lee, Y.J.; Chiu, S.J.; Lee, K.T.; Chien, W.C.; Lin, C.T.; Huang, C.A. Preparation of a PVA/HAP composite polymer membrane for a direct ethanol fuel cell (DEFC). J. Appl. Electrochem. 2008, 38, 1329–1337. [Google Scholar] [CrossRef]
- Sun, S.; Gebauer, D.; Cölfen, H. A solvothermal method for synthesizing monolayer protected amorphous calcium carbonate clusters. Chem. Commun. 2016, 52, 7036–7038. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, Y.; Lv, Y.; Yin, P.; Lei, T. Porous calcite CaCO3 microspheres: Preparation, characterization and release behavior as doxorubicin carrier. Colloids Surf. B Biointerfaces 2020, 186, 110720. [Google Scholar] [CrossRef]
- Freyman, T.M.; Yannas, I.V.; Gibson, L.J. Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 2001, 46, 273–282. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Minagar, S.; Lin, J.; Li, Y.; Berndt, C.C.; Wen, C. Nanotopography and surface chemistry of TiO2-ZrO2-ZrTiO4 nanotubular surfaces and the influence on their bioactivity and cell responses. In Metallic Foam Bone: Processing, Modification and Characterization and Properties; Woodhead Publishing: Sawston, UK, 2017; pp. 181–202. ISBN 9780081012901. [Google Scholar]
- De Odontología, F. Universidad Complutense de Madrid Tesis Doctoral un Composite Nuevo de Fosfato Cálcico-Silicato Cálcico Para la Regeneración Ósea: Caracterización Y Comportamiento Memoria Para Optar al Grado de Doctor Presentada Por Lucas Aparicio; Universidad Complutense de Madrid: Madrd, Spain, 2014. [Google Scholar]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmaksiz, M.; Lalegül-Ülker, Ö.; Vurat, M.T.; Elçin, A.E.; Elçin, Y.M. Magneto-sensitive decellularized bone matrix with or without low frequency-pulsed electromagnetic field exposure for the healing of a critical-size bone defect. Mater. Sci. Eng. C 2021, 124, 112065. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, N.; Ghiaseddin, A.; Irani, S.; Rabbani, S.; Tafti, S.H.A.; Soufizomorrod, M.; Soleimani, M. Cartilage tissue engineering using injectable functionalized Demineralized Bone Matrix scaffold with glucosamine in PVA carrier, cultured in microbioreactor prior to study in rabbit model. Mater. Sci. Eng. C 2021, 120, 111677. [Google Scholar] [CrossRef]
- Neves, N.; Campos, B.B.; Almeida, I.F.; Costa, P.C.; Cabral, A.T.; Barbosa, M.A.; Ribeiro, C.C. Strontium-rich injectable hybrid system for bone regeneration. Mater. Sci. Eng. C 2016, 59, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; Colonna, C.; Genta, I.; De Trizio, A.; Modena, T.; Klöss, H.; Conti, B. In vitro characterization of an injectable in situ forming composite system for bone reconstruction. Polym. Degrad. Stab. 2015, 119, 151–158. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable preformed scaffolds with shape-memory properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thai, V.V.; Lee, B.T. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J. Mater. Sci. Mater. Med. 2010, 21, 1867–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neill, R.O.; Mccarthy, H.O.; Montufar, E.B.; Ginebra, M.; Wilson, D.I.; Lennon, A.; Dunne, N. Acta Biomaterialia Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, C.; Epple, M. Carbonated calcium phosphates are suitable pH-stabilising fillers for biodegradable polyesters. Biomaterials 2003, 24, 2037–2043. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Artigas, N.; Gámez, B.; Rosa, J.L.; Ventura, F. Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signalling. PLoS ONE 2017, 12, e0178158. [Google Scholar] [CrossRef]
