Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances
Abstract
:1. Introduction
2. Polymeric Nanofibers and Electrospun Scaffolds
3. Nanofiber Action towards Bacteria
4. Entrapment of Antimicrobial Agents into Nanofibers: Classification
4.1. Metal, Metal Oxides and Metal Nanoparticles
4.2. Carbon Materials
4.3. Antimicrobial Peptides (AMPs)
4.4. Natural Extracts
5. Surface Chemical Functionalization via Monomer Grafting
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Centers for Disease Control Prevention Centre (CDC). Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 9 September 2021).
- Ventola, C.L. The antibiotic resistance crisis part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Sprenger, M.; Fukuda, K. New mechanisms, new worries. Science 2016, 351, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munita, J.; Arias, C. Mechanisms of Antibiotic Resistance. Microbial. Spectr. 2016, 4, 4.2.15. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Kadhum, H.A.; Hasan, T.H. The study of bacillus subtils antimicrobial activity on some of the pathological isolates. Int. J. Drug Deliv. Technol. 2019, 9, 193–196. [Google Scholar] [CrossRef]
- Henrichfreise, B.; Wiegand, I.; Pfister, W.; Wiedemann, B. Resistance mechanisms of multiresistant Pseudomonas aeruginosa strains from Germany and correlation with hypermutation. Antimicrob. Agents Chemother. 2007, 51, 4062–4070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Motta, S.; Aldana, M. Adaptive resistance to antibiotics in bacteria: A systems biology perspective. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 253–267. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Namdari, M.; Eatemadi, A. Nanofibrous bioengineered heart valve—Application in paediatric medicine. Biomed. Pharmacother. 2016, 84, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Farnaz-Sadat, F.; Khoddami, A.; Avinc, O. Poly(lactic acid) (PLA) Nanofibers for Bone Tissue Engineering. J. Text Polym. 2019, 7, 47. [Google Scholar]
- Obregón, R.; Ramón-Azcón, J.; Ahadian, S. Nanofiber composites in blood vessel tissue engineering. In Nanofiber Composites for Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 483–506. [Google Scholar]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Jingcheng, L.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent polymers, fibers and applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.R.; Guarino, V.; Oliveira, M.J.; Ribeiro, C.C.; Barbosa, M.A.; Ambrosio, L. Ibuprofen-loaded poly (trimethylene carbonate-co-ϵ-caprolactone) electrospun fibres for nerve regeneration. J. Tissue Eng. Regen. Med. 2013, 10, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Akhmetova, A.; Heinz, A. Electrospinning proteins for wound healing purposes: Opportunities and challenges. Pharmaceutics. 2021, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Bombin, J.A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Sabra, S.; Ragab, D.M.; Agwa, M.M.; Rohani, S. Recent advances in electrospun nanofibers for some biomedical applications. Eur. J. Pharm. Sci. 2020, 144, 105224. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, B.; Nagy, Z.K.; Szabó, D.; Zsidai, L.; Kocsis, B. Polymer structure and antimicrobial activity of polyvinylpyrrolidone-based iodine nanofibers prepared with high-speed rotary spinning technique. Int. J. Pharm. 2013, 458, 99–103. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [Green Version]
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A. Electrospun wound dressings containing bioactive natural products: Physico-chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, Q.; Wang, K.; Chen, H. Improving Antibacterial Activity and Biocompatibility of Bioinspired Electrospinning Silk Fibroin Nanofibers Modified by Graphene Oxide. ACS Omega 2018, 3, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Calcaterra, A.; Ruggiero, V.; Pichichero, E.; Martino, A.; Iosi, F. Functionalized Graphene Derivatives: Antibacterial Properties and Cytotoxicity. J. Nanomater. 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Balasubramanian, K. Bioabsorbable Engineered Nanobiomaterials for Antibacterial Therapy. In Engineering of Nanobiomaterials: Applications of Nanobiomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 77–117. [Google Scholar]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Dai, J.; Dai, J.; Wang, H.; Chen, S.; Liu, Y. Extracellular matrix imitation utilizing nanofibers-embedded biomimetic scaffolds for facilitating cartilage regeneration. Chem. Eng. J. 2021, 410, 128379. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, X.; Liu, H.; Xu, P.; Teng, G.; Xiao, Z. Neuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.L.; Erickson, A.E.; Chang, F.C.; Zhang, M. Chitosan-poly(caprolactone) nanofibers for skin repair. J. Mater. Chem. B 2017, 5, 1822–1833. [Google Scholar] [CrossRef] [Green Version]
