Recent Advancements in the Technologies Detecting Food Spoiling Agents
Abstract
:1. Introduction
2. Spoilage of Food by Adventitious Agents
2.1. Microorganisms
2.1.1. Gram-Negative Rod-Shaped Bacteria
2.1.2. Gram-Positive Spore-Forming Bacteria
2.1.3. Lactic Acid Bacteria
2.1.4. Yeast and Molds
2.2. Non-Biological Contaminants
3. Methods of Detection of Adventitious Agents in Food
3.1. Single Cell Droplet Microfluidic System
3.2. Analytical Devices-Based Onmicrofluidic Paper System
3.3. Aptasensing for Detecting Microbes Their Toxins and Other Impurities
3.4. Electrochemical Biosensor Devices
3.5. Omics Tools for Detection: PCR-Based and LAMP-Based Detection
3.6. Elisa-Based Detection
3.7. Microextraction and Chromatographic Techniques
3.8. Biosensors
3.8.1. Nanobiosensor
3.8.2. DNA Biosensor
3.9. Smartphone-Based Biosensing
3.9.1. Smartphone-Based Optical Biosensors
3.9.2. Smartphone-Based Electrochemical Biosensor
3.10. DNA Microarray
- Longer probe length and increased specificity chip was designed by Stanford University, but it has a disadvantage of low chip density.
- In-situ synthesis technology produces chips by photolithography with probes of only 25 mer length. Multiple probes have been used to avoid misjudgment for a single gene.
- Micro bead placement method of microarray preparation where nucleic acid probes are put loaded on micro particles on a particular slide.
- qPCR array where RT-PCR primer and probe were synthesized in well plates microfluidic disk, and the detection was carried out by quantitative PCR.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behera, B.K.; Rout, P.K.; Behera, S. Move towards Zero Hunger; World Hunger and Poverty; Springer: Singapore, 2019; pp. 183–201. [Google Scholar]
- Bain, L.E.; Awah, P.K.; Geraldine, N.; Kindong, N.P.; Siga, Y.; Bernard, N.; Tanjeko, A.T. Malnutrition in Sub–Saharan Africa: Burden, causes and prospects. Pan Afr. Med. J. 2013, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; Food & Agriculture Organization: Rome, Italy, 2020. [Google Scholar]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste-FAO Report; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Abraham, J. Microbial Contamination and Food Degradation; Microbial Contamination, Prevention, and Early Detection in Food Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 21–47. [Google Scholar]
- Dogan, B.; Boor, J. Genetic diversity and spoilage potentials among Pseudomonas from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 2003, 69, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Raposo, A.; Pérez, E.; de Faria, C.T.; Ferrús, M.A.; Carrascosa, C. Food Borne Pathogens and Antibiotic Resistance; Food Spoilage by Pseudomonas spp.—An Overview; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 41–58. [Google Scholar]
- Dainty, H.; Mackey, M. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Bacteriol. 1992, 73, 113–114. [Google Scholar] [CrossRef]
- Walker, S.J.; Archer, P.; Banks, J.G. Growth of Pathogenic Bacteria at Chill Temperatures; Campden Food and Drink Research Association Technical Memorandum; Campden Food and Drink Research Association: Chipping Campden, UK, 1989; p. 535. [Google Scholar]
- Elliot, E.L.; Kaysner, C.A.; Jackson, L.; Tamplin, M.L. V. cholerae, V. parahaemolyticus, V. vulnificus, and other Vibrio spp. In Food and Drug Administration Bacteriological Analytical Manual, 8th ed.; (revision A); (CD-ROM version); Merker, R.L., Ed.; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Borch, E.; Kant-Muermans, M.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Arslan, S.; Eyi, A.; Ozdemir, F. Spoilage potentials and antimicrobial resistance of Pseudomonas spp. isolated from cheeses. J. Dairy Sci. 2011, 94, 5851–5856. [Google Scholar] [CrossRef] [Green Version]
- Molina, G.; Pimentel, M.; Pastore, G. Pseudomonas: A promising biocatalyst for the bioconversion of terpenes. Appl. Microbiol. Biotechnol. 2013, 97, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Resp. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Gaynes, R.; Edwards, J. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar]
- Kolleff, H.; Shorr, A.; Tabak, Y.; Gupta, V.; Liu, L.; Johannes, R. Epidemiology and outcomes of health-care associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest 2005, 128, 3854–3862. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.J.; Farmer, J.J., III. Bergey’s Manual of Systematics of Archaea and Bacteria; Enterobacteriaceae; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 1–24. [Google Scholar]
- Silvetti, T.; Morandi, S.; Brasca, M. Growth factors affecting gas production and reduction potential of vegetative cell and spore inocula of dairy-related Clostridium species. LWT 2018, 92, 32–39. [Google Scholar] [CrossRef]
- Cousin, M.A. Presence and activity of psychotrophic microorganisms in milk and dairy products: A review. J. Food Prot. 1982, 45, 172–207. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.J. Major spoilage organisms in milk and dairy products. J. Soc. Dairy Technol. 1988, 41, 91–92. [Google Scholar] [CrossRef]
- Podrzaj, L.; Burtscher, J.; Küller, F.; Domig, K.J. Strain-Dependent Cheese Spoilage Potential of Clostridium tyrobutyricum. Microorganisms 2020, 8, 1836. [Google Scholar] [CrossRef]
- Clark, F.M.; Dehr, A. A study of butyric acid-producing anaerobes isolated from spoiled canned tomatoes. Food Res. 1946, 12, 122–128. [Google Scholar] [CrossRef]
- Townsend, C.T. Spore-forming anaerobes causing spoilage in acid canned foods. Food Res. 1939, 4, 231–237. [Google Scholar] [CrossRef]
- Spiegelberg, C.H. Clostridium pasteurianum associated with spoilage of an acid canned fruit. Food Res. 1940, 5, 115–130. [Google Scholar] [CrossRef]
- Feng, G.; Churey, J.J.; Worobo, R.W. Thermoaciduric Clostridium pasteurianum spoilage of shelf-stable apple juice. J. Food Prot. 2010, 73, 1886–1890. [Google Scholar] [CrossRef]
- Jensen, T.Ø.; Kvist, T.; Mikkelsen, M.J.; Christensen, P.V.; Westermann, P. Fermentation of crude glycerol from biodiesel production by Clostridium pasteurianum. J. Ind. Microbiol. Biotechnol. 2012, 39, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.M.; Grosse-Honebrink, A.; Derecka, K.; Rotta, C.; Zhang, Y.; Minton, N.P. Towards improved butanol production through targeted genetic modification of Clostridium pasteurianum. Metab. Eng. 2017, 40, 124–137. [Google Scholar] [CrossRef]
- Sangeetha, R.; Karunanithi, T. Response Surface Methodological Analysis on Growth of Clostridium pasteurianum. Int. J. Chem. Technol. Res. 2011, 3, 303–308. [Google Scholar]
- Broda, D.M.; Saul, D.J.; Lawson, P.A.; Bell, R.G.; Musgrave, D.R. Clostridium gasigenes sp. nov., a psychrophile causing spoilage of vacuum-packed meat. Int. J. Syst. Evol. Microbiol. 2000, 50, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Lawson, P.; Dainty, R.H.; Kristiansen, N.; Berg, J.; Collins, M.D. Characterization of a psychrotrophic Clostridium causing spoilage in vacuum-packed cooked pork: Description of Clostridium algidicarnis sp. nov. Lett. Appl. Microbiol. 1994, 19, 153–157. [Google Scholar] [CrossRef]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M. Spoilage of vacuum-packed beef by a clostridium sp. J. Sci. Food Agric. 1989, 49, 153–157. [Google Scholar] [CrossRef]
