Electron Beam-Treated Enzymatically Mineralized Gelatin Hydrogels for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation
2.2. Electron Beam Treatment
2.3. Enzymatic Mineralization
2.4. Scanning Electron Microscopy (SEM)
2.5. Raman Spectroscopy
2.6. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.7. Dry Mass
2.8. Uniaxial Compression Modulus
2.9. Cytocompatibility
2.9.1. Sample Preparation and Cell Seeding
2.9.2. Fluorescence Microscopy
2.9.3. Proliferation Assay (MTS)
3. Results
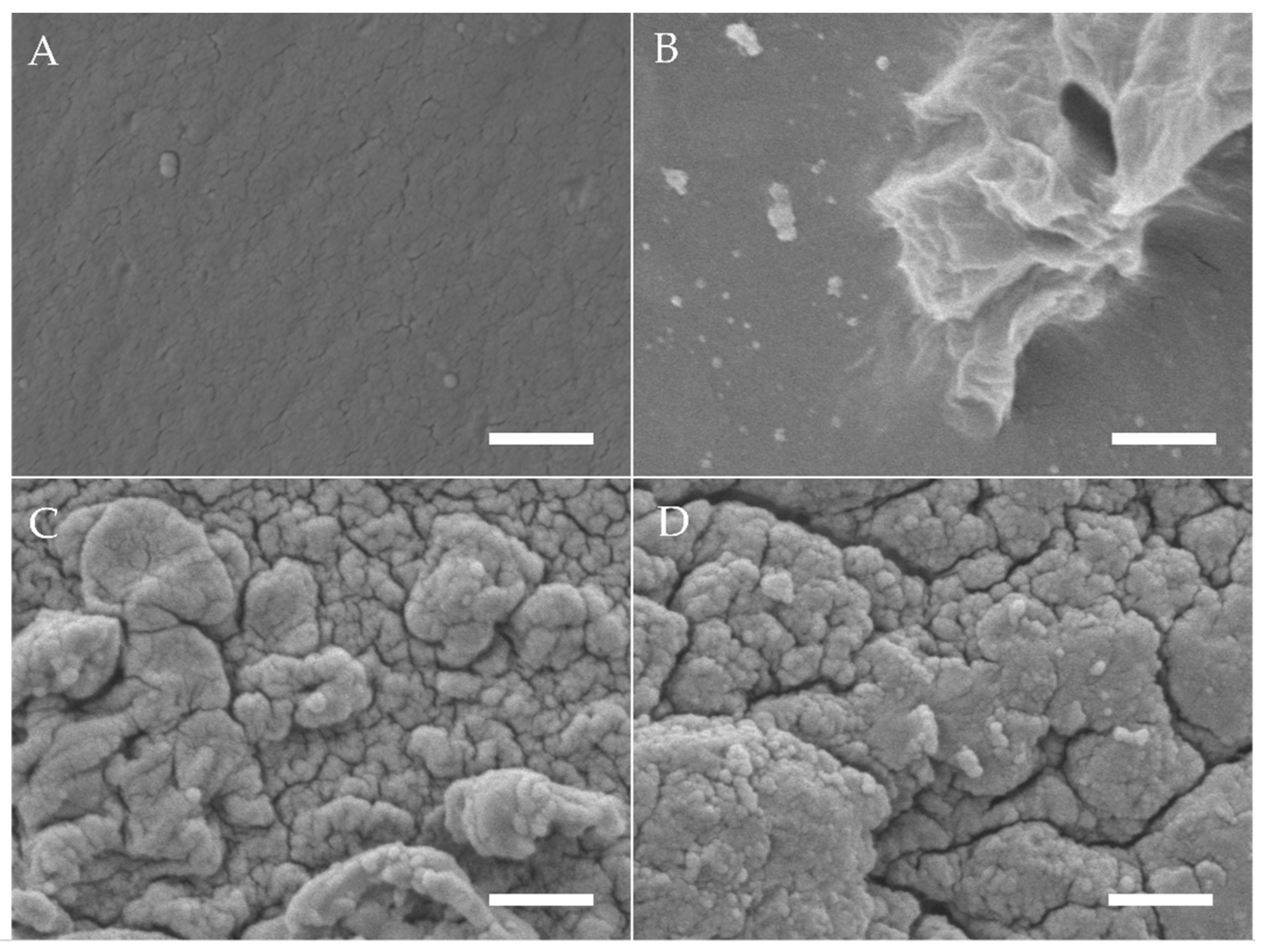
3.1. Scanning Electron Microscopy (SEM)
3.2. Raman Spectroscopy
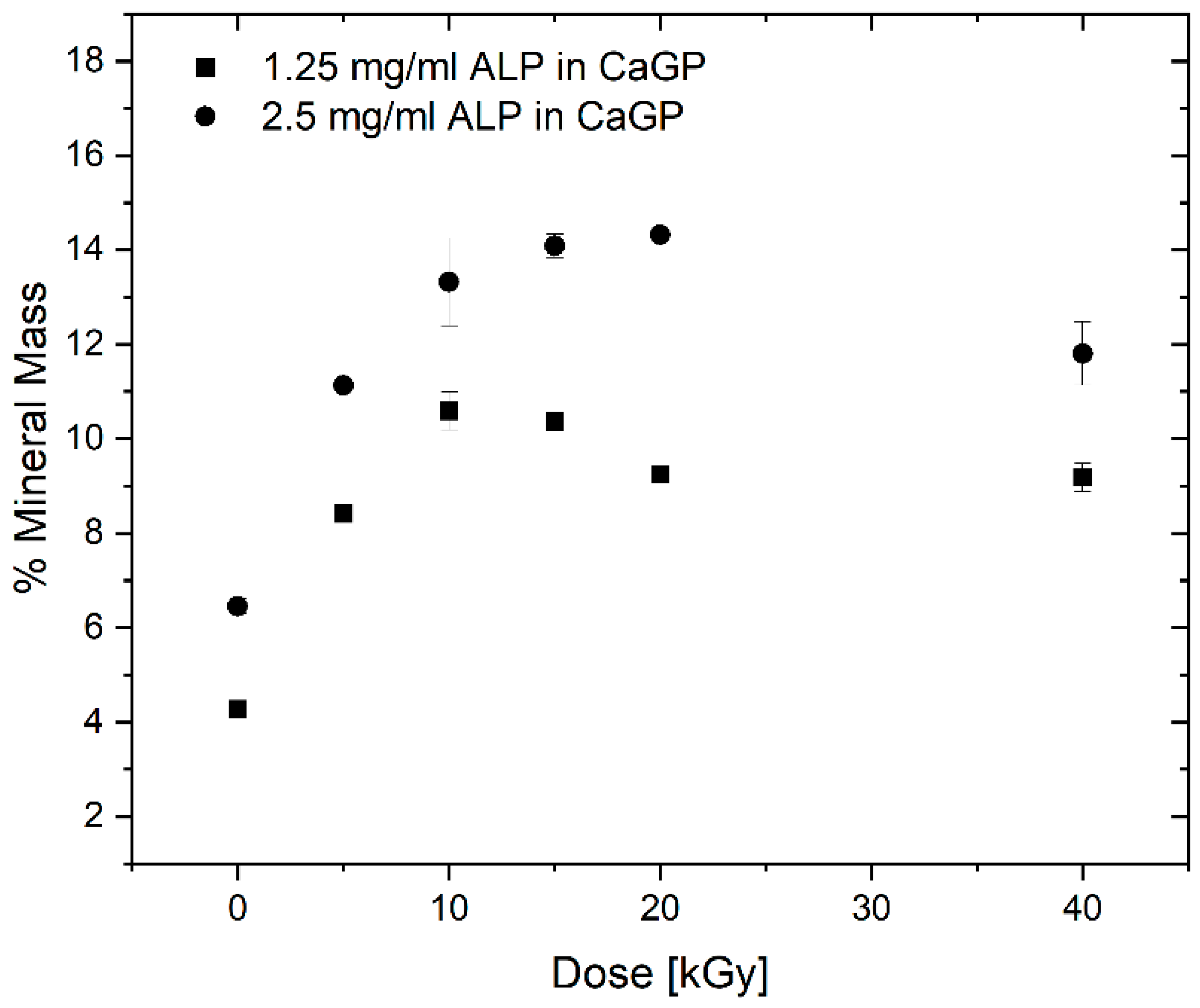
3.3. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
3.4. Dry Mass
3.5. Uniaxial Compression Modulus
3.6. Cytocompatibility
3.6.1. Cell Culture
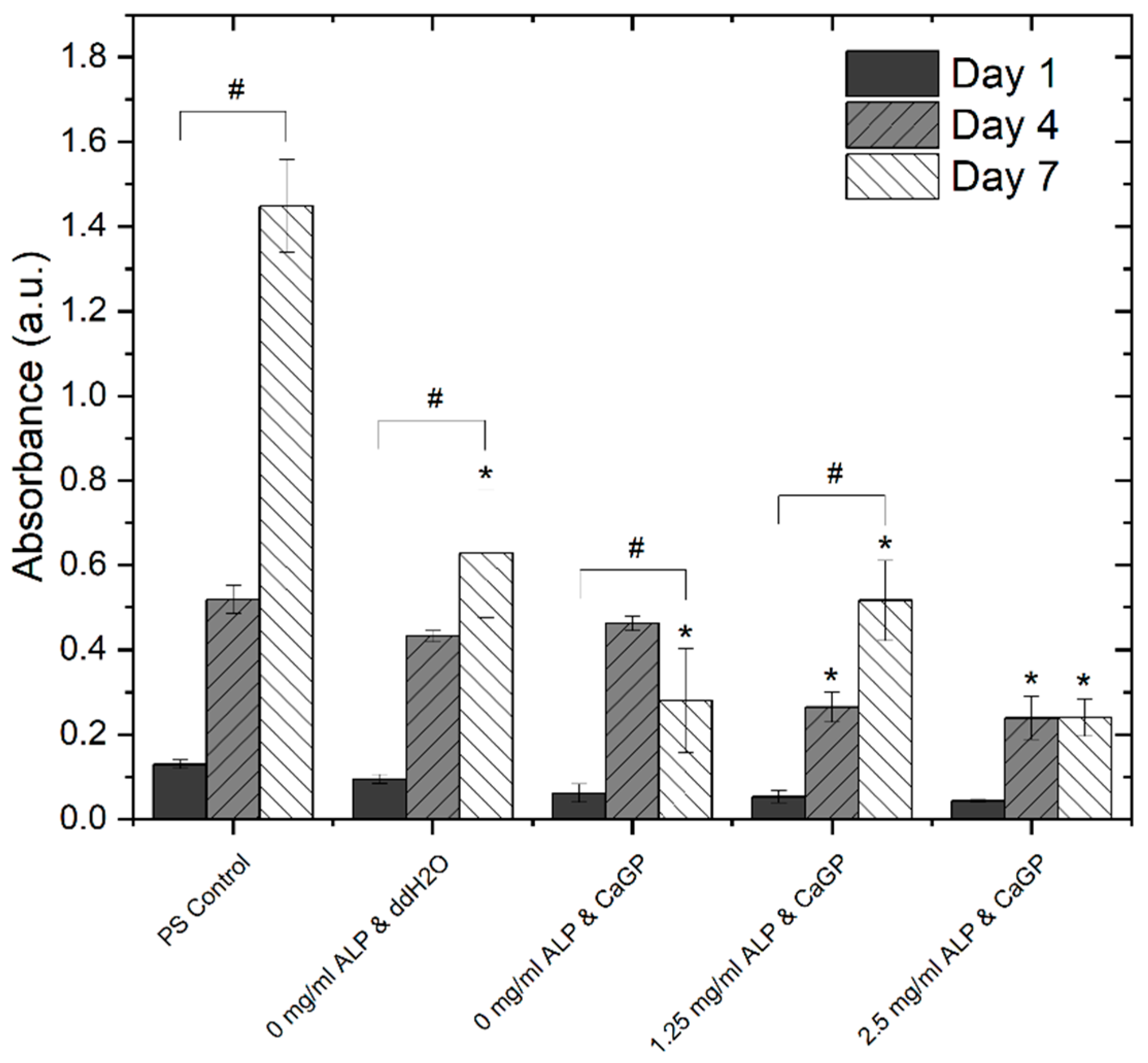
3.6.2. Proliferation Assay (MTS)
4. Discussion
4.1. Formation of CaP
4.1.1. Scanning Electron Microscopy (SEM)
4.1.2. Raman Spectroscopy
4.1.3. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
4.1.4. Dry Mass
4.2. Uniaxial Compression Modulus
4.3. Cytocompatibility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglas, T.E.L.; Messersmith, P.B.; Chasan, S.; Mikos, A.G.; de Mulder, E.L.W.; Dickson, G.; Schaubroeck, D.; Balcaen, L.; Vanhaecke, F.; Dubruel, P.; et al. Enzymatic Mineralization of Hydrogels for Bone Tissue Engineering by Incorporation of Alkaline Phosphatase. Macromol. Biosci. 2012, 12, 1077–1089. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Jansen, J.A.; Mikos, A.G. Functionalization of Oligo(Poly(Ethylene Glycol)Fumarate) Hydrogels with Finely Dispersed Calcium Phosphate Nanocrystals for Bone-Substituting Purposes. J. Biomater. Sci. Polym. Ed. 2007, 18, 1547–1564. [Google Scholar] [CrossRef]