- Alven, S.; Buyana, B.; Feketshane, Z.; Aderibigbe, B.A. Electrospun nanofibers/nanofibrous scaffolds loaded with silver nanoparticles as effective antibacterial wound dressing materials. Pharmaceutics 2021, 13, 964. [Google Scholar] [CrossRef]
- Wang, P.Y.; Ji, Q.T.; Xiang, H.M.; Zhang, T.H.; Zeng, D.; Zhou, X. Assembling Anthracene-Tailored Amphiphiles: Charge-Transfer Interactions Directed Hierarchical Nanofibers with Ameliorative Antibacterial Activity toward Plant Pathogens. J. Agric. Food Chem. 2020, 68, 5579–5585. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Exp. Rev. Med. Dev. 2016, 13, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Saracino, E.; Cirillo, V.; Marrese, M.; Guarino, V.; Benfenati, V.; Zamboni, R.; Ambrosio, L. Structural and functional properties of astrocytes on PCL based electrospun fibres. Mater. Sci. Eng. C 2021, 118, 111363. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Nelson, M.T.; Xue, R.; DeJesus, J.; Viapiano, M.S.; Lannutti, J.J. Mimicking white matter tract topography using core-shell electrospun nanofibers to examine migration of malignant brain tumors. Biomaterials 2013, 34, 5181–5190. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.F.; Zhang, J.; Liu, J.J.; Zhou, Q.H.; Liu, Z.; Hu, P.Y. Bifunctional CuS composite nanofibers via in situ electrospinning for outdoor rapid hemostasis and simultaneous ablating superbug. Chem. Eng. J. 2020, 401, 126096. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Chen, Y.; Shen, Y.; Yu, F.; Wang, W. Conjugate Electrospun 3D Gelatin Nanofiber Sponge for Rapid Hemostasis. Adv. Healthc. Mater. 2021, 10, 2100918. [Google Scholar] [CrossRef]
- Chen, S.; Carlson, M.A.; Zhang, Y.S.; Hu, Y.; Xie, J. Fabrication of injectable and superelastic nanofiber rectangle matrices (“peanuts”) and their potential applications in hemostasis. Biomaterials 2018, 179, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Gaiser, S.; Luisier, N.; Hegemann, D. pH-responsive chitosan/alginate polyelectrolyte complexes on electrospun PLGA nanofibers for controlled drug release. Nanomaterials 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311. [Google Scholar] [CrossRef]
- Kowalczyk, T. Functional micro- and nanofibers obtained by nonwoven post-modification. Polymers 2020, 12, 1087. [Google Scholar] [CrossRef]
- Ko, Y.M.; Choi, D.Y.; Jung, S.C.; Kim, B.H. Characteristics of plasma treated electrospun polycaprolactone (PCL) nanofiber scaffold for bone tissue engineering. J. Nanosci. Nanotechnol. 2015, 15, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Belgacem, M.N.; Bras, J. Effect of variable aminoalkyl chains on chemical grafting of cellulose nanofiber and their antimicrobial activity. Mater. Sci. Eng. C 2017, 75, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Giram, P.S.; Shitole, A.; Nande, S.S.; Sharma, N.; Garnaik, B. Fast dissolving moxifloxacin hydrochloride antibiotic drug from electrospun Eudragit L-100 nonwoven nanofibrous Mats. Mater. Sci. Eng. C 2018, 92, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res.—Part A 2013, 101, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, R.; Wang, Z.; Wang, W.; Yu, D. Eco-fabrication of antibacterial nanofibrous membrane with high moisture permeability from wasted wool fabrics. Waste Manag. 2020, 102, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Truong, Y.B.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131, 9041–9053. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Ryšánek, P.; Malý, M.; Čapková, P.; Kormunda, M.; Kolská, Z.; Gryndler, M. Antibacterial modification of nylon-6 nanofibers: Structure, properties and antibacterial activity. J. Polym. Res. 2017, 24, 208. [Google Scholar] [CrossRef]
- Ferraris, S.; Guarino, V.; Cochis, A.; Varesano, A.; Cruz Maya, I.; Vineis, C.; Rimondini, L.; Spriano, S. Aligned keratin submicrometric-fibers for fibroblasts guidance onto nanogrooved titanium surfaces for transmucosal implants. Mater. Lett. 2018, 229, 1–4. [Google Scholar] [CrossRef]
- Coelho, C.S.; Estevinho, N.B.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I.S.; Schiffman, J.D. Current and emerging approaches to engineer antibacterial and antifouling electrospun nanofibers. Materials 2018, 11, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; Kingshott, P.; Mcarthur, S.L. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Bahrami, H.; Ranjbar-Mohammadi, M. Fabrication, optimization and characterization of electrospun poly(caprolactone)/gelatin/graphene nanofibrous mats. Mater. Sci. Eng. C 2017, 78, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Jalaja, K.; Sreehari, V.S.; Kumar, P.R.A.; Nirmala, R.J. Graphene oxide decorated electrospun gelatin nanofibers: Fabrication, properties and applications. Mater. Sci. Eng. C 2016, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Cruz-Maya, I.; Altobelli, R.; Abdul Khodir, W.K.; Ambrosio, L.; Pèrez, A.M.A.; Flores, A.A. Electrospun polycaprolactone nanofibres decorated by drug loaded chitosan nano-reservoirs for antibacterial treatments. Nanotechnology 2017, 28, 202103. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Ganesh, M.; Ubaidulla, U.; Young Choi, E.; Tae Jang, H. Preparation, characterization, and in vitro diffusion study of nonwoven electrospun nanofiber of curcumin-loaded cellulose acetate phthalate polymer. Saudi Pharm. J. 2017, 25, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Arai, Y.; Ahn, J.C.; Park, H.; Lee, S.H. Influence of cationic lipid concentration on properties of lipid–polymer hybrid nanospheres for gene delivery. Int. J. Nanomed. 2015, 10, 5367–5382. [Google Scholar]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, J.S.; Koo, W.T.; Choi, S.J.; Kim, S.J.; Kim, I.D. Hierarchically interconnected porosity control of catalyst-loaded WO3 nanofiber scaffold: Superior acetone sensing layers for exhaled breath analysis. Sensors Actuators B Chem. 2018, 259, 616–625. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: How small is small enough? Biofilms Microbiomes 2015, 1, 15022. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; McCarthy, A.; John, J.V.; Su, Y.; Xie, J. Converting 2D Nanofiber Membranes to 3D Hierarchical Assemblies with Structural and Compositional Gradients Regulates Cell Behavior. Adv. Mater. 2020, 32, 2003754. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.D.M.; Correa, D.S.; Medeiros, E.S.; Oliveira, J.E.; Mattoso, L.H.C. Advances in Functional Polymer Nanofibers: From Spinning Fabrication Techniques to Recent Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 45673–45701. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Barakat, N.A.M.; Tirupathi, P.P.B.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.S. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Guarino, V.; Alvarez-Perez, M.A. Protein based devices for oral tissue repair and regeneration. Mater. Sci. 2018, 5, 156–170. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Chen, W.; Mulchandani, A. Bactericidal activity of elastin-like polypeptide biopolymer with polyhistidine domain and silver. Colloids Surf. B Biointerfaces 2014, 119, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef] [Green Version]
- Guarino, V.; Branda, F.; Ausanio, G.; Iannotti, V.; Lanotte, L.; Ambrosio, L. Elastomagnetic NI-PDMS nanofibers via coaxial electrospinning. Mater. Res. Express 2018, 111, 085029. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Heuzey, M.C.; Ajji, A. Mechanism of action of electrospun chitosan-based nanofibers against meat spoilage and pathogenic bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, S.; Siyamak, S.; Ibrahim, N.A.; Wan Yunus, W.M.Z.; Ab Rahman, M.Z.; Azizi, S. Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. Int. J. Mol. Sci. 2012, 13, 4508–4522. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Intoduction. In Science and Technology of Polymer Nanofibers; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2008; Volume 30, pp. 1–26. [Google Scholar]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J.M. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Mulholland, E.J.; Ali, A.; Robson, T.; Dunne, N.J.; McCarthy, H.O. Delivery of RALA/siFKBPL nanoparticles via electrospun bilayer nanofibres: An innovative angiogenic therapy for wound repair. J. Control. Release 2019, 316, 53–65. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Pouranvari, S.; Ebrahimi, F.; Javadi, G.; Maddah, B. Chemical cross-linking of chitosan/polyvinyl alcohol electrospun nanofibers. Mater. Tehnol. 2016, 50, 663–666. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Fabrication and characterization of PCL/gelatin/chitosan ternary nanofibrous composite scaffold for tissue engineering applications. J. Mater. Sci. 2014, 49, 1076–1089. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L. Temperature-driven processing techniques for manufacturing fully interconnected porous scaffolds in bone tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1389–1400. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Holtzl, T.; Szekely, G. Nanofiber engineering of microporous polyimides through electrospinning: Influence of electrospinning parameters and salt addition. Mater. Des. 2021, 198, 109280. [Google Scholar] [CrossRef]
- Hamdan, N.; Darnis, D.S.; Khodir, W.K.W.A. In vitro evaluation of crosslinked polyvinyl alcohol/chitosan-gentamicin sulfate electrospun nanofibers. Malays. J. Chem. 2021, 23, 1–10. [Google Scholar]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 2019, 94, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidonium majus L. Incorporated emulsion electrospun pcl/pva_pec nanofibrous meshes for antibacterial wound dressing applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef]
- Yu, L.; Dou, S.; Ma, J.; Gong, Q.; Zhang, M.; Zhang, X. An Antimicrobial Peptide-Loaded Chitosan/Polyethylene Oxide Nanofibrous Membrane Fabricated by Electrospinning Technology. Front. Mater. 2021, 8, 70. [Google Scholar] [CrossRef]