- Collins, M.D.; Rodrigues, U.M.; Dainty, R.H.; Edwards, R.A.; Roberts, T.A. Taxonomic studies on a phsychrophilic Clostridium from vacuum-packed beef: Description of Clostridium estertheticum sp. FEMS Microbiol. Lett. 1992, 96, 235–240. [Google Scholar] [CrossRef]
- Adam, K.H.; Flint, S.H.; Brightwell, G. Psychrophilic and psychrotrophic clostridia: Sporulation and germination processes and their role in the spoilage of chilled, vacuum-packaged beef, lamb and venison. Int. J. Food Sci. Technol. 2010, 45, 1539–1544. [Google Scholar] [CrossRef]
- Coghill, D.; Juff, H.S. Incidence of psychotrophic spore forming bacteria in pasteurized milk and cream products and effect of temperature on their growth. Aust. J. Dairy Technol. 1979, 3, 150–153. [Google Scholar]
- Despina, K.V. Biochemical activities of Bacillus species isolated from flat sour evaporated milk. J. Dairy Sci. 1992, 75, 2681–2686. [Google Scholar] [CrossRef]
- Scheldeman, P.; Herman, L.; Foster, S.; Heyndrickx, M. Bacillus sporothermodurans and other highly heat-resistant spore formers in milk. J. Appl. Microbiol. 2006, 101, 542–555. [Google Scholar] [CrossRef]
- Crielly, E.; Logan, N.; Anderton, A. Studies on the Bacillus flora of milk and milk products. J. Appl. Bacteriol. 1994, 77, 256–263. [Google Scholar] [CrossRef]
- Dhakal, R.; Seale, R.B.; Deeth, H.C.; Craven, H.; Turner, M.S. Draft genome comparison of representatives of the three dominant genotype groups of dairy Bacillus licheniformis strains. Appl. Environ. Microbiol. 2014, 80, 3453–3462. [Google Scholar] [CrossRef] [Green Version]
- Jonghe, V.D.; Coorevits, A.; Block, J.D.; Coillie, E.V.; Grijspeerdt, K.; Herman, L.; Vos, P.D.; Heyndrickx, M. Toxinogenic and spoilage potential of aerobic spore-formers isolated from raw milk. Int. J. Food Microbiol. 2010, 136, 318–325. [Google Scholar] [CrossRef]
- Reginensi, S.M.; Gonzalez, M.J.; Olivera, J.A.; Sosa, M.; Juliano, P.; Bermudez, J. RAPD-based screening for spore-forming bacterial populations in Uruguayan commercial powdered milk. Int. J. Food Microbiol. 2011, 148, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hammer, P.; Lembke, F.; Suhren, G.; Heeschen, W. Characterization of a heat resistant mesophilic Bacillus species affecting quality of UHT-milk: A preliminary report. Kiel. Milchwirtsch. Forschungsber. 1995, 47, 297–305. [Google Scholar]
- Klijn, N.; Herman, L.; Langeveld, L.; Vaerewijck, M.; Wagendorp, A.A.; Huemer, I.; Weerkamp, A.H. Genotypical and phenotypical characterization of Bacillus sporothermodurans strains, surviving UHT sterilisation. Int. Dairy J. 1997, 7, 421–428. [Google Scholar] [CrossRef]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The prevalence and control of Bacillus and related spore-forming bacteria in the dairy industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef] [PubMed]
- Veld, H.I.; Jos, H.J. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Khalid, K. An overview of lactic acid bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Xu, Z.; Luo, Y.; Mao, Y.; Peng, R.; Chen, J.; Soteyome, T.; Bai, C.; Chen, L.; Liang, Y.; Su, J.; et al. Spoilage lactic acid bacteria in the brewing industry. J. Microbiol. Biotechnol. 2020, 30, 955–961. [Google Scholar] [CrossRef]
- Buu-Hoi, A.; Branger, C.; Acar, J.F. Vancomycin-resistant streptococci or Leuconostoc sp. Antimicrob. Agents Chemother. 1985, 28, 458–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, M.; Collins, M.D. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 1993, 75, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W. Yeasts in food spoilage: An update. Food Technol. 1979, 33, 76680. [Google Scholar]
- dos Santos Couto, M.M.B. Development and Implementation of Molecular Typing Techniques for Identification of Food Spoilage Yeasts. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 1995. [Google Scholar]
- Fowler, J.L.; Clark., W.S. Microbiology of delicatessen salads. J. Milk Food Technol. 1975, 38, 146–149. [Google Scholar] [CrossRef]
- Koburger, J.A. Fungi in foods. II. Some observations on acidulants used to adjust media pH for yeasts and mold counts. J. Milk Food Technol. 1971, 34, 4755477. [Google Scholar]
- Koburger, J.A. Fungi in foods. III. The enumeration of lipolytic and proteolytic organisms. J. Milk Food Technol. 1972, 35, 117–118. [Google Scholar] [CrossRef]
- Winter, F.H.; York, G.K.; El-Nakhal, H. Quick counting method for estimating the number of viable microbes on food and food processin equipment. Appl. Microbiol. 1971, 22, 89–92. [Google Scholar] [CrossRef]
- Dagnas, S.; Membré, J.M. Predicting and preventing mold spoilage of food products. J. Food Prot. 2013, 76, 538–551. [Google Scholar] [CrossRef]
- Aiko, V.; Mehta, A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015, 40, 943–954. [Google Scholar] [CrossRef]
- Liu, B.H.; Yu, F.Y.; Wu, T.S.; Li, S.Y.; Su, M.C.; Wang, M.C.; Shih, S.M. Evaluation of genotoxic risk and oxidative DNA damage in mammalian cells exposed to mycotoxins, patulin and citrinin. Toxicol. Appl. Pharmacol. 2003, 191, 255–263. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, J.; Sachdev, T.; Basu, T.; Malhotra, B.D. Recent advances in mycotoxins detection. Biosens. Bioelectron. 2016, 81, 532–545. [Google Scholar] [CrossRef]
- Oliveira, I.S.; da Silva Junior, A.G.; de Andrade, C.A.S.; Oliveira, M.D.L. Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr. Opin. Food Sci. 2019, 29, 64–79. [Google Scholar] [CrossRef]
- Hutton, M. Human health concerns of lead, mercury, cadmium and arsenic. In Lead, Mercury, Cadmium and Arsenic in the Environment; Hutchinson, T.C., Meema, K.M., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1987; Volume 31, pp. 53–68. [Google Scholar]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania Modified Gum Tragacanth Based Hydrogel Nanocomposite for Water Remediation. J. Environ. Chem. Eng. 2020, 9, 104608. [Google Scholar] [CrossRef]
- Abnet, C. Carcinogenic food contaminants. Cancer Investig. 2007, 25, 189–196. [Google Scholar] [CrossRef]
- Schantz, S.L.; Gardiner, J.C.; Gasior, D.M.; McCaffrey, R.J.; Sweeney, A.M.; Humphrey, H.E. Much ado about something: The weight of evidence for PCB effects on neuropsychological function. Psychol. Sch. 2004, 41, 669–679. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.P.; Dogliotti, E.; Di Domenico, A.; Fernandez-Cruz, M.L.; Fink-Gremmels, J.; Furst, P.; Galli, C.; et al. Polycyclic Aromatic Hydrocarbons in Food Scientifc Opinion of the Panel on Contaminants in the Food Chain. Food Saf. Auth. J. 2008, 724, 1–114. [Google Scholar]
- Li, Q.Q.; Loganath, A.; Chong, Y.S.; Tan, J.; Obbard, J.P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A 2006, 69, 1987–2005. [Google Scholar]
- Ritter, L.; Solomon, K.R.; Forget, J.; Stemeroff, M.; O’leary, C. A review of selected persistent organic pollutants for the International Programme on Chemical Safety (IPCS).; World Health Organization: Geneva, Switzerland, 1995; Volume 65, p. 66. [Google Scholar]
