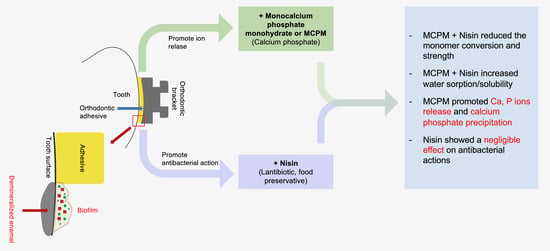
Physical/Mechanical and Antibacterial Properties of Orthodontic Adhesives Containing Calcium Phosphate and Nisin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Degree of Monomer Conversion
2.3. Biaxial Flexural Strength (BFS) and Biaxial Flexural Modulus (BFM)
2.4. Water Sorption and Solubility
2.5. Enamel Shear Bond Strength
- (1)
- Score 0: no adhesive remained on the enamel.
- (2)
- Score 1: less than 50% of the adhesive remained on the enamel surface.
- (3)
- Score 2: more than 50% of the adhesive remained on the enamel surface.
- (4)
- Score 3: all adhesive remained on the enamel surface.
2.6. Calcium Phosphate Precipitation
2.7. Ion Release
2.8. Fluence to S. mutans Growth
2.9. Statistical Analysis
3. Results
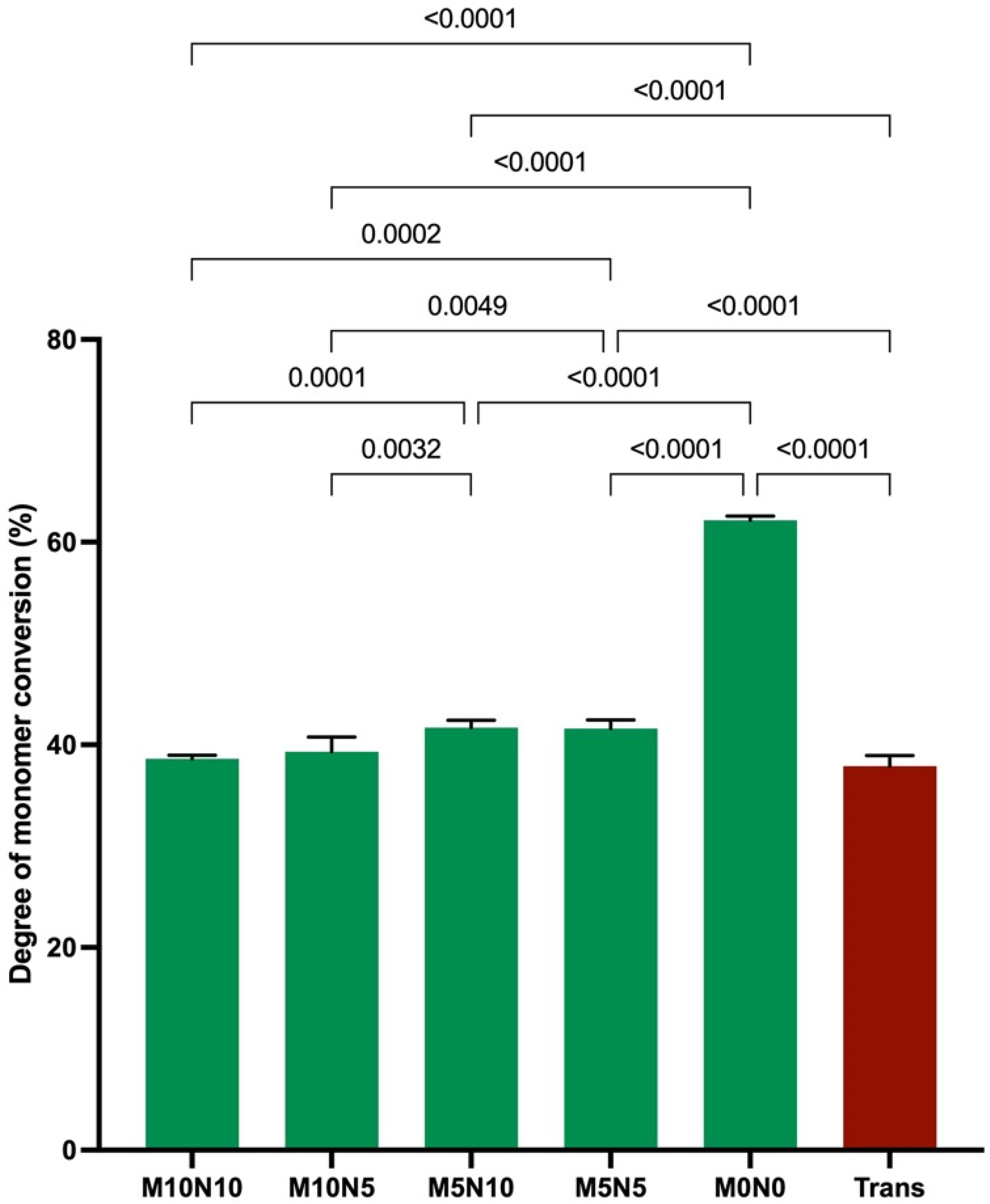
3.1. Degree of Monomer Conversion
3.2. Biaxial Flexural Strength (BFS) and Modulus (BFM)
3.3. Water Sorption (WSP) and Water Solubility (WSL)
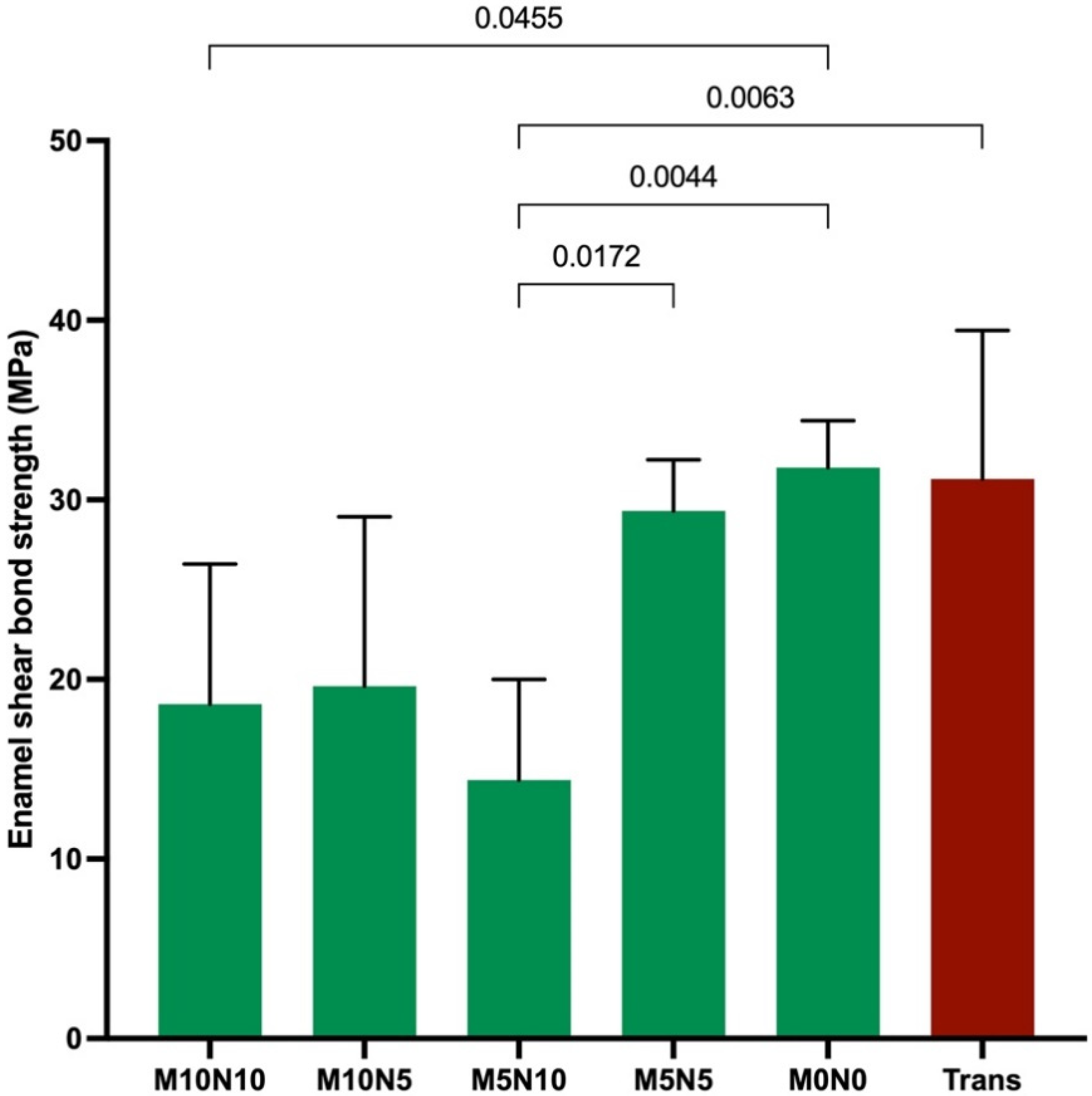
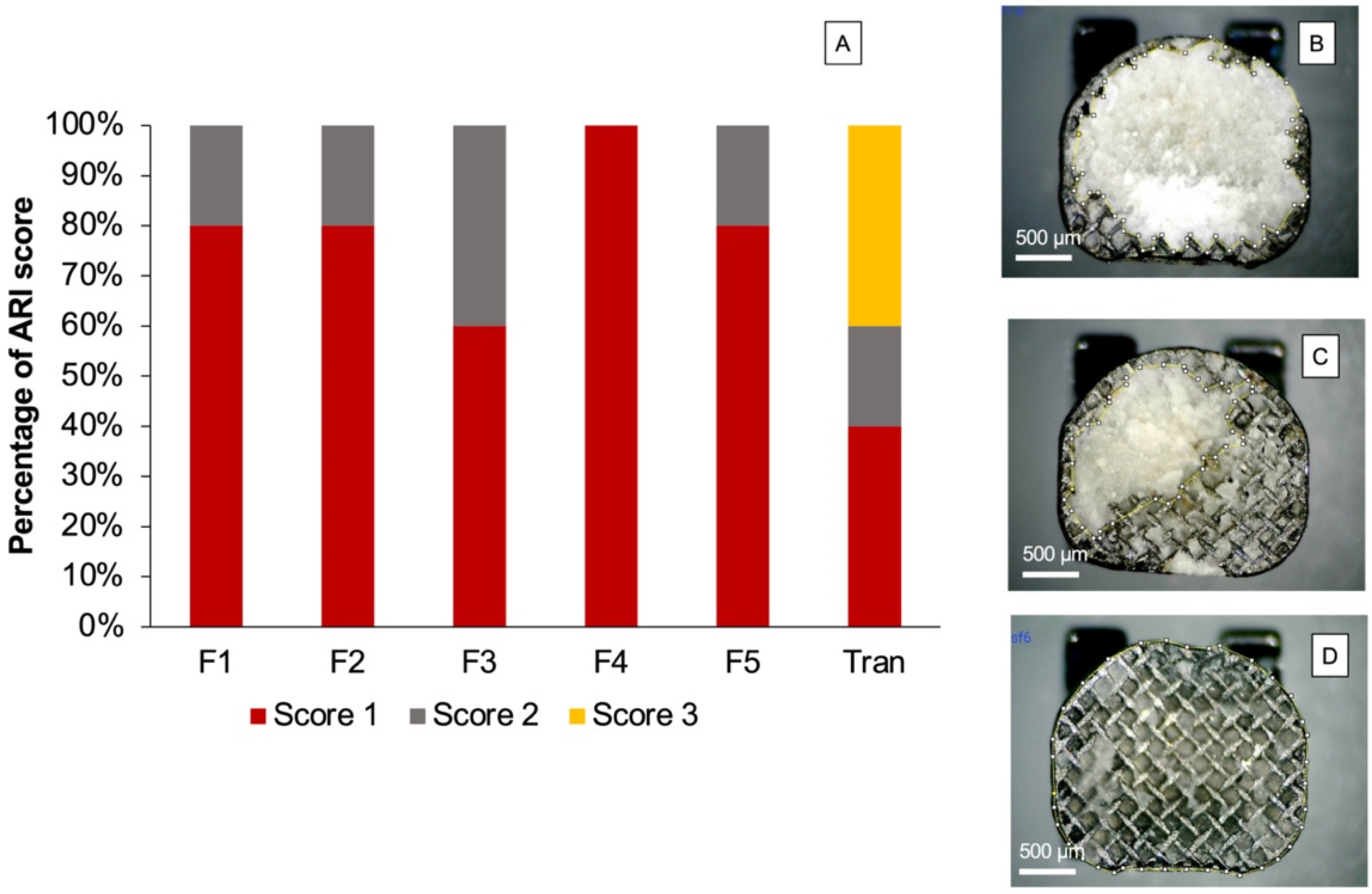
3.4. Enamel Shear Bond Strength (SBS) and Adhesive Remnant Index (ARI) Score
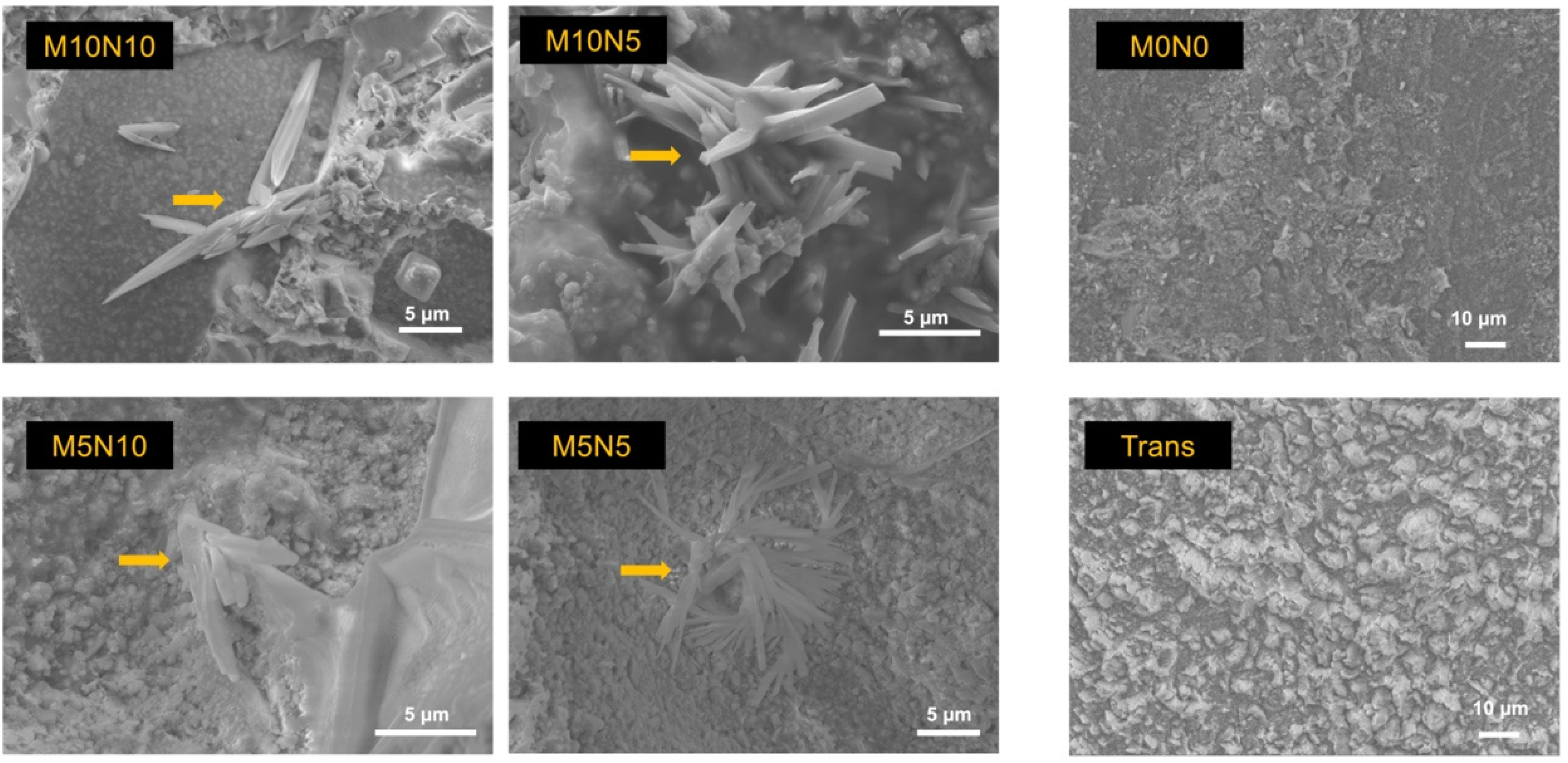
3.5. Calcium Phosphate Precipitation
3.6. Ion Release
3.7. Influence on S. mutans Growth
4. Discussion
4.1. Degree of Monomer Conversion
4.2. Biaxial Flexural Strength and Modulus
4.3. Water Sorption and Solubility
4.4. Enamel Shear Bond Strength (SBS)
4.5. Calcium Phosphate Precipitation
4.6. Ion Release
4.7. Antibacterial Action on S. mutans
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonesson, M.; Brechter, A.; Lindman, R.; Abdulraheem, S.; Twetman, S. Fluoride varnish for white spot lesion prevention during orthodontic treatment: Results of a randomized controlled trial 1 year after debonding. Eur. J. Orthod. 2021, 43, 473–477. [Google Scholar] [CrossRef]
- Livas, C.; Kuijpers-Jagtman, A.M.; Bronkhorst, E.; Derks, A.; Katsaros, C. Quantification of White Spot Lesions around Orthodontic Brackets with Image Analysis. Angle Orthod. 2008, 78, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Dalessandri, D.; Dalessandri, M.; Bonetti, S.; Visconti, L.; Paganelli, C. Effectiveness of an indirect bonding technique in reducing plaque accumulation around braces. Angle Orthod. 2012, 82, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Almosa, N.A.; Alqasir, A.M.; Aldekhayyil, M.A.; Aljelayel, A.; Aldosari, M.A. Enamel demineralization around two different orthodontic bracket adhesive systems: An in vivo study. Saudi Dent. J. 2019, 31, 99–104. [Google Scholar] [CrossRef]
- Al Tuma, R.R.; Yassir, Y.A. Evaluation of a newly developed calcium fluoride nanoparticles-containing orthodontic primer: An in-vitro study. J. Mech. Behav. Biomed. Mater. 2021, 122, 104691. [Google Scholar] [CrossRef]