- Alhusein, N.; Blagbrough, I.S.; Beeton, M.L.; Bolhuis, A.; De Bank, P.A. Electrospun Zein/PCL Fibrous Matrices Release Tetracycline in a Controlled Manner, Killing Staphylococcus Aureus Both in Biofilms and Ex Vivo on Pig Skin, and are Compatible with Human Skin Cells. Pharm. Res. 2016, 33, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Maya, I.; Guarino, V.; Almaguer-Flores, A.; Alvarez-Perez, M.A.; Varesano, A.; Vineis, C. Highly polydisperse keratin rich nanofibers: Scaffold design and in vitro characterization. J. Biomed. Mater. Res.—Part A 2019, 107, 1803–1813. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khodir, W.K.W.; Abdul Razak, A.; Ng, M.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Kolbasov, A.; Sinha-Ray, S.; Joijode, A.; Hassan, M.A.; Brown, D.; Maze, B. Industrial-Scale Solution Blowing of Soy Protein Nanofibers. Ind. Eng. Chem. Res. 2016, 55, 323–333. [Google Scholar] [CrossRef]
- Shojaei, R.T.; Hajalilou, A.; Tabatabaei, M.; Mobli, H.; Aghbashlo, M. Characterization and Evaluation of Nanofiber Materials. In Handbook of Nanofibers; Springer: Berlin/Heidelberg, Germany, 2019; pp. 491–522. [Google Scholar]
- Lu, A.; Gao, Y.; Jin, T.; Luo, X.; Zeng, Q.; Shang, Z. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2020, 46, 6550–6559. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M. Controlling nanofiber morphology by the electrospinning process. In Electrospun Nanofibers; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 109–123. [Google Scholar]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Zahmatkeshan, M.; Adel, M.; Bahrami, S.; Esmaeili, F.; Rezayat, S.M.; Saeedi, Y. Polymer-Based Nanofibers: Preparation, Fabrication, and Applications. In Handbook of Nanofibers; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Orr, S.B.; Chainani, A.; Hippensteel, K.J.; Kishan, A.; Gilchrist, C.; Garrigues, N.W. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015, 24, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arik, N.; Inan, A.; Ibis, F.; Demirci, E.A.; Karaman, O.; Ercan, U.K. Modification of electrospun PVA/PAA scaffolds by cold atmospheric plasma: Alignment, antibacterial activity, and biocompatibility. Polym. Bull. 2019, 76, 797–812. [Google Scholar] [CrossRef]
- Yu, L.; Shao, Z.; Xu, L.; Wang, M. High throughput preparation of aligned nanofibers using an improved bubble-electrospinning. Polymers 2017, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Kharaghani, D.; Gitigard, P.; Ohtani, H.; Kim, K.O.; Ullah, S. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019, 9, 12640. [Google Scholar] [CrossRef] [Green Version]
- Coimbra, P.; Freitas, J.P.; Gonçalves, T.; Gil, M.H.; Figueiredo, M. Preparation of gentamicin sulfate eluting fiber mats by emulsion and by suspension electrospinning. Mater. Sci. Eng. C 2019, 94, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Han, S.; Zhang, R.; Liu, G.; Wu, J. Progress in electrospun composite nanofibers: Composition, performance and applications for tissue engineering. J. Mater. Chem. B 2019, 7, 7075–7089. [Google Scholar] [CrossRef] [PubMed]
- Calamak, S.; Shahbazi, R.; Eroglu, I.; Gultekinoglu, M.; Ulubayram, K. An overview of nanofiber-based antibacterial drug design. Expert Opin. Drug Discov. 2017, 12, 391–406. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Zhang, J.; Ji, D.; Qin, X.; Ge, Y.; Xie, S. A novel approach for fabricating antibacterial nanofiber/cotton hybrid yarns. Fibers Polym. 2017, 18, 987–992. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Zheng, F.; Liu, H.; Yu, J.; Shi, X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Colloids Surfaces B Biointerfaces 2013, 110, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G. Antibacterial, antiviral, and self-cleaning mats with sensing capabilities based on electrospun nanofibers decorated with ZnO nanorods and Ag nanoparticles for protective clothing applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Choong, T.S.Y.; Rashid, U. Recent progress in the design and synthesis of nanofibers with diverse synthetic methodologies: Characterization and potential applications. New J. Chem. 2020, 44, 9581–9606. [Google Scholar] [CrossRef]
- Motamedi, A.S.; Mirzadeh, H.; Hajiesmaeilbaigi, F.; Bagheri-Khoulenjani, S.; Shokrgozar, M. Effect of electrospinning parameters on morphological properties of PVDF nanofibrous scaffolds. Prog. Biomater. 2017, 6, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Kwak, S.; Gupta, K.C.; Kang, I.K. Antibacterial activity and cytocompatibility of PLGA/CuO hybrid nanofiber scaffolds prepared by electrospinning. J. Nanomater. 2015, 2015, 832762. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; Ossa, J.G.; Linari, S.; Lazzeri, A. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Goh, Y.F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef]
- Moreira, A.; Lawson, D.; Onyekuru, L.; Dziemidowicz, K.; Angkawinitwong, U.; Costa, P.F. Protein encapsulation by electrospinning and electrospraying. J. Control. Release 2021, 329, 1172–1197. [Google Scholar] [CrossRef] [PubMed]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 325–347. [Google Scholar]