- Mostafavi, H.A.; Fathollahi, H.; Motamedi, F.; Mirmajlessi, S.M. Food irradiation: Applications, public acceptance and global trade. Afr. J. Biotechnol. 2010, 9, 2826–2833. [Google Scholar]
- Walker, J.S.; Don, G.W. Mathematics and Music; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Khan, M.F.; Wesley, S.G. Assessment of health safety from ingestion of natural radionuclides in seafoods from a tropical coast, India. Mar. Pollut. Bull. 2011, 62, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P.; Oliveira, J.M. Uranium isotopes in the Balkan’s environment and foods following the use of depleted uranium in the war. Environ. Int. 2010, 36, 352–360. [Google Scholar] [CrossRef]
- Brennwald, M.S.; Van Dorp, F. Radiological risk assessment and biosphere modelling for radioactive waste disposal in Switzerland. J. Environ. Radioact. 2009, 100, 1058–1061. [Google Scholar] [CrossRef]
- Pröhl, G.; Olyslaegers, G.; Kanyar, B.; Pinedo, P.; Bergstrom, U.; Mobbs, S.; Eged, K.; Katona, T.; Simon, I.; Hallberg, U.B.; et al. Development and comparison of five site-specifc biosphere models for safety assessment of radioactive waste disposal. J. Radiol. Prot. 2005, 25, 343–373. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental chemical contaminants in food: Review of a global problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Introduction to foodborne pathogens. In Modern Food Microbiology. Food Science Text Series; Springer: Berlin/Heidelberg, Germany, 2005; pp. 519–544. [Google Scholar]
- An, X.; Zuo, P.; Ye, B.C. A single cell droplet microfluidic system for quantitative determination of food-borne pathogens. Talanta 2020, 209, 120571. [Google Scholar] [CrossRef]
- Palchetti, I.; Mascini, M. Electroanalytical biosensors and their potential for food pathogen and toxin detection. Anal. Bioanal. Chem. 2008, 391, 455–471. [Google Scholar] [CrossRef]
- Zhu, X.D.; Shi, X.; Chu, J.; Ye, B.; Zuo, P.; Wang, Y.H. Quantitative analysis of the growth of individual Bacillus coagulans cells by microdroplet technology. Biores. Bioprocess. 2018, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.D.; Shi, X.; Wang, S.W.; Chu, J.; Zhu, W.H.; Ye, B.C.; Zuo, P.; Wang, Y.H. High-throughput screening of high lactic acid-producing Bacillus coagulans by droplet microfluidic based flow cytometry with fluorescence activated cell sorting. RSC Adv. 2019, 9, 4507–4513. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Jing, F.; Li, G.; Fan, X.; Jia, C.; Zhou, H.; Jin, Q.; Zhao, J. A microfluidic droplet digital PCR for simultaneous detection of pathogenic Escherichia coli O157 and Listeria monocytogenes. Biosens. Bioelectron. 2015, 74, 770–777. [Google Scholar] [CrossRef]
- Jang, M.; Jeong, S.W.; Bae, N.H.; Song, Y.; Lee, T.J.; Lee, M.K.; Lee, S.J.; Lee, K.G. Droplet-based digital PCR system for detection of single-cell level of foodborne pathogens. BioChip J. 2017, 11, 329–337. [Google Scholar] [CrossRef]
- Oh, J.H.; Park, M.K. Immunosensors combined with a light microscopic imaging system for rapid detection of Salmonella. Food Control 2016, 59, 780–786. [Google Scholar] [CrossRef]
- Srisa-Art, M.; Boehle, K.E.; Geiss, B.J.; Henry, C.S. Highly Sensitive Detection of Salmonella typhimurium Using a Colorimetric Paper-Based Analytical Device Coupled with Immunomagnetic Separation. Anal. Chem. 2018, 90, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Walt, D.R. Detection of Salmonella spp. Using Microsphere-Based, Fiber-Optic DNA Microarrays. Anal. Chem. 2005, 77, 5041–5047. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar] [CrossRef]
- Xu, X.; Ma, X.; Wang, H.; Wang, Z. Aptamer based SERS detection of Salmonella typhimurium using DNA-assembled gold nanodimers. Microchim. Acta 2018, 185, 325. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Chen, Y.; Zilberman, Y.; Mostafalu, P.; Sonkusale, S.R. Paper based platform for colorimetric sensing of dissolved NH3 and CO2. Biosens. Bioelectron. 2015, 67, 477–484. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Gehlot, P.; Sidapra, K.; Edwards, A.D.; Reis, N.M. Portable smartphone quantitation of prostate specific antigen (PSA) in a fluoropolymer microfluidic device. Biosens. Bioelectron. 2015, 70, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.Y.; Jia, C.P.; Yang, J.; Li, G.; Mao, H.J.; Jin, Q.H.; Zhao, J.L. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens. Bioelectron. 2015, 71, 380–386. [Google Scholar] [CrossRef]
- Martins, D.; Levicky, R.; Song, Y.A. Enhancing the speed of morpholino-DNA biosensor by electrokinetic concentration of DNA in a microfluidic chip. Biosens. Bioelectron. 2015, 72, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.F.; Tang, T.T.; Xu, H.S.; Zhu, C.Q.; Cunningham, B.T. High sensitivity automated multiplexed immunoassays using photonic crystal enhanced fluorescence microfluidic system. Biosens. Bioelectron. 2015, 73, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, S.T.; Lewis, G.G. The expanding role of paper in point-of-care diagnostics. Expert Rev. Mol. Diagnostics 2014, 14, 123–125. [Google Scholar] [CrossRef]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012, 84, 487–515. [Google Scholar] [CrossRef]
- Warsinke, A. Point-of-care testing of proteins. Anal. Bioanal. Chem. 2009, 393, 1393–1405. [Google Scholar] [CrossRef]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, S.Q.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 11–301. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.; Kumar Narasimhan, A.; Gangodkar, D.; Dhanasekaran, S.; Kumar Jha, N.; Dua, K.; Thakur, V.K.; Kumar Gupta, P. Aptameric Nanobiosensors for the Diagnosis of COVID-19: An Update. Mater. Lett. 2021, 131237. [Google Scholar] [CrossRef]
- Anthony, P.C.; Perez, C.F.; García-García, C.; Block, S.M. Folding energy landscape of the thiamine pyrophosphate riboswitch aptamer. Proc. Natl. Acad. Sci. USA 2012, 109, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Frieda, K.L.; Block, S.M. Direct observation of cotranscriptional folding in an adenine riboswitch. Science 2012, 338, 397–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmadadi, M.; Shayeh, J.S.; Omidi, M.; Yazdian, F.; Alebouyeh, M.; Tayebi, L. A glassy carbon electrode modified with reduced graphene oxide and gold nanoparticles for electrochemical aptasensing of lipopolysaccharides from Escherichia coli bacteria. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Xu, W.; Tian, J.; Shao, X.; Zhu, L.; Huang, K.; Luo, Y. A rapid and visual aptasensor for lipopolysaccharides detection based on the bulb-like triplex turn-on switch coupled with HCR-HRP nanostructures. Biosens. Bioelectron. 2017, 89, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Chai, Y.; Pu, X.; Yuan, R. A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 2014, 6, 2902–2908. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Miao, P. Electrochemical sensing strategies for the detection of endotoxin: A review. RSC Adv. 2013, 3, 9606–9617. [Google Scholar] [CrossRef]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [Green Version]