- Al-Eesa, N.A.; Johal, A.; Hill, R.G.; Wong, F.S.L. Fluoride containing bioactive glass composite for orthodontic adhesives—Apatite formation properties. Dent. Mater. 2018, 34, 1127–1133. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Niu, L.N.; Yu, T.; Xu, H.H.K.; Weir, M.D.; Oates, T.W.; Tay, F.R.; Chen, J.H. Antibacterial and remineralizing orthodontic adhesive containing quaternary ammonium resin monomer and amorphous calcium phosphate nanoparticles. J. Dent. 2018, 72, 53–63. [Google Scholar] [CrossRef]
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-situ Raman spectroscopy of amorphous calcium phosphate to crystalline hydroxyapatite transformation. MethodsX 2018, 5, 1241–1250. [Google Scholar] [CrossRef]
- Kangwankai, K.; Sani, S.; Panpisut, P.; Xia, W.; Ashley, P.; Petridis, H.; Young, A.M. Monomer conversion, dimensional stability, strength, modulus, surface apatite precipitation and wear of novel, reactive calcium phosphate and polylysine-containing dental composites. PLoS ONE 2017, 12, e0187757. [Google Scholar] [CrossRef] [Green Version]
- Panpisut, P.; Khan, M.A.; Main, K.; Arshad, M.; Xia, W.; Petridis, H.; Young, A.M. Polymerization kinetics stability, volumetric changes, apatite precipitation, strontium release and fatigue of novel bone composites for vertebroplasty. PLoS ONE 2019, 14, e0207965. [Google Scholar] [CrossRef] [Green Version]
- Panpisut, P.; Liaqat, S.; Zacharaki, E.; Xia, W.; Petridis, H.; Young, A.M. Dental composites with calcium/strontium phosphates and polylysine. PLoS ONE 2016, 11, e0164653. [Google Scholar] [CrossRef]
- Panpisut, P.; Suppapatpong, T.; Rattanapan, A.; Wongwarawut, P. Monomer conversion, biaxial flexural strength, apatite forming ability of experimental dual-cured and self-adhesive dental composites containing calcium phosphate and nisin. Dent. Mater. J. 2021, 40, 399–406. [Google Scholar] [CrossRef]
- Aljabo, A.; Abou Neel, E.A.; Knowles, J.C.; Young, A.M. Development of dental composites with reactive fillers that promote precipitation of antibacterial-hydroxyapatite layers. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 285–292. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates as a dental regenerative material. In Advanced Dental Biomaterials; Elsevier: Duxford, UK, 2019; pp. 377–452. [Google Scholar]
- Aljabo, A.; Xia, W.; Liaqat, S.; Khan, M.A.; Knowles, J.C.; Ashley, P.; Young, A.M. Conversion, shrinkage, water sorption, flexural strength and modulus of re-mineralizing dental composites. Dent. Mater. 2015, 31, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Araujo, J.; Alvim, M.M.A.; Campos, M.; Apolonio, A.C.M.; Carvalho, F.G.; Lacerda-Santos, R. Analysis of Chlorhexidine Modified Cement in Orthodontic Patients: A Double-Blinded, Randomized, Controlled Trial. Eur. J. Dent. 2021, 15, 639–646. [Google Scholar] [CrossRef]