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion electrospinning of polycaprolactone: Influence of surfactant type towards the scaffold properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75. [Google Scholar] [CrossRef]
- Ji, X.; Li, R.; Jia, W.; Liu, G.; Luo, Y.; Cheng, Z. Co-Axial Fibers with Janus-Structured Sheaths by Electrospinning Release Corn Peptides for Wound Healing. ACS Appl. Bio Mater. 2020, 3, 6430–6438. [Google Scholar] [CrossRef]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ma, Q.; Yu, W.; Dong, X.; Yang, Y.; Zhao, B. An electrospun flexible Janus nanoribbon array endowed with simultaneously tuned trifunctionality of electrically conductive anisotropy, photoluminescence and magnetism. New J. Chem. 2017, 41, 13983–13992. [Google Scholar] [CrossRef]
- Labbaf, S.; Ghanbar, H.; Stride, E.; Edirisinghe, M. Preparation of multilayered polymeric structures using a novel four-needle coaxial electrohydrodynamic device. Macromol. Rapid Commun. 2014, 35, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Yu, D.G.; Wang, M.; Ning, T. Nanofabrication of Janus Fibers through Side-by-Side Electrospinning—A Mini Review. Mater. Highlights 2021, 2, 18. [Google Scholar] [CrossRef]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Li, Y. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019, 204, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Santa, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewell, E.W.C.; Brown, E.D. Taking aim at wall teichoic acid synthesis: New biology and new leads for antibiotics. Nature 2014, 67, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistretta, N.; Brossaud, M.; Telles, F.; Sanchez, V.; Talaga, P. Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions. Nature 2019, 9, 3212. [Google Scholar] [CrossRef] [Green Version]
- Filimon, A.; Olaru, N.; Doroftei, F.; Coroaba, A.; Dunca, S. Processing of quaternized polysulfones solutions as tool in design of electrospun nanofibers: Microstructural characteristics and antimicrobial activity. J. Mol. Liq. 2021, 330, 115664. [Google Scholar] [CrossRef]
- Ahamed, M.I.; Sharma, G.; Khan, A.; Asiri, A.M. Turmeric/polyvinyl alcohol Th(IV) phosphate electrospun fibers: Synthesis, characterization and antimicrobial studies. J. Taiwan Inst. Chem. Eng. 2016, 68, 407–414. [Google Scholar] [CrossRef]
- Wang, H.; Gill, C.J.; Lee, S.H.; Mann, P.; Zuck, P.; Meredith, T.C. Discovery of wall teichoic acid inhibitors as potential anti-MRSA β-lactam combination agents. Chem. Biol. 2013, 20, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Antimicrobial activity of chitosan nanofibers obtained by electrospinning. Polym. Int. 2011, 60, 1663–1669. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.; Hussain, I.; Rotello, V. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Abadi, S.S.M.; Mirzaei, E.; Bazargani, A.; Gholipour, A.; Heidari, H.; Hadi, N. Antibacterial activity and mechanism of action of chitosan nanofibers against toxigenic Clostridioides (Clostridium) difficile Isolates. Ann. Med. Prev. Comunita 2020, 32, 72–80. [Google Scholar]
- Hajipour, M.J.; Fromm, K.M.; Akbar, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Landis, R.F.; Rotello, V.M. Nanoparticle-Based Antimicrobials: Surface Functionality is Critical. F1000Research 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Lin, N.; Berton, P.; Moraes, C.; Rogers, R.D.; Tufenkji, N. Nanodarts, nanoblades, and nanospikes: Mechano-bactericidal nanostructures and where to find them. Adv. Colloid Interface Sci. 2018, 252, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloids Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; Kingshott, P.; Mcarthur, S.L. Bacterial response to different surface chemistries fabricated by plasma polymerization on electrospun nanofibers. Biointerphases 2015, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 2041731418790694. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Boegli, L.; Hancock, J.; Bowersock, L.; Parker, A.; Kinney, B.M. Bacterial Adhesion and Biofilm Formation on Textured Breast Implant Shell Materials. Aesthetic Plast. Surg. 2019, 43, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M. Bacteria-targeted clindamycin loaded polymeric nanoparticles: Effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics 2019, 11, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [Green Version]
- Hanna, S.K.; Bustos, A.M.; Peterson, A.W.; Reipa, V.; Scanlan, D.; Coskun, S.H.; Cho, T.J.; Johnson, M.E.; Hackley, V.A.; Nelson, B.C.; et al. Agglomeration of Escherichia coli with positively charged nanoparticles can lead to artifacts in a standard Caenorhabditis elegans toxicity assay. Environ. Sci. Technol. 2018, 52, 5968–5978. [Google Scholar] [CrossRef] [PubMed]