- Lan, M.; Wu, J.; Liu, W.; Zhang, W.; Ge, J.; Zhang, H.; Sun, J.; Zhao, W.; Wang, P. Copolythiophene-derived colorimetric and fluorometric sensor for visually supersensitive determination of lipopolysaccharide. J. Am. Chem. Soc. 2012, 134, 6685–6694. [Google Scholar] [CrossRef]
- Liu, L.; Jin, H.; Sun, L.; Ma, S.; Lin, R. Determination of Aflatoxins in Medicinal Herbs by High-performance Liquid Chromatography–Tandem Mass Spectrometry. Phytochem. Analusis 2012, 23, 469–476. [Google Scholar] [CrossRef]
- Kolosova, A.Y.; Shim, W.B.; Yang, Z.Y.; Eremin, S.A.; Chung, D.H. Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B 1. Stabilization of ELISA kit components and application to grain samples. Anal. Bioanal. Chem. 2006, 384, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luan, C.; Wang, L.; Wang, S.; Shao, L. Simultaneous determination of six mycotoxins in peanut by high-performance liquid chromatography with a fluorescence detector. J. Sci. Food Agric. 2017, 97, 1805–1810. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z. A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin B1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef]
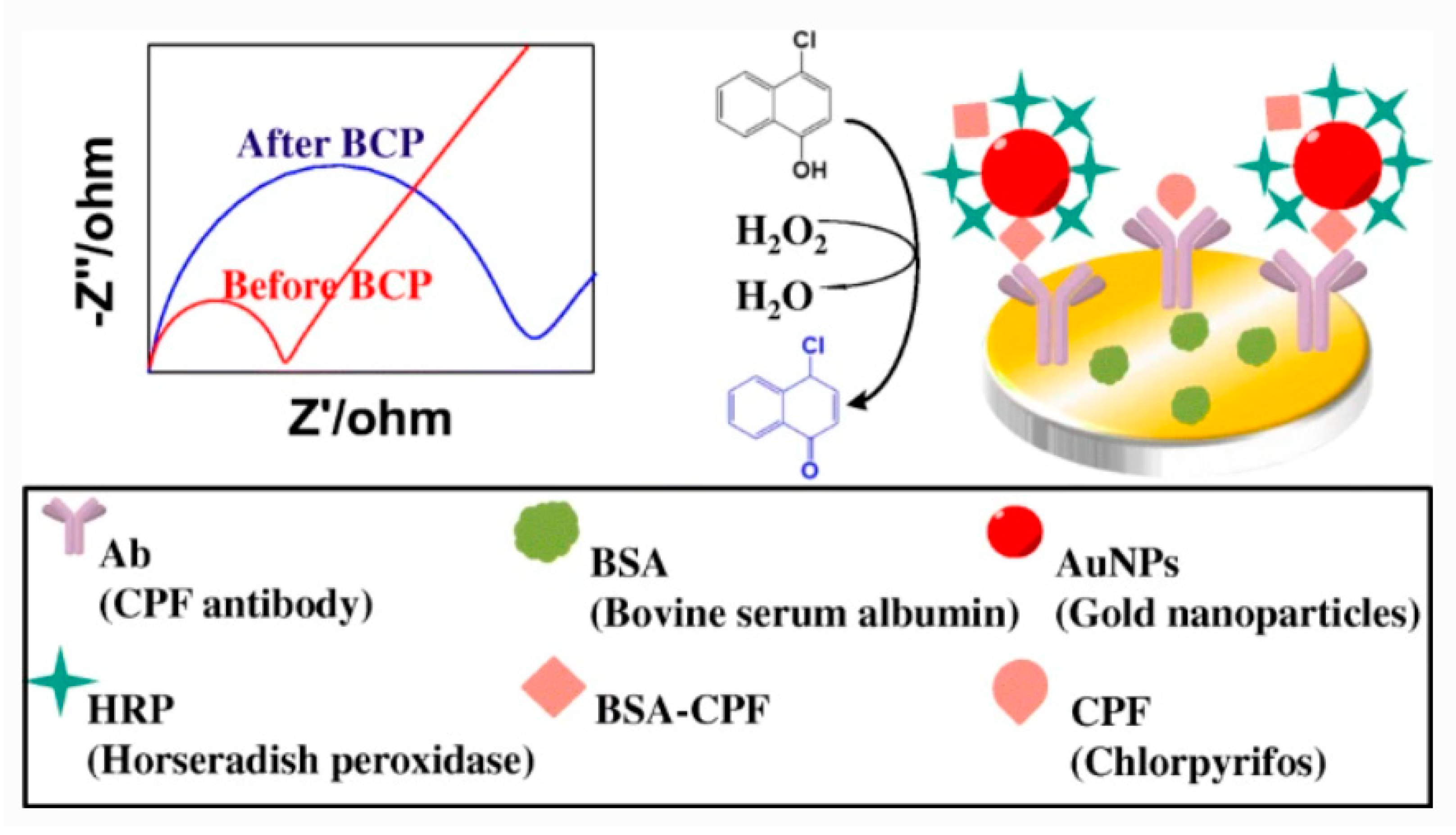
- Roushani, M.; Nezhadali, A.; Jalilian, Z. An electrochemical chlorpyrifos aptasensor based on the use of a glassy carbon electrode modified with an electropolymerized aptamer-imprinted polymer and gold nanorods. Microchim. Acta 2018, 185, 551. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Xu, W.; Leng, X.; Wang, H.; Guo, Y.; Huang, J. A novel sandwich-type electrochemical aptasensor based on GR-3D Au and aptamer-AuNPs-HRP for sensitive detection of oxytetracycline. Biosens. Bioelectron. 2017, 88, 181–187. [Google Scholar] [CrossRef]
- Shrivas, K.; Shankar, R.; Dewangan, K. Gold nanoparticles as a localized surface plasmon resonance based chemical sensor for on-site colorimetric detection of arsenic in water samples. Sens. Actuators B Chem. 2015, 220, 1376–1383. [Google Scholar] [CrossRef]
- Cui, H.; Yang, W.; Li, X.; Zhao, H.; Yuan, Z. An electrochemical sensor based on a magnetic Fe3O4 nanoparticles and gold nanoparticles modified electrode for sensitive determination of trace amounts of arsenic (III). Anal. Methods 2012, 4, 4176–4181. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemifard, N.; Rezaei, B. A simple and sensitive fluorimetricaptasensor for the ultrasensitive detection of arsenic (III) based on cysteamine stabilized CdTe/ZnS quantum dots aggregation. Biosens. Bioelectron. 2016, 77, 499–504. [Google Scholar] [CrossRef]
- Song, L.; Mao, K.; Zhou, X.; Hu, J. A novel biosensor based on Au@ Ag core–shell nanoparticles for SERS detection of arsenic (III). Talanta 2016, 146, 285–290. [Google Scholar] [CrossRef]
- Pooja, D.; Saini, S.; Thakur, A.; Kumar, B.; Tyagi, S.; Nayak, M.K. A “Turn-On” thiol functionalized fluorescent carbon quantum dot based chemosensory system for arsenite detection. J. Hazard. Mater. 2017, 328, 117–126. [Google Scholar]
- Vadahanambi, S.; Lee, S.H.; Kim, W.J.; Oh, I.K. Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures. Environ. Sci. Technol. 2013, 47, 10510–10517. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhang, C.; Liang, R.; Chi, B.; Yuan, Y.; Qiu, J. Highly sensitive voltammetric determination of arsenite by exploiting arsenite-induced conformational change of ssDNA and the electrochemical indicator Methylene Blue. Microchim. Acta 2017, 184, 4047–4054. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, X.; Jingjing, Y.U.; Wang, X. Determination of arsenic and its species in dry seafood by high performance liquid chromatography-inductively coupled plasma mass spectrometry. Chin. J. Anal. Chem. 2009, 37, 1738–1742. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, N.; Fang, Z.; Feng, Y.; Ke, L. Simultaneous multi-channel hydride generation atomic fluorescence spectrometry determination of arsenic, bismuth, tellurium and selenium in tea leaves. Food Chem. 2011, 124, 1185–1188. [Google Scholar] [CrossRef]
- Hassanpoor, S.; Khayatian, G.; Azar, A.R.J. Ultra-trace determination of arsenic species in environmental waters, food and biological samples using a modified aluminum oxide nanoparticle sorbent and AAS detection after multivariate optimization. Microchim. Acta 2015, 182, 1957–1965. [Google Scholar] [CrossRef]
- Rabieh, S.; Bagheri, M.; Planer-Friedrich, B. Speciation of arsenite and arsenate by electrothermal AAS following ionic liquid dispersive liquid-liquid microextraction. Microchim. Acta 2013, 180, 415–421. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Zeng, G.; Wu, Y.; Liu, Y.; Jiang, Q.; Gu, S. Three dimensional graphene based materials: Synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv. Colloid Interface Sci. 2015, 221, 41–59. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.Y.; He, L.; Zhang, X.; Liu, C.S. Fe (III)-based metal–organic framework-derived core–shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Akbarian, F.; Heydari-Soureshjani, E.; Rezaei, B. A novel aptasensor based on 3D-reduced graphene oxide modified gold nanoparticles for determination of arsenite. Biosens. Bioelectron. 2018, 122, 25–31. [Google Scholar] [CrossRef]
- Fritzen-Garcia, M.B.; Monteiro, F.F.; Cristofolini, T.; Acuña, J.J.S.; Zanetti-Ramos, B.G.; Oliveira, I.R.W.; Soldi, V.; Pasa, A.A.; Creczynski-Pasa, T.B. Characterization of horseradish peroxidase immobilized on PEGylated polyurethane nanoparticles and its application for dopamine detection. Sens. Actuators B Chem. 2013, 182, 264–272. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef]
- Venton, B.J.; Wightman, R.M. Psychoanalytical electrochemistry: Dopamine and behavior. Anal. Chem. 2003, 75, 414A–421A. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, F.; Zaidi, S.A.; Koo, C.M. Highly sensitive electrochemical sensor based on environmentally friendly biomass-derived sulfur-doped graphene for cancer biomarker detection. Sens. Actuators B Chem. 2017, 241, 716–724. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Zheng, X.Q.; Xu, J.Y.; Bao, W.J.; Wang, F.B.; Xia, X.H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Electrochemical aptasensors. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1237–1250. [Google Scholar] [CrossRef]