- Opstrup, M.S.; Jemec, G.B.E.; Garvey, L.H. Chlorhexidine Allergy: On the Rise and Often Overlooked. Curr. Allergy Asthma Rep. 2019, 19, 23. [Google Scholar] [CrossRef]
- Pemberton, M.N. Allergy to Chlorhexidine. Dent. Update 2016, 43, 272–274. [Google Scholar] [CrossRef]
- Saleem, H.G.; Seers, C.A.; Sabri, A.N.; Reynolds, E.C. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016, 16, 214. [Google Scholar] [CrossRef] [Green Version]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Webber, J.L.; Namivandi-Zangeneh, R.; Drozdek, S.; Wilk, K.A.; Boyer, C.; Wong, E.H.H.; Bradshaw-Hajek, B.H.; Krasowska, M.; Beattie, D.A. Incorporation and antimicrobial activity of nisin Z within carrageenan/chitosan multilayers. Sci. Rep. 2021, 11, 1690. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Prince, A.; Sandhu, P.; Ror, P.; Dash, E.; Sharma, S.; Arakha, M.; Jha, S.; Akhter, Y.; Saleem, M. Lipid-II Independent Antimicrobial Mechanism of Nisin Depends on Its Crowding and Degree of Oligomerization. Sci. Rep. 2016, 6, 37908. [Google Scholar] [CrossRef]
- Zhao, M.; Qu, Y.; Liu, J.; Mai, S.; Gu, L. A universal adhesive incorporating antimicrobial peptide nisin: Effects on Streptococcus mutans and saliva-derived multispecies biofilms. Odontology 2020, 108, 376–385. [Google Scholar] [CrossRef]
- Su, M.; Yao, S.; Gu, L.; Huang, Z.; Mai, S. Antibacterial effect and bond strength of a modified dental adhesive containing the peptide nisin. Peptides 2018, 99, 189–194. [Google Scholar] [CrossRef]
- Delgado, A.H.S.; Young, A.M. Methacrylate peak determination and selection recommendations using ATR-FTIR to investigate polymerisation of dental methacrylate mixtures. PLoS ONE 2021, 16, e0252999. [Google Scholar] [CrossRef]
- British Standard. BS EN ISO 4049:2019. In Dentistry-Polymer-Based Restorative Materials; BSI Standards Limited: London, UK, 2019. [Google Scholar]
- British Standard. PD ISO/TS 11405:2015. In Dentistry-Testing of Adhesion to Tooth Structure; BSI Standards Limited: London, UK, 2015. [Google Scholar]
- Thepveera, W.; Potiprapanpong, W.; Toneluck, A.; Channasanon, S.; Khamsuk, C.; Monmaturapoj, N.; Tanodekaew, S.; Panpisut, P. Rheological Properties, Surface Microhardness, and Dentin Shear Bond Strength of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. J. Funct. Biomater. 2021, 12, 42. [Google Scholar] [CrossRef]