- Leeuwenburgh, S.C.G.; Ana, I.D.; Jansen, J.A. Sodium Citrate as an Effective Dispersant for the Synthesis of Inorganic–Organic Composites with a Nanodispersed Mineral Phase. Acta Biomater. 2010, 6, 836–844. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium Phosphates in Oral Biology and Medicine. Monogr. Oral Sci. 1991, 15, 1–201. [Google Scholar] [PubMed]
- Ruhé, P.Q.; Boerman, O.C.; Russel, F.G.M.; Spauwen, P.H.M.; Mikos, A.G.; Jansen, J.A. Controlled Release of RhBMP-2 Loaded Poly(Dl-Lactic-Co-Glycolic Acid)/Calcium Phosphate Cement Composites In Vivo. J. Control. Release 2005, 106, 162–171. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Pamula, E.; Leeuwenburgh, S.C.G. Biomimetic Mineralization of Hydrogel Biomaterials for Bone Tissue Engineering. In Biomimetics; Ramalingam, M., Wang, X., Chen, G., Ma, P., Cui, F.-Z., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 51–67. ISBN 978-1-118-81040-8. [Google Scholar]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A. Raman Spectroscopic Evidence for Octacalcium Phosphate and Other Transient Mineral Species Deposited during Intramembranous Mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Xi, Y.; Beganovic, M.; Belotti, F.M.; Scholz, R. Vibrational Spectroscopy of the Phosphate Mineral Lazulite—(Mg, Fe)Al2(PO4)2·(OH)2 Found in the Minas Gerais, Brazil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 241–247. [Google Scholar] [CrossRef][Green Version]
- Frushour, B.G.; Koenig, J.L. Raman Scattering of Collagen, Gelatin, and Elastin. Biopolymers 1975, 14, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yao, H.; Wang, G.; Wang, Y.; Li, Y.; Feng, M. NIR Raman Spectroscopic Investigation of Single Mitochondria Trapped by Optical Tweezers. Opt. Express 2007, 15, 12708. [Google Scholar] [CrossRef]