- Deng, C.Y.; Chen, J.H.; Nie, Z.; Wang, M.D.; Chu, X.C.; Chen, X.L.; Xiao, X.L.; Lei, C.Y.; Yao, S.Z. Impedimetric aptasensor with femtomolar sensitivity based on the enlargement of surface-charged gold nanoparticles. Anal. Chem. 2009, 81, 739–745. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.G.; Wang, D.W.; Dai, H.J. Noncovalent sidewall functionalization of single-walled carbon 381 nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Jin, H.; Gui, R.J.; Wang, Z.H. Facile fabrication of dual-ratiometric electrochemical sensors based on a bare electrode for dual-signal sensing of analytes in electrolyte solution. Sens. Actuators B Chem. 2017, 409, 71–78. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Kong, M.; Jiang, G.; Sun, Y.; Mo, W.; Lin, T.; Ye, F.; Zhao, S. A competitive immunoassay for electrochemical impedimetric determination of chlorpyrifos using a nanogold-modified glassy carbon electrode based on enzymatic biocatalytic precipitation. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, C.; Gui, R.; Gao, X.; Wang, Z. Reduced graphene oxide/nile blue/gold nanoparticles complex-modified glassy carbon electrode used as a sensitive and label-free aptasensor for ratiometric electrochemical sensing of dopamine. Anal. Chim. Acta 2018, 1025, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Zhang, Y.; Fang, J.; Xu, P.; Xu, J.; Li, X.; Liu, C.C.; Wen, W. Highly effective and specific way for the trace analysis of carbaryl insecticides based on Au 42 Rh 58 alloy nanocrystals. J. Mater. Chem. A 2017, 5, 7064–7071. [Google Scholar] [CrossRef]
- Ruan, C.; Yang, L.; Li, Y. Rapid detection of viable Salmonella typhimurium in a selective medium by monitoring oxygen consumption with electrochemical cyclic voltammetry. J. Electroanal. Chem. 2002, 519, 33–38. [Google Scholar] [CrossRef]
- Berg, J.D.; Fiksdal, L. Rapid detection of total and fecal coliforms in water by enzymatic hydrolysis of 4-methylumbelliferone-beta-D-galactoside. Appl. Environ. Microbiol. 1988, 54, 2118–2122. [Google Scholar] [CrossRef] [Green Version]
- Tryland, I.; Fiksdal, L. Enzyme characteristics of β-d-galactosidase-and β-d-glucuronidase-positive bacteria and their interference in rapid methods for detection of waterborne coliforms and Escherichia coli. Appl. Environ. Microbiol. 1998, 64, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar-Mathur, P.; Sunkara, S.; Bhatnagar-Panwar, M.; Waliyar, F.; Sharma, K.K. Biotechnological advances for combating Aspergillus flavus and aflatoxin contamination in crops. Plant Sci. 2015, 234, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, D.; Rajasekaran, K.; Payne, G.; Brown, R.; Yu, J.; Cleveland, T. The ‘omics’ tools: Genomics, proteomics, metabolomics and their potential for solving the aflatoxin contamination problem. World Mycotoxin J. 2008, 1, 3–12. [Google Scholar] [CrossRef]
- Bergholz, T.M.; Switt, A.I.M.; Wiedmann, M. Omics approaches in food safety: Fulfilling the promise? Trends Microbiol. 2014, 22, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Cela, E.; Verheecke-Vaessen, C.; Magan, N.; Medina, A. The “-omics” contributions to the understanding of mycotoxin production under diverse environmental conditions. Curr. Opin. Food Sci. 2018, 23, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Leng, Y.; Sun, K.; Chen, X.; Li, W. Suspension arrays based on nanoparticle-encoded microspheres for high-throughput multiplexed detection. Chem. Soc. Rev. 2015, 44, 5552–5595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in analysis and detection of major mycotoxins in foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- Righetti, L.; Dall’Asta, C.; Hajslova, J.; Rubert, J. Metabolomics approaches and their hidden potential for explaining the mycotoxin contamination problem. In Metabolomics:Fundamentals and Applications; IntechOpen: London, UK, 2016; p. 119. [Google Scholar]
- Balmer, D.; Flors, V.; Glauser, G.; Mauch-Mani, B. Metabolomics of cereals under biotic stress: Current knowledge and techniques. Front. Plant Sci. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to decipher the chemical defense of cereals against Fusarium graminearum and deoxynivalenol accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef]
- Varga, E.; Glauner, T.; Berthiller, F.; Krska, R.; Schuhmacher, R.; Sulyok, M. Development and validation of a (semi-) quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal. Bioanal. Chem. 2013, 405, 5087–5104. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.A. Detection of Aspergillus flavus in Stored Peanuts Using Real-Time PCR and the Expression of Aflatoxin Genes in Toxigenic and Atoxigenic A. flavus Isolates. Foodborne Pathog. Dis. 2015, 12, 289–296. [Google Scholar] [CrossRef]
- Zhang, J.; Chiodini, R.; Badr, A.; Zhang, G. The impact of next-generation sequencing on genomics. J. Genet. Genom. 2011, 38, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Ronning, C.M.; Wilkinson, J.R.; Campbell, B.C.; Payne, G.A.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C. Gene profiling for studying the mechanism of aflatoxin biosynthesis in Aspergillus flavus and A. parasiticus. Food Addit. Contam. 2007, 24, 1035–1042. [Google Scholar] [PubMed]
- Moore, G.G.; Mack, B.M.; Beltz, S.B. Genomic sequence of the aflatoxigenic filamentous fungus Aspergillus nomius. BMC Genom. 2015, 16, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Varga, J.; Bhatnagar, D.; Cleveland, T.E.; Nierman, W.C.; Campbell, B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008, 122, 49–60. [Google Scholar] [CrossRef]
- Cleveland, T.E.; Bhatnagar, D.; Yu, J. Elimination and control of aflatoxin contamination in agricultural crops through Aspergillus flavus genomics. In Mycotoxin Prevention and Control in Agriculture; Appell, M., Kendra, F.D.F., Trucksess, M.W., Eds.; ACS eBooks Publications: Washington, DC, USA, 2010; pp. 93–106. [Google Scholar]
- Faustinelli, P.C.; Wang, X.M.; Palencia, E.R.; Arias, R.S. Genome sequences of eight Aspergillus flavus spp. and one A. parasiticus sp., isolated from peanut seeds in georgia. Genome Announc. 2016, 4, e00278-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrotty, D.M.; Morley, M.P.; Cappola, T.P. Transcriptomic biomarkers of cardiovascular disease. Prog. Cardiovasc. Dis. 2012, 55, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macaulay, I.C.; Carr, P.; Gusnanto, A.; Ouwehand, W.; Fitzgerald, D.; Watkins, N. Platelet genomics and proteomics in human health and disease. J. Clin. Investig. 2005, 115, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Kanawati, B.; Schmitt-Kopplin, P. Foodomics as a promising tool to investigate the mycobolome. TrAC Trends Anal. Chem. 2017, 96, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Alwine, J.C.; Kemp, D.J.; Stark, G.R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 1977, 74, 5350–5354. [Google Scholar] [CrossRef] [Green Version]
- Malone, J.H.; Oliver, B. Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol. 2011, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [Green Version]
- Castellá, G.; Bragulat, M.R.; Puig, L.; Sanseverino, W.; Cabañes, F.J. Genomic diversity in ochratoxigenic and non ochratoxigenic strains of Aspergillus carbonarius. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Musungu, B.M.; Bhatnagar, D.; Brown, R.L.; Payne, G.A.; OBrian, G.; Fakhoury, A.M.; Geisler, M. A Network Approach of Gene Co-Expression in the Zea mays/Aspergillus flavus Pathosystem to Map Host/Pathogen Interaction Pathways. Front. Genet. 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.N.; Agarwal, G.; Pandey, M.K.; Sudini, H.K.; Jayale, A.S.; Purohit, S.; Desai, A.; Wan, L.; Guo, B.; Liao, B.; et al. Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, D.; Rajasekaran, K.; Gilbert, M.; Cary, J.W.; Magan, N. Advances in molecular and genomic research to safeguard food and feed supply from aflatoxin contamination. World Mycotoxin J. 2018, 11, 47–72. [Google Scholar] [CrossRef]