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Zhou, J.; Li, L. An investigation of chitosan and its derivatives on red blood cell 982 agglutination. RSC Adv. 2017, 7, 12247. [Google Scholar] [CrossRef] [Green Version]
- Lüdecke, C.; Roth, M.; Yu, W.; Horn, U.; Bossert, J.; Jandt, K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surfaces B Biointerfaces 2016, 145, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Ghosh, A.; Bhadury, P.; De, P. Side-Chain Amino Acid-Based Cationic Antibacterial Polymers: Investigating the Morphological Switching of a Polymer-Treated Bacterial Cell. ACS Omega 2017, 2, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Tran, N.; Rifai, A.; Brandt, M.; Tran, P.A.; Leary, M. Rational design of additively manufactured Ti6Al4V implants to control Staphylococcus aureus biofilm formation. Materialia 2019, 5, 100250. [Google Scholar] [CrossRef]
- Yusuf, Y.; Ghazali, M.J.; Otsuka, Y.; Ohnuma, K.; Morakul, S.; Nakamura, S. Antibacterial properties of laser surface-textured TiO2/ZnO ceramic coatings. Ceram. Int. 2020, 46, 3949–3959. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure-Activity Relationship of Cationic Surfactants as Antimicrobial Agents. Curr. Opin. Colloid Interface Sci. 2019, 45, 28–43. [Google Scholar] [CrossRef]
- Liu, S.; Fukushima, K.; Venkataraman, S. Supramolecular nanofibers self-assembled from cationic small molecules derived from repurposed poly (ethylene teraphthalate) for antibiotic delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Nitanan, T.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Opanasopit, P. Fabrication of Cationic Exchange Polystyrene Nanofibers for Drug Delivery. Trop. J. Pharm. Res. 2014, 13, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Kim, K.S. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as promising alternative in the infection treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [Green Version]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.P.; Wang, L.; Benicewicz, B.C.; Decho, A.W. Inorganic nanoparticles engineered to attack bacteria. Chem. Soc. Rev. 2015, 44, 7787–7807. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-step green synthesis of antibacterial silver nanoparticles embedded in electrospun cyclodextrin nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, A.W.; Kim, I.S.; Ni, Q.Q. Cellulose acetate nanofibers embedded with AgNPs anchored TiO2 nanoparticles for long term excellent antibacterial applications. Carbohydr. Polym. 2019, 207, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.N.; Rebia, R.A.; Saito, Y.; Kharaghani, D.; Khatri, M.; Tanaka, T. Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J. Ind. Eng. Chem. 2020, 85, 258–268. [Google Scholar] [CrossRef]
- Sekar, A.D.; Kumar, V.; Muthukumar, H.; Gopinath, P.; Matheswaran, M. Electrospinning of Fe-doped ZnO nanoparticles incorporated polyvinyl alcohol nanofibers for its antibacterial treatment and cytotoxic studies. Eur. Polym. J. 2019, 118, 27–35. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Yang, Y.N.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.; Hoyos-Nogués, M.; Gil, F.J. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polym. Test. 2019, 79, 106022. [Google Scholar] [CrossRef]
- Celebi, H.; Gurbuz, M.; Koparal, S.; Dogan, A. Development of antibacterial electrospun chitosan/poly (vinyl alcohol) nanofibers containing silver ion-incorporated HAP nanoparticles. Compos. Interfaces 2013, 20, 799–812. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, J.; Hwang, T.I.; Park, C.H.; Kim, C.S. A multifunctional, one-step gas foaming strategy for antimicrobial silver nanoparticle-decorated 3D cellulose nanofiber scaffolds. Carbohydr. Polym. 2021, 273, 118603. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Fu, R.Q.; Yu, C.P.; Li, Z.H.; Guan, H.Y.; Hu, D.Q. Silver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofibers as wound dressings: A preclinical study. Int. J. Nanomed. 2013, 8, 4131–4145. [Google Scholar]
- Nairn, B.L.; Lonergan, Z.R.; Wang, J.; Braymer, J.J.; Zhang, Y.; Calcutt, M.W. The Response of Acinetobacter baumannii to Zinc Starvation. Cell Host Microbe 2016, 19, 826–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- De Faria, A.F.; Shaulsky, E.; Chavez, L.H.A.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide—Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Piao, J.G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Yang, L. Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Lu, C.J.; Jiang, X.F.; Junaid, M.; Ma, Y.B.; Jia, P.P.; Wang, H.B.; Pei, D.S. Graphene oxide nanosheets induce DNA damage and activate the base excision repair (BER) signaling pathway both in vitro and in vivo. Chemosphere 2017, 184, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.M.; Sekol, R.C.; Taylor, A.D.; Pfefferle, L.D.; Zimmerman, J.B. Realizing comparable oxidative and cytotoxic potential of single- and multiwalled carbon nanotubes through annealing. Environ. Sci. Technol. 2013, 47, 8775–8783. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Iqbal, M.A.; Qasim, M.; Park, C.H.; Yoo, H.; Hwang, J.H.; Uhm, S.J.; Song, H.; Park, C.; Do, J.T.; et al. Evaluation of graphene oxide induced cellular toxicity and transcriptome analysis in human embryonic kidney cells. Nanomaterials 2019, 9, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Jiang, C.; Wu, S.; Chen, W.; Lv, P.; Wang, Q.; Liu, J.; Narh, C.; Cao, X.; Ghiladi, R.A.; et al. Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J. Photochem. Photobiol. B Biol. 2020, 206, 111864. [Google Scholar] [CrossRef] [PubMed]