- Sharma, A.; Goring, A.; Staines, K.A.; Emery, R.J.H.; Pitsillides, A.A.; Oreffo, R.O.C.; Mahajan, S.; Clarkin, C.E. Raman Spectroscopy Links Differentiating Osteoblast Matrix Signatures to Pro-Angiogenic Potential. Matrix Biol. Plus 2020, 5, 100018. [Google Scholar] [CrossRef]
- Derbel, N.; Hernández, B.; Pflüger, F.; Liquier, J.; Geinguenaud, F.; Jaïdane, N.; Ben Lakhdar, Z.; Ghomi, M. Vibrational Analysis of Amino Acids and Short Peptides in Hydrated Media. I. L-Glycine and L-Leucine. J. Phys. Chem. B 2007, 111, 1470–1477. [Google Scholar] [CrossRef]
- Wisotzki, E.I.; Hennes, M.; Schuldt, C.; Engert, F.; Knolle, W.; Decker, U.; Käs, J.A.; Zink, M.; Mayr, S.G. Tailoring the Material Properties of Gelatin Hydrogels by High Energy Electron Irradiation. J. Mater. Chem. B 2014, 2, 4297–4309. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Anthony, S.G.; Stupp, S.I. Enzyme Directed Templating of Artificial Bone Mineral. Adv. Mater. 2009, 21, 425–430. [Google Scholar] [CrossRef]
- Mandair, G.S.; Morris, M.D. Contributions of Raman Spectroscopy to the Understanding of Bone Strength. BoneKEy Rep. 2015, 4. [Google Scholar] [CrossRef]
- Kazanci, M.; Fratzl, P.; Klaushofer, K.; Paschalis, E.P. Complementary Information on In Vitro Conversion of Amorphous (Precursor) Calcium Phosphate to Hydroxyapatite from Raman Microspectroscopy and Wide-Angle X-Ray Scattering. Calcif. Tissue Int. 2006, 79, 354–359. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Krawczyk, G.; Pamula, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; Van Der Voort, P.; Bliznuk, V.; van den Vreken, N.M.F.; et al. Generation of Composites for Bone Tissue-Engineering Applications Consisting of Gellan Gum Hydrogels Mineralized with Calcium and Magnesium Phosphate Phases by Enzymatic Means: Gellan Gum and Calcium and Magnesium Phosphate Composites. J. Tissue Eng. Regen. Med. 2016, 10, 938–954. [Google Scholar] [CrossRef]