- Årtun, J.; Bergland, S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am. J. Orthod. 1984, 85, 333–340. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, C.; Baena, E.; Fuentes, M.V.; Albaladejo, A.; Miguez-Contreras, M.; Lagravere, M.O.; Ceballos, L. Shear bond strength of a flash-free orthodontic adhesive system after thermal aging procedure. J. Clin. Exp. Dent. 2019, 11, e154–e161. [Google Scholar] [CrossRef]
- Potiprapanpong, W.; Thepveera, W.; Khamsuk, C.; Channasanon, S.; Tanodekaew, S.; Patntirapong, S.; Monmaturapoj, N.; Panpisut, P. Monomer Conversion, Dimensional Stability, Biaxial Flexural Strength, Ion Release, and Cytotoxicity of Resin-Modified Glass Ionomer Cements Containing Methacrylate-Functionalized Polyacids and Spherical Pre-Reacted Glass Fillers. Polymers 2021, 13, 2742. [Google Scholar] [CrossRef]
- Lygidakis, N.N.; Allan, E.; Xia, W.; Ashley, P.F.; Young, A.M. Early Polylysine Release from Dental Composites and Its Effects on Planktonic Streptococcus mutans Growth. J. Funct. Biomater. 2020, 11, 53. [Google Scholar] [CrossRef]
- Ferreira, C.J.; Leitune, V.C.B.; Balbinot, G.S.; Degrazia, F.W.; Arakelyan, M.; Sauro, S.; Mezzomo Collares, F. Antibacterial and Remineralizing Fillers in Experimental Orthodontic Adhesives. Materials 2019, 12, 652. [Google Scholar] [CrossRef] [Green Version]
- Panpisut, P.; Toneluck, A. Monomer conversion, dimensional stability, biaxial flexural strength, and fluoride release of resin-based restorative material containing alkaline fillers. Dent. Mater. J. 2020, 39, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Putzeys, E.; Nys, S.; Cokic, S.M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Meerbeek, B.V.; Van Landuyt, K.L. Long-term elution of monomers from resin-based dental composites. Dent. Mater. 2019, 35, 477–485. [Google Scholar] [CrossRef]
- Bationo, R.; Rouamba, A.; Diarra, A.; Beugre-Kouassi, M.L.A.; Beugre, J.B.; Jordana, F. Cytotoxicity evaluation of dental and orthodontic light-cured composite resins. Clin. Exp. Dent. Res. 2021, 7, 40–48. [Google Scholar] [CrossRef]
- Boonen, I.; De Nys, S.; Vervliet, P.; Covaci, A.; Van Landuyt, K.L.; Duca, R.C.; Godderis, L.; Denison, M.S.; Elskens, M. Assessing the estrogenic activity of chemicals present in resin based dental composites and in leachates of commercially available composites using the ERalpha-CALUX bioassay. Dent. Mater. 2021, 37, 1834–1844. [Google Scholar] [CrossRef]
- Kim, K.; An, J.S.; Lim, B.S.; Ahn, S.J. Effect of Bisphenol A Glycol Methacrylate on Virulent Properties of Streptococcus mutans UA159. Caries Res. 2019, 53, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.N.; Lim, B.S.; Ahn, S.J. Urethane Dimethacrylate Influences the Cariogenic Properties of Streptococcus Mutans. Materials 2021, 14, 1015. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bakkal, M.; Zengin Kurt, B. Structural and mechanical analysis of three orthodontic adhesive composites cured with different light units. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020901716. [Google Scholar] [CrossRef] [Green Version]