- Alhashimi, R.A.; Mannocci, F.; Sauro, S. Bioactivity, cytocompatibility and thermal properties of experimental Bioglass-reinforced composites as potential root-canal filling materials. J. Mech. Behav. Biomed. Mater. 2017, 69, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Al-Wafi, R.; Eldera, S.S.; Hamzawy, E.M.A. Characterization and in vitro bioactivity study of a new glass ceramic from mica/apatite glass mixtures. J. Mater. Res. Technol. 2020, 9, 7558–7569. [Google Scholar] [CrossRef]
- Radin, S.R.; Ducheyne, P. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. J. Biomed. Mater. Res. 1993, 27, 35–45. [Google Scholar] [CrossRef]
- Yuan, H.; Barbieri, D.; Luo, X.; Van Blitterswijk, C.A.; De Bruijn, J.D. Calcium phosphates and bone induction. Compr. Biomater. II 2017, 1, 333–349. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Laurencin, C.T.; Jiang, T. Bone Graft Substitutes and Bone Regenerative Engineering, 2nd ed.; ASTM International: West Conshahoken, PA, USA, 2014; ISBN 9780803170605. [Google Scholar]
- Adkisson, H.D.; Strauss-Schoenberger, J.; Gillis, M.; Wilkins, R.; Jackson, M.; Hruska, K.A. Rapid quantitative bioassay of osteoinduction. J. Orthop. Res. 2000, 18, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J. A review of osteoinductive testing methods and sterilization processes for demineralized bone. Cell Tissue Bank. 2005, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.C.; Yuan, H.; van Blitterswijk, C.A.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cell. Mater. 2011, 21, 407–429. [Google Scholar] [CrossRef]
- Katz, J.M.; Nataraj, C.; Jaw, R.; Deigl, E.; Bursac, P. Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; White, A.A.; Best, S.M. Properties and characterization of bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–102. [Google Scholar]
- Zhao, X.; Liang, M.; Li, X.; Qiu, X.; Cui, L. Identification of key genes and pathways associated with osteogenic differentiation of adipose stem cells. J. Cell. Physiol. 2018, 233, 9777–9785. [Google Scholar] [CrossRef]
- Han, B.; Tang, B.; Nimni, M.E. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J. Orthop. Res. 2003, 21, 648–654. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Li, X.; Li, Y.; Zhang, Y.; Wu, H.; Wen, Z.; Dai, C. Evaluation of long-term biocompatibility and osteogenic differentiation of graphene nanosheet doped calcium phosphate-chitosan AZ91D composites. Mater. Sci. Eng. C 2018, 90, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Y.; Zhou, L.; Feng, B.; Weng, X.; Liang, R. Biological evaluation of bone substitute. Clin. Chim. Acta 2020, 510, 544–555. [Google Scholar] [CrossRef] [PubMed]




| Approx. Weight Composition (%) | ||||||
|---|---|---|---|---|---|---|
| Formulation | Powders | Liquids | ||||
| DBM | CaCO3 | PVA | Glycerol | Borax | Water | |
| 1 | 26 | 2 | 2 | 6.5 | 0.5 | 63 |
| 2 | 25 | 5 | 2 | 6.5 | 0.5 | 61 |
| 3 | 24 | 5 | 3.6 | 6.5 | 0.4 | 60.5 |
| Formulation | Max. Compression Load (N) | Injectability (%) |
|---|---|---|
| 1 | 50 ± 2 | 93 ± 1 |
| 2 | 13 ± 1 | 94 ± 1 |
| 3 | 193 ± 29 | 59 ± 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano-David, D.; Lopera, A.M.; Londoño, M.E.; Araque-Marín, P. Formulation and Characterization of a New Injectable Bone Substitute Composed PVA/Borax/CaCO3 and Demineralized Bone Matrix. J. Funct. Biomater. 2021, 12, 46. https://doi.org/10.3390/jfb12030046
Medrano-David D, Lopera AM, Londoño ME, Araque-Marín P. Formulation and Characterization of a New Injectable Bone Substitute Composed PVA/Borax/CaCO3 and Demineralized Bone Matrix. Journal of Functional Biomaterials. 2021; 12(3):46. https://doi.org/10.3390/jfb12030046
Chicago/Turabian StyleMedrano-David, Daniela, Aura María Lopera, Martha Elena Londoño, and Pedronel Araque-Marín. 2021. "Formulation and Characterization of a New Injectable Bone Substitute Composed PVA/Borax/CaCO3 and Demineralized Bone Matrix" Journal of Functional Biomaterials 12, no. 3: 46. https://doi.org/10.3390/jfb12030046