- Wisotzki, E.I.; Friedrich, R.P.; Weidt, A.; Alexiou, C.; Mayr, S.G.; Zink, M. Cellular Response to Reagent-Free Electron-Irradiated Gelatin Hydrogels. Macromol. Biosci. 2016, 16, 914–924. [Google Scholar] [CrossRef]
- Osathanon, T.; Giachelli, C.M.; Somerman, M.J. Immobilization of Alkaline Phosphatase on Microporous Nanofibrous Fibrin Scaffolds for Bone Tissue Engineering. Biomaterials 2009, 30, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Borau, C.; Kamm, R.D.; García-Aznar, J.M. Mechano-Sensing and Cell Migration: A 3D Model Approach. Phys. Biol. 2011, 8, 066008. [Google Scholar] [CrossRef] [PubMed]
- Moreo, P.; García-Aznar, J.M.; Doblaré, M. Modeling Mechanosensing and Its Effect on the Migration and Proliferation of Adherent Cells. Acta Biomater. 2008, 4, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of Cell Adhesion, Proliferation and Differentiation on Materials Designed for Body Implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.; Qu, R.; Li, Q.; Rong, D.; Fan, T.; Yang, Y.; Sun, B.; Bi, Z.; Khan, A.U.; et al. Synthesis of Aligned Porous Polyethylene Glycol/Silk Fibroin/Hydroxyapatite Scaffolds for Osteoinduction in Bone Tissue Engineering. Stem Cell Res. Ther. 2020, 11, 522. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem Cells: Their Source, Potency and Use in Regenerative Therapies with Focus on Adipose-Derived Stem Cells—A Review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Atif, A.R.; Pujari-Palmer, M.; Tenje, M.; Mestres, G. A Microfluidics-Based Method for Culturing Osteoblasts on Biomimetic Hydroxyapatite. Acta Biomater. 