- Vettorazzi, A.; van Delft, J.; López de Cerain, A. A review on ochratoxin: A transcriptomic studies. Food Chem. Toxicol. 2013, 59, 766–783. [Google Scholar] [CrossRef]
- Eshelli, M.; Qader, M.M.; Jambi, E.J.; Hursthouse, A.S.; Rateb, M.E. Current status and future opportunities of omics tools in mycotoxin research. Toxins 2018, 10, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, R.E. PCR detection of aflatoxin producing fungi and its limitations. Int. J. Food Microbiol. 2012, 156, 1–6. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2018, 28, e63. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Niessen, L.; Bechtner, J.; Fodil, S.; Taniwaki, M.H.; Vogel, R.F. LAMP-based group specific detection of aflatoxin producers within Aspergillus section Flavi in food raw materials, spices, and dried fruit using neutral red for visible-light signal detection. Int. J. Food Microbiol. 2018, 266, 241–250. [Google Scholar] [CrossRef]
- Niessen, L.; Luo, J.; Denschlag, C.; Vogel, R.F. The application of loop-mediated isothermal amplification (LAMP) in food testing for bacterial pathogens and fungal contaminants. Food Microbiol. 2013, 36, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Parida, M.; Sannarangaiah, S.; Dash, P.K.; Rao, P.; Morita, K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification techniques; perspectives in clinical diagnosis of infectious diseases. Rev. Med. Virol. 2008, 18, 407–421. [Google Scholar] [CrossRef]
- Niessen, L. Current state and future perspectives of loop-mediated isothermal amplification (LAMP)-based diagnosis of filamentous fungi and yeasts. Appl. Microbiol. Biotechnol. 2015, 99, 553–574. [Google Scholar] [CrossRef]
- Luo, J.; Taniwaki, M.H.; Iamanaka, B.T.; Vogel, R.F.; Niessen, L. Application of loop-mediated isothermal amplification assays for direct identification of pure cultures of Aspergillus flavus, A. nomius, and A. caelatus and for their rapid detection in shelled Brazil nuts. Int. J. Food Microbiol. 2014, 172, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Vogel, R.F.; Niessen, L. Development and application of a loop-mediated isothermal amplification assay for rapid identification of aflatoxigenic molds and their detection in food samples. Int. J. Food Microbiol. 2012, 159, 214–224. [Google Scholar] [CrossRef]
- Luo, J.; Vogel, R.F.; Niessen, L. Rapid detection of aflatoxin producing fungi in food by real-time quantitative loop-mediated isothermal amplification. Food Microbiol. 2014, 44, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Reddy, C.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Cook, N.A. Rapid LAMP-Based Method for Screening Poultry Samples for Campylobacter without Enrichment. Front Microbiol. 2018, 9, 2401. [Google Scholar] [CrossRef]
- Wang, P. Nucleic acid-based rapid methods for the detection of foodborne pathogens. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 1759, p. 012023. [Google Scholar]
- Sadhasivam, S.; Britzi, M.; Zakin, V.; Kostyukovsky, M.; Trostanetsky, A.; Quinn, E.; Sionov, E. Rapid detection and identification of mycotoxigenic fungi and mycotoxins in stored wheat grain. Toxins 2017, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Sun, J.; Liu, B.; Zhang, Y.; Song, Y.; Wang, S. Development of a sensitivity-improved immunoassay for the determination of carbaryl in food samples. J. Sci. Food Agric. 2010, 90, 1106–1112. [Google Scholar] [CrossRef]
- Wang, S.; Yu, C.D.; Wang, J.P. Enzyme immunoassay for the determination of carbaryl residues in agricultural products. Food Addit. Contam. 2005, 22, 735–742. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, G.; Fang, G.; Zhang, Y.; Wang, S. Development of a capillary electrophoresis-based immunoassay with laser-induced fluorescence for the detection of carbaryl in rice samples. J. Agric. Food Chem. 2008, 56, 8832–8837. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.Z.M.; Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. Two-dimensional 1T-phase transition metal dichalcogenides as nanocarriers to enhance and stabilize enzyme activity for electrochemical pesticide detection. ACS Nano 2017, 11, 5774–5784. [Google Scholar] [CrossRef] [PubMed]
- Croci, L.; Delibato, E.; Volpe, G.; De Medici, D.; Palleschi, G. Comparison of PCR, electrochemical enzyme-linked immunosorbent assays, and the standard culture method for detecting Salmonella in meat products. Appl. Environ. Microbiol. 2004, 70, 1393–1396. [Google Scholar] [CrossRef] [Green Version]
- Brewster, J.D.; Mazenko, R.S. Filtration capture and immunoelectrochemical detection for rapid assay of Escherichia coli O157: H7. J. Immunol. Methods 1998, 211, 1–8. [Google Scholar] [CrossRef]
- Lazcka, O.; Del Campo, F.J.; Munoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef]
- Mittelmann, A.S.; Ron, E.Z.; Rishpon, J. Amperometric Quantification of Total Coliforms and Specific Detection of Escherichia coli. Anal. Chem. 2002, 74, 903–907. [Google Scholar] [CrossRef]
- Bodur, S.; Özlü, C.; Tışlı, B.; Fırat, M.; Chormey, D.S.; Bakırdere, S. Analytical protocol for determination of endosulfan beta, propham, chlorpyrifos, and acibenzolar-s-methyl in lake water and wastewater samples by gas chromatography–mass spectrometry after dispersive liquid–liquid microextraction. Environ. Monit. Assess. 2020, 192, 1–9. [Google Scholar] [CrossRef]
- Crutchfield, C.A.; Lu, W.; Melamud, E.; Rabinowitz, J.D. Mass spectrometry-based metabolomics of yeast. Methods Enzymol. 2010, 470, 393–426. [Google Scholar]
- Santos, I.C.; Schug, K.A. Recent advances and applications of gas chromatography vacuum ultraviolet spectroscopy. J. Sep. Sci. 2017, 40, 138–151. [Google Scholar] [CrossRef]
- Stashenko, E.; Ren, J. Advances in Gas Chromatography; Gas Chromatography-Mass Spectrometry; InTech: London, UK, 2014. [Google Scholar]
- Viñas, P.; Campillo, N.; Andruch, V. Recent achievements in solidified floating organic drop microextraction. TrAC Trends Anal. Chem. 2015, 68, 48–77. [Google Scholar] [CrossRef]
- Zeng, C.; Ji, L.; Zhou, C.; Zhang, F.; Liu, M.; Xie, Q. Chemical vapor generation of bismuth in non-aqueous phase based on cloud point extraction coupled with thermospray flame furnace atomic absorption spectrometric determination. Microchem. J. 2015, 119, 1–5. [Google Scholar] [CrossRef]
- Román, I.P.; Chisvert Alberto, A.; Canals, A. Dispersive solid-phase extraction basedo n oleic acid-coated magnetic nanoparticles followed by gas chromatography mass spectrometry for UV-filter determination in water samples. J. Chromatogr. A 2011, 1218, 2467–2475. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, B. Comparison of hollow fiber liquid phase microextraction and dispersive liquid-liquid microextraction for the determination of organosulfur pesticides in environmental and beverage samples by gas chromatography with flame photometric detection. J. Chromatogr. A 2008, 1193, 7–18. [Google Scholar] [CrossRef]