- Lempel, E.; Ori, Z.; Kincses, D.; Lovasz, B.V.; Kunsagi-Mate, S.; Szalma, J. Degree of conversion and in vitro temperature rise of pulp chamber during polymerization of flowable and sculptable conventional, bulk-fill and short-fibre reinforced resin composites. Dent. Mater. 2021, 37, 983–997. [Google Scholar] [CrossRef]
- Walters, N.J.; Xia, W.; Salih, V.; Ashley, P.F.; Young, A.M. Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent. Mater. 2016, 32, 264–277. [Google Scholar] [CrossRef] [Green Version]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Shortall, A.C.; Palin, W.M.; Burtscher, P. Refractive index mismatch and monomer reactivity influence composite curing depth. J. Dent. Res. 2008, 87, 84–88. [Google Scholar] [CrossRef]
- Fujita, K.; Nishiyama, N.; Nemoto, K.; Okada, T.; Ikemi, T. Effect of base monomer’s refractive index on curing depth and polymerization conversion of photo-cured resin composites. Dent. Mater. J. 2005, 24, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Ghani, F.; Fareed, M.A.; Riaz, S.; Khurshid, Z.; Zafar, M.S. Bi-axial flexural strength of resin based dental composites—Influence and reliability of the testing method configuration. Mater. Technol. 2021, 1–7. [Google Scholar] [CrossRef]
- Ilie, N.; Hilton, T.; Heintze, S.; Hickel, R.; Watts, D.; Silikas, N.; Stansbury, J.; Cadenaro, M.; Ferracane, J. Academy of dental materials guidance—Resin composites: Part I—Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef]
- Miura, D.; Ishida, Y.; Miyasaka, T.; Aoki, H.; Shinya, A. Reliability of Different Bending Test Methods for Dental Press Ceramics. Materials 2020, 13, 5162. [Google Scholar] [CrossRef] [PubMed]
- Azad, E.; Atai, M.; Zandi, M.; Shokrollahi, P.; Solhi, L. Structure-properties relationships in dental adhesives: Effect of initiator, matrix monomer structure, and nano-filler incorporation. Dent. Mater. 2018, 34, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Araujo-Neto, V.; Sebold, M.; Fernandes de Castro, E.; Feitosa, V.P.; Giannini, M. Evaluation of physico-mechanical properties and filler particles characterization of conventional, bulk-fill, and bioactive resin-based composites. J. Mech. Behav. Biomed. Mater. 2021, 115, 104288. [Google Scholar] [CrossRef]