2021, 127, 327–337. [Google Scholar] [CrossRef]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A Novel Strontium(II)-Modified Calcium Phosphate Bone Cement Stimulates Human-Bone-Marrow-Derived Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation In Vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedel, S.; Ward, D.; Kudláčková, R.; Mazur, K.; Bačáková, L.; Kerns, J.G.; Allinson, S.L.; Ashton, L.; Koniezcny, R.; Mayr, S.G.; et al. Electron Beam-Treated Enzymatically Mineralized Gelatin Hydrogels for Bone Tissue Engineering. J. Funct. Biomater. 2021, 12, 57. https://doi.org/10.3390/jfb12040057
Riedel S, Ward D, Kudláčková R, Mazur K, Bačáková L, Kerns JG, Allinson SL, Ashton L, Koniezcny R, Mayr SG, et al. Electron Beam-Treated Enzymatically Mineralized Gelatin Hydrogels for Bone Tissue Engineering. Journal of Functional Biomaterials. 2021; 12(4):57. https://doi.org/10.3390/jfb12040057
Chicago/Turabian StyleRiedel, Stefanie, Daniel Ward, Radmila Kudláčková, Karolina Mazur, Lucie Bačáková, Jemma G. Kerns, Sarah L. Allinson, Lorna Ashton, Robert Koniezcny, Stefan G. Mayr, and et al. 2021. "Electron Beam-Treated Enzymatically Mineralized Gelatin Hydrogels for Bone Tissue Engineering" Journal of Functional Biomaterials 12, no. 4: 57. https://doi.org/10.3390/jfb12040057
APA StyleRiedel, S., Ward, D., Kudláčková, R., Mazur, K., Bačáková, L., Kerns, J. G., Allinson, S. L., Ashton, L., Koniezcny, R., Mayr, S. G., & Douglas, T. E. L. (2021). Electron Beam-Treated Enzymatically Mineralized Gelatin Hydrogels for Bone Tissue Engineering. Journal of Functional Biomaterials, 12(4), 57. https://doi.org/10.3390/jfb12040057