- Kanagasubbulakshmi, S.; Lakshmi, K.; Kadirvelu, K. Carbon quantum dots-embedded electrospun antimicrobial and fluorescent scaffold for reepithelialization in albino wistar rats. J. Biomed. Mater. Res.-Part A 2021, 109, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Photocatalytic self-cleaning poly(l-lactide) materials based on a hybrid between nanosized zinc oxide and expanded graphite or fullerene. Mater. Sci. Eng. C 2016, 60, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Eriksen, T.H.B.; Skovsen, E.; Fojan, P. Release of antimicrobial peptides from electrospun nanofibres as a drug delivery system. J. Biomed. Nanotechnol. 2013, 9, 492–498. [Google Scholar] [CrossRef]
- Seo, M.D.; Won, H.S.; Kim, J.H.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti-Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, R.; Zhang, Q.; Yang, S.; Liu, P.; Chen, L.; Wang, K.; Zhang, X.; Wei, Y. Nanoclay cross-linked semi-IPN silk sericin/poly(NIPAm/LMSH) nanocomposite hydrogel: An outstanding antibacterial wound dressing. Mater. Sci. Eng. C 2017, 81, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef]
- Amariei, G.; Kokol, V.; Boltes, K.; Letón, P.; Rosal, R. Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications. RSC Adv. 2018, 8, 28013–28023. [Google Scholar] [CrossRef] [Green Version]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Wang, K.; Zhang, X.; Wei, Y. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018, 9, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Vanhauteghem, D.; Janssens, G.P.J.; Lauwaerts, A.; Sys, S.; Boyen, F.; Cox, E.; Meyer, E. Exposure to the Proton Scavenger Glycine under Alkaline Conditions Induces Escherichia coli Viability Loss. PLoS ONE 2013, 8, e60328. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, J.M.; Asally, M. The Microbiologist’s Guide to Membrane Potential Dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Ge, L.; Gombart, A.F.; Shuler, F.D.; Carlson, M.A.; Reilly, D.A.; Xie, J. Nanofiber-based sutures induce endogenous antimicrobial peptide. Nanomedicine 2017, 12, 2597–2609. [Google Scholar] [CrossRef]
- Garcia-Orue, I.; Gainza, G.; Gutierrez, F.B.; Aguirre, J.J.; Evora, C.; Pedraz, J.L.; Hernandez, R.M.; Delgado, A.; Igartua, M. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int. J. Pharm. 2017, 523, 556–566. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun polycaprolactone/Aloe Vera_chitosan nanofibrous asymmetric membranes aimed for wound healing applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Fayemi, O.E.; Ekennia, A.C.; Katata-Seru, L.; Ebokaiwe, A.P.; Ijomone, O.M.; Onwudiwe, D.C.; Ebenso, E.E. Antimicrobial and Wound Healing Properties of Polyacrylonitrile-Moringa Extract Nanofibers. ACS Omega 2018, 3, 4791–4797. [Google Scholar] [CrossRef]
- Kegere, J.; Ouf, A.; Siam, R.; Mamdouh, W. Fabrication of Poly (vinyl alcohol)/Chitosan/Bidens pilosa Composite Electrospun Nanofibers with Enhanced Antibacterial Activities. ACS Omega 2019, 4, 8778–8785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar]
- Venkateswaran, S.; Wu, M.; Gwynne, P.J.; Hardman, A.; Lilienkampf, A.; Pernagallo, S.; Blakely, G.; Swann, D.G.; Gallagher, M.P.; Bradley, M. Bacteria repelling poly(methylmethacrylate-codimethylacrylamide) coatings for biomedical devices. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 6723–6729. [Google Scholar] [CrossRef] [Green Version]
- Lukowiak, M.C.; Wettmarshausen, S.; Hidde, G.; Landsberger, P.; Boenke, V.; Rodenacker, K.; Braun, U.; Friedrich, J.F.; Gorbushina, A.A.; Haag, R. Polyglycerol coated polypropylene surfaces for protein and bacteria resistance. Polym. Chem. 2014, 6, 1350–1359. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Chakraborty, P.; Saha, P.; Acharya, S.; Ray, M. Polymer based nanoformulation of methylglyoxal as an antimicrobial agent: Efficacy against resistant. RSC Adv. 2014, 4, 23251–23261. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Zhitnyak, I.Y.; Gloushankova, N.A. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [Green Version]
- Miguel, S.P.; Figueira, D.R.; Simoes, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surfaces B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Konar, M.; Sahoo, H.; Hota, G. Surface functionalization of electrospun PAN nanofibers with ZnO-Ag heterostructure nanoparticles: Synthesis and antibacterial study. Nanotechnology 2021, 30, 205704. [Google Scholar] [CrossRef] [PubMed]