- Odermatt, R.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Bioactivity and physico-chemical properties of dental composites functionalized with nano-vs. micro-sized bioactive glass. J. Clin. Med. 2020, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Mechanical properties of experimental composites containing bioactive glass after artificial aging in water and ethanol. Clin. Oral Investig. 2019, 23, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Natale, L.C.; Rodrigues, M.C.; Alania, Y.; Chiari, M.D.S.; Boaro, L.C.C.; Cotrim, M.; Vega, O.; Braga, R.R. Mechanical characterization and ion release of bioactive dental composites containing calcium phosphate particles. J. Mech. Behav. Biomed. Mater. 2018, 84, 161–167. [Google Scholar] [CrossRef]
- Xu, H.H.; Moreau, J.L. Dental glass-reinforced composite for caries inhibition: Calcium phosphate ion release and mechanical properties. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R. Calcium phosphates as ion-releasing fill.lers in restorative resin-based materials. Dent. Mater. 2019, 35, 3–14. [Google Scholar] [CrossRef]
- Mehdawi, I.M.; Pratten, J.; Spratt, D.A.; Knowles, J.C.; Young, A.M. High strength re-mineralizing, antibacterial dental composites with reactive calcium phosphates. Dent. Mater. 2013, 29, 473–484. [Google Scholar] [CrossRef]
- Suiter, E.A.; Watson, L.E.; Tantbirojn, D.; Lou, J.S.; Versluis, A. Effective Expansion: Balance between Shrinkage and Hygroscopic Expansion. J. Dent. Res. 2016, 95, 543–549. [Google Scholar] [CrossRef]
- Sokolowski, K.; Szczesio-Wlodarczyk, A.; Bociong, K.; Krasowski, M.; Fronczek-Wojciechowska, M.; Domarecka, M.; Sokolowski, J.; Lukomska-Szymanska, M. Contraction and Hydroscopic Expansion Stress of Dental Ion-Releasing Polymeric Materials. Polymers 2018, 10, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgórski, M.; Becka, E.; Claudino, M.; Flores, A.; Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Ester-free thiol–ene dental restoratives—Part A: Resin development. Dent. Mater. 2015, 31, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, I. A review of direct orthodontic bonding. Br. J. Orthod. 1975, 2, 171–178. [Google Scholar] [CrossRef]
- Bakhadher, W.; Halawany, H.; Talic, N.; Abraham, N.; Jacob, V. Factors Affecting the Shear Bond Strength of Orthodontic Brackets—A Review of In Vitro Studies. Acta Medica 2015, 58, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Mitwally, R.A.; Bakhsh, Z.T.; Feteih, R.M.; Bakry, A.S.; Abbassy, M.A. Orthodontic Bracket Bonding Using Self-adhesive Cement to Facilitate Bracket Debonding. J. Adhes. Dent. 2019, 21, 551–556. [Google Scholar] [CrossRef]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In-Vitro Study. Nanomaterials 2020, 10, 1466. [Google Scholar] [CrossRef]