- Šrámková, I.H.; Horstkotte, B.; Fikarová, K.; Sklenářová, H.; Solich, P. Direct-immersion single-drop microextraction and in-drop stirring microextraction for the determination of nanomolar concentrations of lead using automated Lab-In-Syringe technique. Talanta 2018, 184, 162–172. [Google Scholar] [CrossRef]
- Vidal, M.; Ospina, M.; Serafim, A.B.; Calafat, A.M.; Baker, S.E.; Morales-Agudelo, P. Quantification of DEET and neonicotinoid pesticide biomarkers in human urine by online solid-phase extraction high-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 411, 669–678. [Google Scholar]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Ranjbari, E.; Hadjmohammadi, M.R.; Kiekens, F.; De Wael, K. Mixed Hemi/Ad-Micelle Sodium Dodecyl Sulfate-Coated Magnetic Iron Oxide Nanoparticles for the Efficient Removal and Trace Determination of Rhodamine-B and Rhodamine-6G. Anal. Chem. 2015, 87, 7894–7901. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.D.S.; Laube, T.; Yamanaka, H.; Alegret, S.; Pividori, M.I. Magneto Immunoassays for Plasmodium falciparum Histidine-Rich. Anal. Chem. 2011, 83, 5570–5577. [Google Scholar] [CrossRef]
- Chaichi, M.J.; Ehsani, M. A novel glucose sensor based on immobilization of glucose oxidase on the chitosan-coated Fe3O4 nanoparticles and the luminol-H2O2 -gold nanoparticle chemiluminescence detection system. Sens. Actuators B Chem. 2016, 223, 713–722. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Mayorga-Martinez, C.C.; Watanabe, T.; Ivandini, T.A.; Honda, Y.; Pino, F.; Nakata, A.; Fujishima, A.; Einaga, Y.; Merkoçi, A. Microfluidic platform for environmental contaminants sensing and degradation based on boron-doped diamond electrodes. Biosens. Bioelectron. 2016, 75, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.F.M.; Neto, S.Y.; Luz, R.D.C.S.; Damos, F.S.; Yamanaka, H. Ultrasensitive determination of malathion using acetylcholinesterase immobilized on chitosan-functionalized magnetic iron nanoparticles. Biosensors 2018, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunney, J.; Williamson, S.; Atkin, D.; Jeanneret, M.; Cozzolino, D.; Chapman, J.; Power, A.; Chandra, S. The use of electrochemical biosensors in food analysis. Curr. Res. Nutr. Food Sci. J. 2017, 5, 183–195. [Google Scholar] [CrossRef]
- Lucarelli, F.; Marrazza, G.; Turner, A.P.F.; Mascini, M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens. Bioelectron. 2004, 19, 515–530. [Google Scholar] [CrossRef]
- Kerman, K.; Vestergaard, M.D.; Nagatani, N.; Takamura, Y.; Tamiya, E. Electrochemical genosensor based on peptide nucleic acid-mediated PCR and asymmetric PCR techniques: Electrostatic interactions with a metal cation. Anal. Chem. 2006, 78, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Palecek, E.; Fojta, M. Electrochemical DNA sensors. In Bioelectronics: From Theory to Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 127–192. [Google Scholar]
- Wang, J. Electrochemical nucleic acid biosensors. Anal. Chim. Acta 2002, 469, 63–71. [Google Scholar] [CrossRef]
- Pividori, M.I.; Merkoci, A.; Alegret, S. Electrochemical genosensor design: Immobilisation of oligonucleotides onto transducer surfaces and detection methods. Biosens. Bioelectron. 2002, 15, 291–303. [Google Scholar] [CrossRef]
- Farabullini, F.; Lucarelli, F.; Palchetti, I.; Marrazza, G.; Mascini, M. Disposable electrochemical genosensor for the simultaneous analysis of different bacterial food contaminants. Biosens. Bioelectron. 2007, 22, 1544–1549. [Google Scholar] [CrossRef]
- Lermo, A.; Campoy, S.; Barbe, J.; Hernandez, S.; Alegret, S.; Pividori, M.I. In situ DNA amplification with magnetic primers for the electrochemical detection of food pathogens. Biosens. Bioelectron. 2007, 22, 2010–2017. [Google Scholar] [CrossRef]
- Elsholz, B.; Wörl, R.; Blohm, L.; Albers, J.; Feucht, H.; Grunwald, T.; Jurgen, T.; Schweder, T.; Hintsche, R. Automated detection and quantitation of bacterial RNA by using electrical microarrays. Anal. Chem. 2006, 78, 4794–4802. [Google Scholar] [CrossRef]
- Baeumner, A.J.; Cohen, R.N.; Miksic, V.; Min, J. RNA biosensor for the rapid detection of viable Escherichia coli in drinking water. Biosens. Bioelectron. 2003, 18, 405–413. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Lin, M. Using standing gold nanorod arrays as surface-enhanced Raman spectroscopy (SERS) substrates for detection of carbaryl residues in fruit juice and milk. J. Agric. Food Chem. 2017, 65, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly sensitive, printable nanostructured conductive polymer wireless sensor for food spoilage detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef]
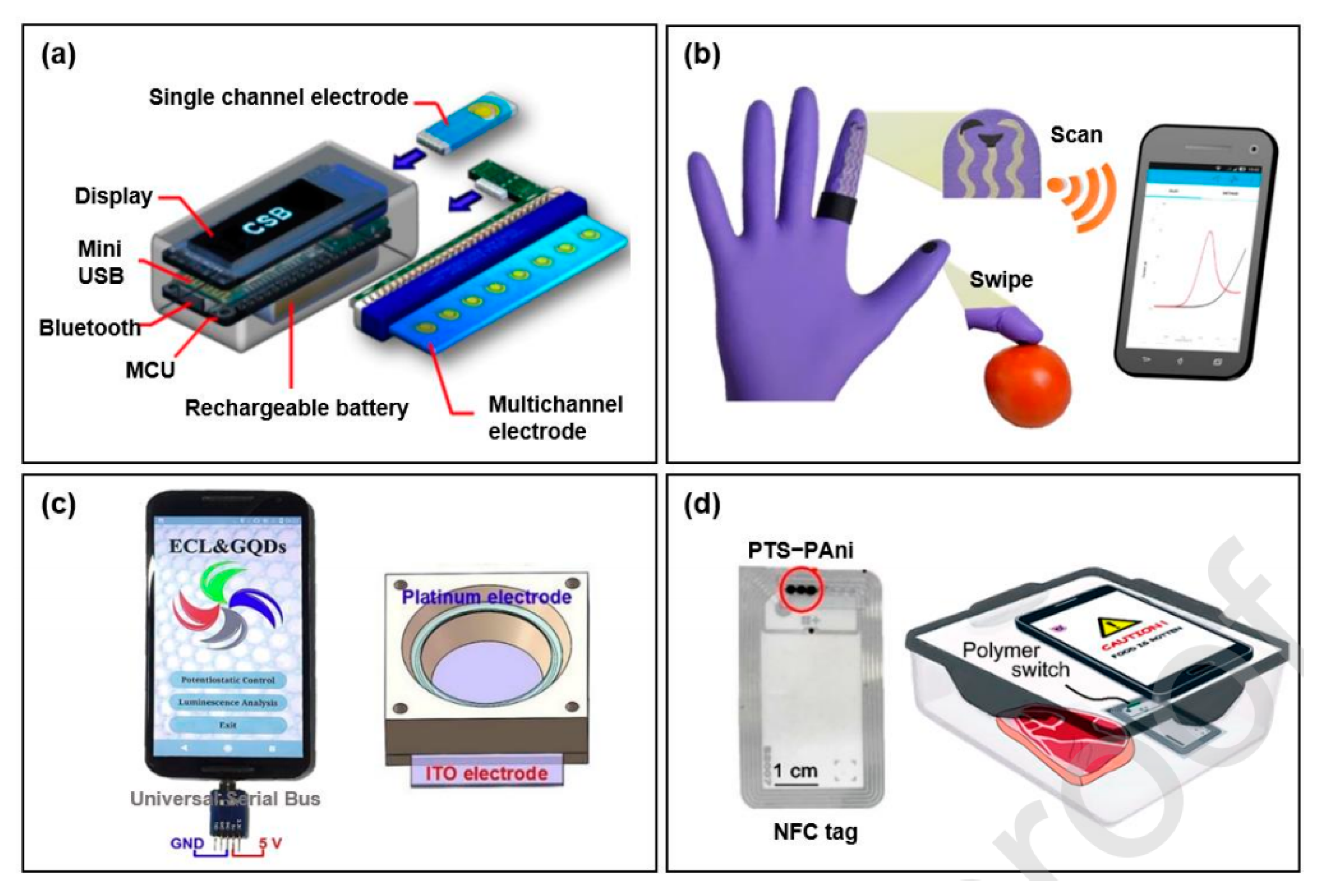
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Bioelectronics: Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Ross, G.M.S.; Bremer, M.G.E.G.; Nielen, M. Consumer-friendly food allergen detection: Moving towards smartphone-based immunoassays. Anal. Bioanal. Chem. 2018, 410, 5353–5371. [Google Scholar] [CrossRef] [Green Version]
- Lillehoj, P.B.; Huang, M.C.; Truong, N.; Ho, C.M. Rapid electrochemical detection on a mobile phone. Lab Chip 2013, 13, 2950–2955. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants–trends and perspective. TrAC Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Li, L.; Pan, L.; Ma, Z.; Yan, K.; Cheng, W.; Shi, Y.; Yu, G. All inkjet-printed amperometric multiplexed biosensors based on nanostructured conductive hydrogel electrodes. Nano Lett. 2018, 18, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- Quesada-González, D.; Merkoçi, A. Bioelectronics: Mobile phone-based biosensing: An emerging “diagnostic and communication” technology. Biosens. Bioelectron. 2017, 92, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Chen, Z.; Lu, Y.; Low, S.S.; Zhu, L.; Cheng, C.; He, Y.; Chen, Q.; Su, B.; et al. Electrogenerated chemiluminescence on smartphone with graphene quantum dots nanocomposites for Escherichia Coli detection. Sens. Actuators B Chem. 2019, 297, 126811. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Zeinhom, M.M.; Chang, Y.C.; Sheng, L.; Li, H.; Du, D.; Li, L.; Zhu, M.J.; Luo, Y.; et al. Nanozyme-mediated dual immunoassay integrated with smartphone for use in simultaneous detection of pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef]
- Shan, Y.; Wang, B.; Huang, H.; Jian, D.; Wu, X.; Xue, L.; Wang, S.; Liu, F. On-site quantitative Hg2+ measurements based on selective and sensitive fluorescence biosensor and miniaturized smartphone fluorescence microscope. Biosens. Bioelectron. 2019, 132, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mei, Q.; Yu, L.; Ge, H.; Yue, J.; Zhang, K.; Hayat, T.; Alsaedi, A.; Wang, S. Rapid and onsite detection of uranyl ions via ratiometric fluorescence signals based on a smartphone platform. ACS Appl. Mater. Interfaces 2018, 10, 42225–42232. [Google Scholar] [CrossRef]
- GSMA. The Mobile Economy 2019. Available online: https://www.gsma.com/mobileeconomy/wp-content/uploads/2021/07/GSMA_MobileEconomy2021_3.pdf (accessed on 2 September 2021).