- Semnani, D.; Naghashzargar, E.; Hadjianfar, M.; Dehghan Manshadi, F.; Mohammadi, S.; Karbasi, S.; Effaty, F. Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 149–157. [Google Scholar] [CrossRef]
- Guida, P.; Piscitelli, E.; Marrese, M.; Martino, V.; Cirillo, V.; Guarino, V.; Angeli, E.; Cocola, C.; Pelucchiet, C.; Repetto, L.; et al. Integrating Microstructured Electrospun Scaffolds in an Open Microfluidic System for in Vitro Studies of Human Patient-Derived Primary Cells. ACS Biomater. Sci. Eng. 2020, 6, 3649–3663. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef]
- Nandakumar, A.; Tahmasebi, B.Z.; Santos, D.; Mentink, A.; Auffermann, N.; van Der Werf, K.; Bennink, M.; Moroni, L.; van Blitterswijk, C.; Habibovic, P. Surface modification of electrospun fibre meshes by oxygen plasma for bone regeneration. Biofabrication 2013, 5, 015006. [Google Scholar] [CrossRef] [PubMed]
- Sanni, O.; Chang, C.; Anderson, D.G.; Langer, R.; Davies, M.C.; Williams, P.M.; Williams, P.; Alexander, M.R.; Hook, A.L. Bacterial Attachment to Polymeric Materials Correlates with Molecular Flexibility and Hydrophilicity. Adv. Healthcare Mater. 2014, 4, 695–701. [Google Scholar] [CrossRef]
- Hook, A.L.; Chang, C.; Yang, J.; Luckett, J.; Cockayne, A.; Atkinson, S.; Mei, Y.; Bayston, R.; Irvine, D.J.; Langer, L.; et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol. 2012, 30, 867–874. [Google Scholar] [CrossRef]
- Guarino, V.; Wan Abdul Khodir, W.K.; Ambrosio, L. Biodegradable microparticles and nanoparticles by electrospraying techniques. J. Appl. Biomater. Funct. Mater. 2012, 10, 191–196. [Google Scholar] [CrossRef]
- Lendlein, A.; Pandit, A. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar]
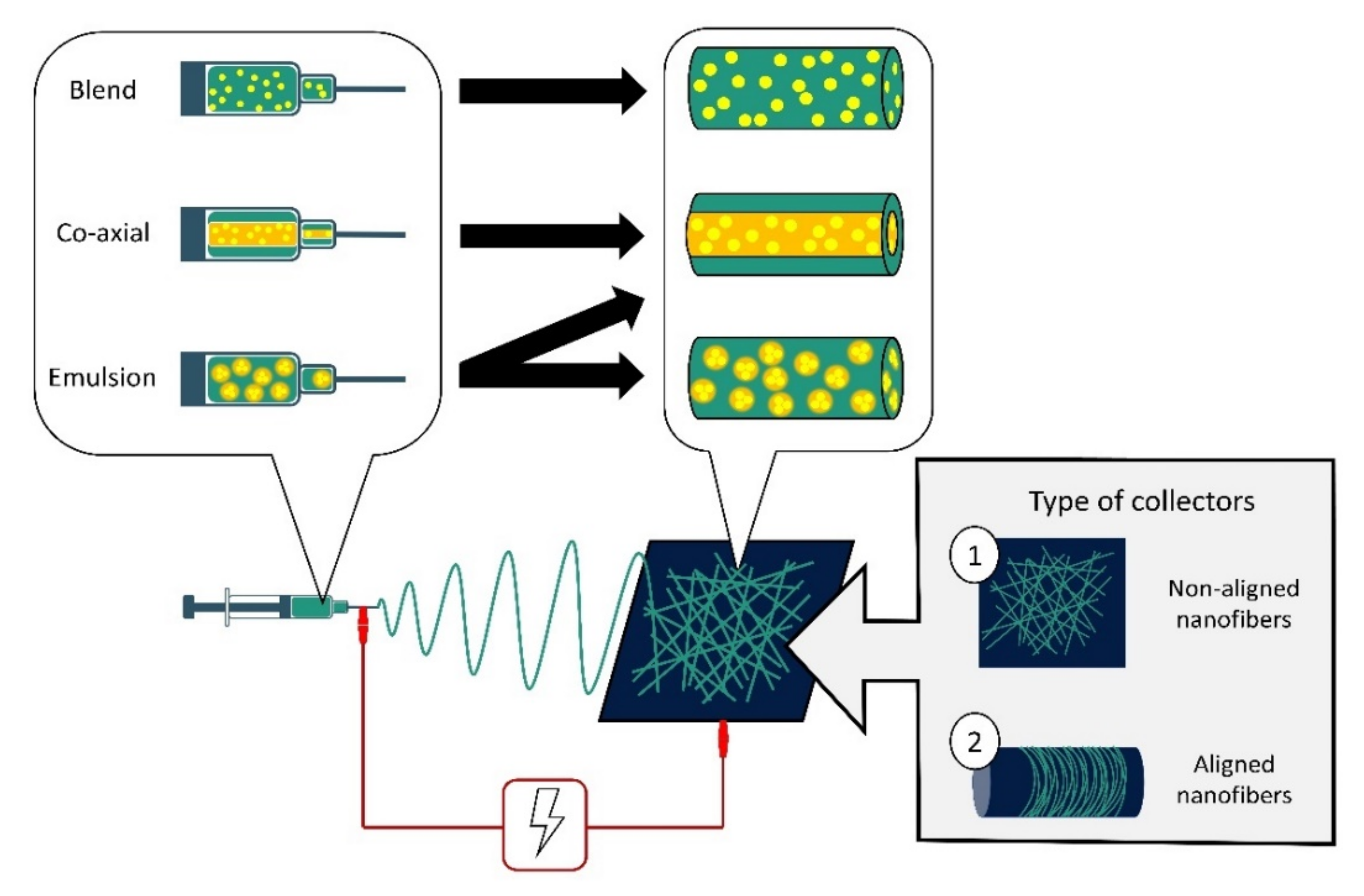

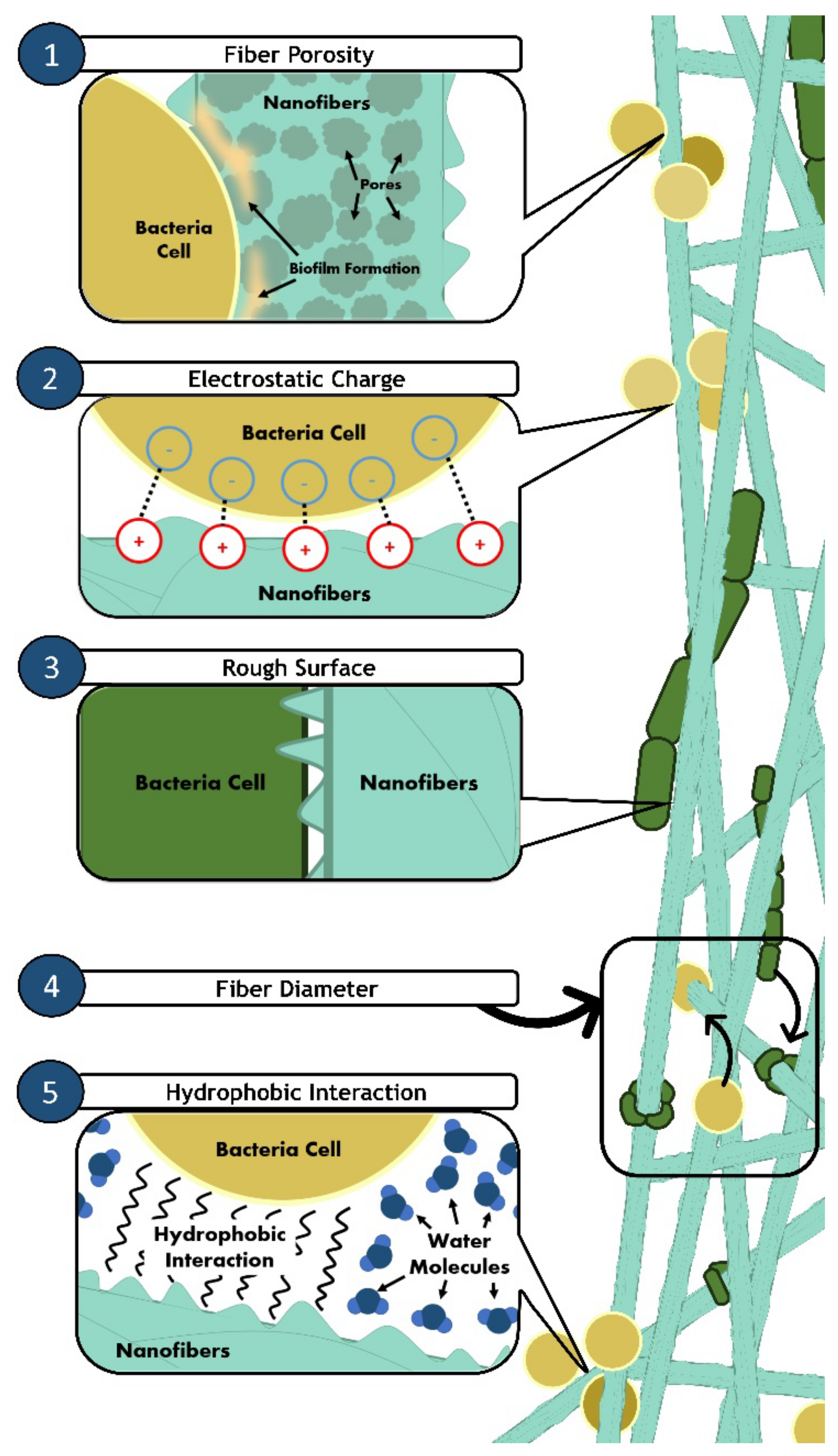

| Properties | Effects | References |
|---|---|---|
| Nano size | Nanofibers, ranging between 100–1000 nm, are similar to bacteria size, thus can enhance bacterial attachment and inhibition. | [62,63,64,65,66] |
| Surface area to volume | Nanofibers with smaller diameters provide a higher surface area-to-volume ratio for efficient encapsulation of antimicrobial therapeutic agents. | [67,68,69,70] |
| High porosity | High porosity allows higher loading of drug or antimicrobial agents into the nanofibers, enhances the surface area, and increases bacteria attachment on the surface of nanofibers. | [71,72,73] |
| Interconnected pores | Promote oxygen and nutrient exchange, provide structural stability, enhance cell proliferation and ensure sustained release of antimicrobial agents. | [72,74,75,76] |
| Polymers | Therapeutic Agent | Findings | References |
|---|---|---|---|
| PVA/Pea protein | Cinnamaldehyde | Inhibition of E. coli and S. aureus increased as the concentration of cinnamaldehyde was increased from 0.5 to 1.5 wt%. | [100] |
| PCL/ Cellulose acetate | Alkanin and shikonin | Higher drug loaded into the scaffolds (1–5 wt%) inhibited the growth of S. aureus and Staphylococcus epidermidis and accelerated wound closure. | [28] |
| PCL/PVA/Pectin | Chelidonium majus L. | The extract was sustained released (65.7%) for up to 30 days and inhibited the growth of S. aureus and Pseudomonas aeruginosa. | [101] |
| Chitosan/PEO | Antimicrobial peptides (AMP) | The addition of AMP into the nanofibers enhanced their antimicrobial activity against E. coli and S. aureus. | [102] |
| PVA/Collagen | Gentamicin | The release of antibiotic gentamicin can be controlled for up to 72 h. | [103] |
| PCL/Gelatin | Graphene oxide, tetracycline hydroxide | Nanofibers demonstrated high antimicrobial activity (99%) against S. aureus and E. coli | [64] |
| Silk Fibroin | Graphene oxide | Incorporation of graphene oxide reduced the survival rate of E. coli and S. aureus by 48%. | [29] |
| PCL/Zein Protein | Tetracycline hydrochloride | Tetracycline was sustained release up to 20 days and the nanofibers inhibited the growth of S. aureus and methicillin-resistant Staphylococcus aureus (MRSA) | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdan, N.; Yamin, A.; Hamid, S.A.; Khodir, W.K.W.A.; Guarino, V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. J. Funct. Biomater. 2021, 12, 59. https://doi.org/10.3390/jfb12040059
Hamdan N, Yamin A, Hamid SA, Khodir WKWA, Guarino V. Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. Journal of Functional Biomaterials. 2021; 12(4):59. https://doi.org/10.3390/jfb12040059
Chicago/Turabian StyleHamdan, Nazirah, Alisa Yamin, Shafida Abd Hamid, Wan Khartini Wan Abdul Khodir, and Vincenzo Guarino. 2021. "Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances" Journal of Functional Biomaterials 12, no. 4: 59. https://doi.org/10.3390/jfb12040059
APA StyleHamdan, N., Yamin, A., Hamid, S. A., Khodir, W. K. W. A., & Guarino, V. (2021). Functionalized Antimicrobial Nanofibers: Design Criteria and Recent Advances. Journal of Functional Biomaterials, 12(4), 59. https://doi.org/10.3390/jfb12040059