- Henkin, F.D.S.; de Oliveira Dias de Macêdo, É.; Santos, K.D.S.; Schwarzbach, M.; Samuel, S.M.W.; Mundstock, K.S. In vitro analysis of shear bond strength and adhesive remnant index of different metal brackets. Dent. Press J. Orthod. 2016, 21, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Tandon, P.; Nagar, A.; Singh, G.P.; Singh, A.; Chugh, V.K. A comparison of shear bond strength of orthodontic brackets bonded with four different orthodontic adhesives. J. Orthod. Sci. 2014, 3, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinagre, A.R.; Messias, A.L.; Gomes, M.A.; Costa, A.L.; Ramos, J.C. Effect of time on shear bond strength of four orthodontic adhesive systems. J. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2014, 55, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; De Stefano, E.D.; Prati, C. Biomimetic remineralization of human dentin using promising innovative calcium-silicate hybrid “smart” materials. Dent. Mater. 2011, 27, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Syed, M.R. A review of bioceramics-based dental restorative materials. Dent. Mater. J. 2019, 38, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Dai, Q.; Weir, M.D.; Melo, M.A.S.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Zhao, Z.; Xu, H.H.K. A nano-CaF2-containing orthodontic cement with antibacterial and remineralization capabilities to combat enamel white spot lesions. J. Dent. 2019, 89, 103172. [Google Scholar] [CrossRef]
- Aoba, T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004, 10, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wanitwisutchai, T.; Monmaturapoj, N.; Srisatjaluk, R.; Subannajui, K.; Dechkunakorn, S.; Anuwongnukroh, N.; Pongprueksa, P. Buffering capacity and antibacterial properties among bioactive glass-containing orthodontic adhesives. Dent. Mater. J. 2021, 2020–2375. [Google Scholar] [CrossRef]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.-A.; Buchalla, W. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef] [Green Version]
- Mehdawi, I.; Neel, E.A.; Valappil, S.P.; Palmer, G.; Salih, V.; Pratten, J.; Spratt, D.A.; Young, A.M. Development of remineralizing, antibacterial dental materials. Acta Biomater. 2009, 5, 2525–2539. [Google Scholar] [CrossRef]
- Sukontapatipark, W.; el-Agroudi, M.A.; Selliseth, N.J.; Thunold, K.; Selvig, K.A. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur. J. Orthod. 2001, 23, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Condò, R.; Mampieri, G.; Pasquantonio, G.; Giancotti, A.; Pirelli, P.; Cataldi, M.E.; La Rocca, S.; Leggeri, A.; Notargiacomo, A.; Maiolo, L. In vitro evaluation of structural factors favouring bacterial adhesion on orthodontic adhesive resins. J. Mater. Sci. 2021, 14, 2485. [Google Scholar] [CrossRef]