- Zeinhom, M.M.A.; Wang, Y.; Sheng, L.; Du, D.; Li, L.; Zhu, M.J.; Lin, Y. Smart phone based immunosensor coupled with nanoflower signal amplification for rapid detection of Salmonella Enteritidis in milk, cheese and water. Sens. Actuators B Chem. 2018, 261, 75–82. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, I.; Zhang, Q.; Liu, X.; Dostalek, J.; Liedberg, B.; Wang, Y. Lipopolysaccharides detection on a grating-coupled surface plasmon resonance smartphone biosensor. Biosens. Bioelectron. 2018, 99, 312–317. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. Bioelectronics: A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157: H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, L.; Huang, F.; Wang, S.; Wang, L.; Liu, N.; Lin, J. A capillary biosensor for rapid detection of Salmonella using Fe-nanocluster amplification and smart phone imaging. Biosens. Bioelectron. 2019, 127, 142–149. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Cai, G.; Liu, N.; Liao, M.; Li, Y.; Zhang, X.; Lin, J. A microfluidic biosensor for online and sensitive detection of Salmonella typhimurium using fluorescence labeling and smartphone video processing. Biosens. Bioelectron. 2019, 140, 111333. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.; Yu, T.; Dai, Z.; Wei, Q. Aptamer-based fluorescent sensor array for multiplexed detection of cyanotoxins on a smartphone. Anal. Chem. 2019, 91, 10448–10457. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Arslan, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. Use of a smartphone for visual detection of melamine in milk based on Au@ carbon quantum dots nanocomposites. Food Chem. 2019, 272, 58–65. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, C.; Liu, Z.; Yuan, W.; Wu, X.; Lu, Y.; Low, S.S.; Liu, J.; Zhu, L.; Ji, D.; et al. Battery-Free and Wireless Epidermal Electrochemical System with All-Printed Stretchable Electrode Array for Multiplexed In Situ Sweat Analysis. Adv. Mater. Technol. 2019, 4, 1800658. [Google Scholar] [CrossRef]
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Bioelectronics: Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cheng, C.; Yuan, W.; Liu, Z.; Zhu, L.; Li, X.; Lu, Y.; Chen, Z.; Liu, J.; Cui, Z.; et al. Smartphone-based battery-free and flexible electrochemical patch for calcium and chloride ions detections in biofluids. Sens. Actuators B Chem. 2019, 297, 126743. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, J.; Chen, J.; Zhang, Q.; Lu, Y.; Yao, Y.; Li, S.; Liu, G.L.; Liu, Q. Smartphone-based portable biosensing system using impedance measurement with printed electrodes for 2, 4, 6-trinitrotoluene (TNT) detection. Biosens. Bioelectron. 2015, 70, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Huang, C.H.; Park, J.; Pathania, D.; Castro, C.M.; Fasano, A.; Weissleder, R.; Lee, H. Integrated magneto-chemical sensor for on-site food allergen detection. ACS Nano 2017, 11, 10062–10069. [Google Scholar] [CrossRef]
- Li, S.; Zhang, D.; Liu, J.; Cheng, C.; Zhu, L.; Li, C.; Lu, Y.; Low, S.S.; Su, B.; Liu, Q. Electrochemiluminescence on smartphone with silica nanopores membrane modified electrodes for nitroaromatic explosives detection. Biosens. Bioelectron. 2019, 129, 284–291. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable flexible and stretchable glove biosensor for on-site detection of organophosphorus chemical threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, Z.; Liu, Q. Smartphone-based biosensors for portable food evaluation. Curr. Opin. Food Sci. 2019, 28, 74–81. [Google Scholar] [CrossRef]
- Zeinhom, M.M.A.; Wang, Y.; Song, Y.; Zhu, M.J.; Lin, Y.; Du, D. A portable smart-phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens. Bioelectron. 2018, 99, 479–485. [Google Scholar] [CrossRef]
- Wilson, W.J.; Strout, C.L.; DeSantis, T.Z.; Stilwell, J.L.; Carrano, A.V.; Andersen, G.L. Sequence-specific identification of 18 pathogenic microorganisms using microarray technology. Mol. Cell. Probes 2002, 16, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Mockler, T.C.; Ecker, J.R. Applications of DNA tiling arrays for whole-genome analysis. Genomics 2005, 85, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bertone, P.; Gerstein, M.; Snyder, M. Applications of DNA tiling arrays to experimental genome annotation and regulatory pathway discovery. Chromosome Res. 2005, 13, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Feng, K.; Jiang, A.; Xiu, Z.; Lao, Y.; Li, Y.; Long, Y. An In Situ-Synthesized Gene Chip for the Detection of Food-Borne Pathogens on Fresh-Cut Cantaloupe and Lettuce. Front. Microbiol. 2020, 10, 3089. [Google Scholar]
- Shridhar, P.B.; Patel, I.R.; Gangiredla, J.; Noll, L.W.; Shi, X.; Bai, J.; Elkins, C.A.; Strockbine, N.; Nagaraja, T.G. DNA microarray-based assessment of virulence potential of Shiga toxin gene-carrying Escherichia coli O104: H7 isolated from feedlot cattle feces. PLoS ONE 2018, 13, e0196490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, R.; Card, R.; Nunes, C.; AbuOun, M.; Bagnall, M.C.; Nunez, J.; Mendonca, N.; Anjum, M.F.; Silva, G.J.D. Virulence Characterization of Salmonella enterica by a New Microarray: Detection and Evaluation of the Cytolethal Distending Toxin Gene Activity in the Unusual Host S. Typhimurium. PLoS ONE 2015, 10, e0135010. [Google Scholar] [CrossRef]





| Organism/Chemical | Name | Food-Borne Diseases and Problems | High-Risk Foods |
|---|---|---|---|
| Gram-Positive bacteria | Listeria monocytogenes | Food borne-listeriosis; Diarrhea | Meat-related products (Deli or ready-to-consume),such ascold smoked-fishery items, meat, sausages, etc. |
| Bacillus cereus | Emetic and diarrheal syndrome | Pasteurized milk and dairy products, red meat, beef, lamb, vension | |
| Bacillus licheniformis, B. coagulans, Geobacillus stearothermophilus, Clostridium algidixylanolyticum, C. algidicarnis, C. gasigenes, C. frigidicarnis and C. estertheticum | Inflammatory bowel disease, Crohn’s disease | Dry milk, and tomato juice (low-acid) | |
| Lactobacillus lactis, Leuconostoc spp. | Diarrhea, wounds and urinary tract infection, bacteremia, pneumonia, and cerebral hemorrhage | Fermented food and beverages, wine, beer, and fruit juices, vacuum packaged meat, fish, and poultry products | |
| Staphylococcus aureus | Suppurative infection, septicemia, pneumonia, sepsis, pericarditis, pseudomembranous colitis | Meat, milk, fish and their products, eggs, and cold food savory | |
| Clostridium botulinum | Respiratory and muscle relaxation paralysis, botulism, blurred vision | Cured meat and Canned products | |
| Gram-Negative bacteria | Pseudomonas | Cystic fibrosis, respiratory and urinary infections, pneumonia as hospital-acquired disease | Vegetables and fruits, red meat, poultry, fish, milk, and milk products |
| Enterobacteriaceae | Diarrheal disease, septicemia; bacteremias, respiratory disease; wound and burn infections; urinary tract infections; and meningitis due to its pathogenicity | Raw meat, chicken and beef, fresh cream desserts | |
| Salmonella typhimurium | Stomach pain, typhoid fever, diarrhea, nausea, headache, gastroenteritis, fever, chills, septicemia | Raw forms ofdairy produce, egg, raw or less cookedmeat, poultry, and seafood. Unprocessedsalads and chocolate. | |
| Escherichia coli | Nausea, diarrhea, stomach pain, fever, headache, and chills | Raw forms of dairy products, raw or less cooked meat, poultry products, such asegg, and seafood | |
| Campylobacter | Nausea, Diarrhea, Stomach pain, fever, and headache | Raw milk, raw or undercooked meat and poultry | |
| Shigella | Bacterial dysentery | Raw and cooked food | |
| Cronobacter | Neonatal meningitis, necrotizing colitis and bacteremia | Milk powder and infant feed | |
| Fungus | Aspergillus, Fusarium,and Penicillium | Athlete’s foot, ringworm, aspergillosis, histoplasmosis and coccidiodomycosis | Fresh seafood, packaged meats, delicatessen salads |
| Parasite | Trematode (Opisthorchisspp; Clonorchisspp; Paragonimusspp; Fasciolaspp) | Trematodiases, Clonorchiasis, fascioliasis, opisthorchiasis, Paragonimiasis, severe lung and liver problem; fever; nausea | Infected raw vegetables, aquatic vegetables, raw fish or raw meat of animals feeding on these, crabs |
| Toxoplasma gondii | Toxoplasmosis | Beef, pork, shellfish, fruits, vegetables | |
| Giardia lamblia | Giardiasis | Shellfish | |
| Entamoeba histolytica | Acute dysentery, Ameboma, perianal ulceration | Raw fruits and vegetables | |
| Trypanosoma cruzi | Chagas disease | Raw fruits and vegetables | |
| Viruses | Hepatitis A virus | Fever, malaise, anorexia, nausea, jaundice | Vegetables, fruits, shellfish, iced drinks, milk, and dairy produce |
| Norovirus | Diarrhea, vomiting, nausea, muscle and stomach cramps | Contaminated drinking water, raw salads, raw shellfish or oysters, berries, and frozen food products | |
| Heavy metals | Arsenic | Lung and bladder diseases, skin infections, heart disorders | Contaminated drinking water, cereals, vegetables |
| Pesticides | Chlorpyrifos | Neuromuscular disorders, nausea, headache, acute poisoning | Contaminated farm produce |
| Carbaryl pesticide | Reproductive and developmental toxicity, cholinesterase inhibition, intestinal agenesis | Contaminated farm produce |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.V.; Vaid, P.; Saini, N.K.; Siwal, S.S.; Gupta, V.K.; Thakur, V.K.; Saini, A.K. Recent Advancements in the Technologies Detecting Food Spoiling Agents. J. Funct. Biomater. 2021, 12, 67. https://doi.org/10.3390/jfb12040067
Saini RV, Vaid P, Saini NK, Siwal SS, Gupta VK, Thakur VK, Saini AK. Recent Advancements in the Technologies Detecting Food Spoiling Agents. Journal of Functional Biomaterials. 2021; 12(4):67. https://doi.org/10.3390/jfb12040067
Chicago/Turabian StyleSaini, Reena V., Prachi Vaid, Neeraj K. Saini, Samarjeet Singh Siwal, Vijai Kumar Gupta, Vijay Kumar Thakur, and Adesh K. Saini. 2021. "Recent Advancements in the Technologies Detecting Food Spoiling Agents" Journal of Functional Biomaterials 12, no. 4: 67. https://doi.org/10.3390/jfb12040067
APA StyleSaini, R. V., Vaid, P., Saini, N. K., Siwal, S. S., Gupta, V. K., Thakur, V. K., & Saini, A. K. (2021). Recent Advancements in the Technologies Detecting Food Spoiling Agents. Journal of Functional Biomaterials, 12(4), 67. https://doi.org/10.3390/jfb12040067