- Hadj-Hamou, R.; Senok, A.C.; Athanasiou, A.E.; Kaklamanos, E.G. Do probiotics promote oral health during orthodontic treatment with fixed appliances? A systematic review. BMC Oral Health 2020, 20, 126. [Google Scholar] [CrossRef]
- Nardi, G.M.; Fais, S.; Casu, C.; Mazur, M.; Di Giorgio, R.; Grassi, R.; Grassi, F.R.; Orru, G. Mouthwash Based on Ozonated Olive Oil in Caries Prevention: A Preliminary In-Vitro Study. Int J. Environ. Res. Public Health 2020, 17, 9106. [Google Scholar] [CrossRef]
- de Almeida, C.M.; da Rosa, W.L.O.; Meereis, C.T.W.; de Almeida, S.M.; Ribeiro, J.S.; da Silva, A.F.; Lund, R.G. Efficacy of antimicrobial agents incorporated in orthodontic bonding systems: A systematic review and meta-analysis. J. Orthod. 2018, 45, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Ateia, I.; Paulus, J.R.; Liu, H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 2015, 6, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Formulations | Boroaluminosilicate Glass (7 μm) | Boroaluminosilicate Glass (0.7 μm) | MCPM | Nisin |
|---|---|---|---|---|
| M10N10 | 40 | 40 | 10 | 10 |
| M10N5 | 42.5 | 42.5 | 10 | 5 |
| M5N10 | 42.5 | 42.5 | 5 | 10 |
| M5N5 | 45 | 45 | 5 | 5 |
| M0N0 | 50 | 50 | 0 | 0 |
| Composition | Amount (wt %) |
|---|---|
| Silane-treated quartz | 70–80 |
| Bisphenol A diglycidyl ether dimethacrylate (Bis-GMA) | 10–20 |
| Bisphenol A bis (2-hydroxyethyl ether) dimethacrylate | 5–10 |
| Silane treated silica | <2 |
| Diphenyliodonium hexafluorophosphate | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanachai, S.; Chaichana, W.; Insee, K.; Benjakul, S.; Aupaphong, V.; Panpisut, P. Physical/Mechanical and Antibacterial Properties of Orthodontic Adhesives Containing Calcium Phosphate and Nisin. J. Funct. Biomater. 2021, 12, 73. https://doi.org/10.3390/jfb12040073
Chanachai S, Chaichana W, Insee K, Benjakul S, Aupaphong V, Panpisut P. Physical/Mechanical and Antibacterial Properties of Orthodontic Adhesives Containing Calcium Phosphate and Nisin. Journal of Functional Biomaterials. 2021; 12(4):73. https://doi.org/10.3390/jfb12040073
Chicago/Turabian StyleChanachai, Supachai, Wirinrat Chaichana, Kanlaya Insee, Sutiwa Benjakul, Visakha Aupaphong, and Piyaphong Panpisut. 2021. "Physical/Mechanical and Antibacterial Properties of Orthodontic Adhesives Containing Calcium Phosphate and Nisin" Journal of Functional Biomaterials 12, no. 4: 73. https://doi.org/10.3390/jfb12040073
APA StyleChanachai, S., Chaichana, W., Insee, K., Benjakul, S., Aupaphong, V., & Panpisut, P. (2021). Physical/Mechanical and Antibacterial Properties of Orthodontic Adhesives Containing Calcium Phosphate and Nisin. Journal of Functional Biomaterials, 12(4), 73. https://doi.org/10.3390/jfb12040073