Dental Poly(methyl methacrylate)-Based Resin Containing a Nanoporous Silica Filler
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porous-Silica Filler
2.3. Characterization of Nanoporous Silica Filler
2.4. Preparation of the Filler-Loaded PMMA-Based Resin
2.5. Characterization of the PMMA-Based Resins
2.6. Statistical Analysis
3. Results
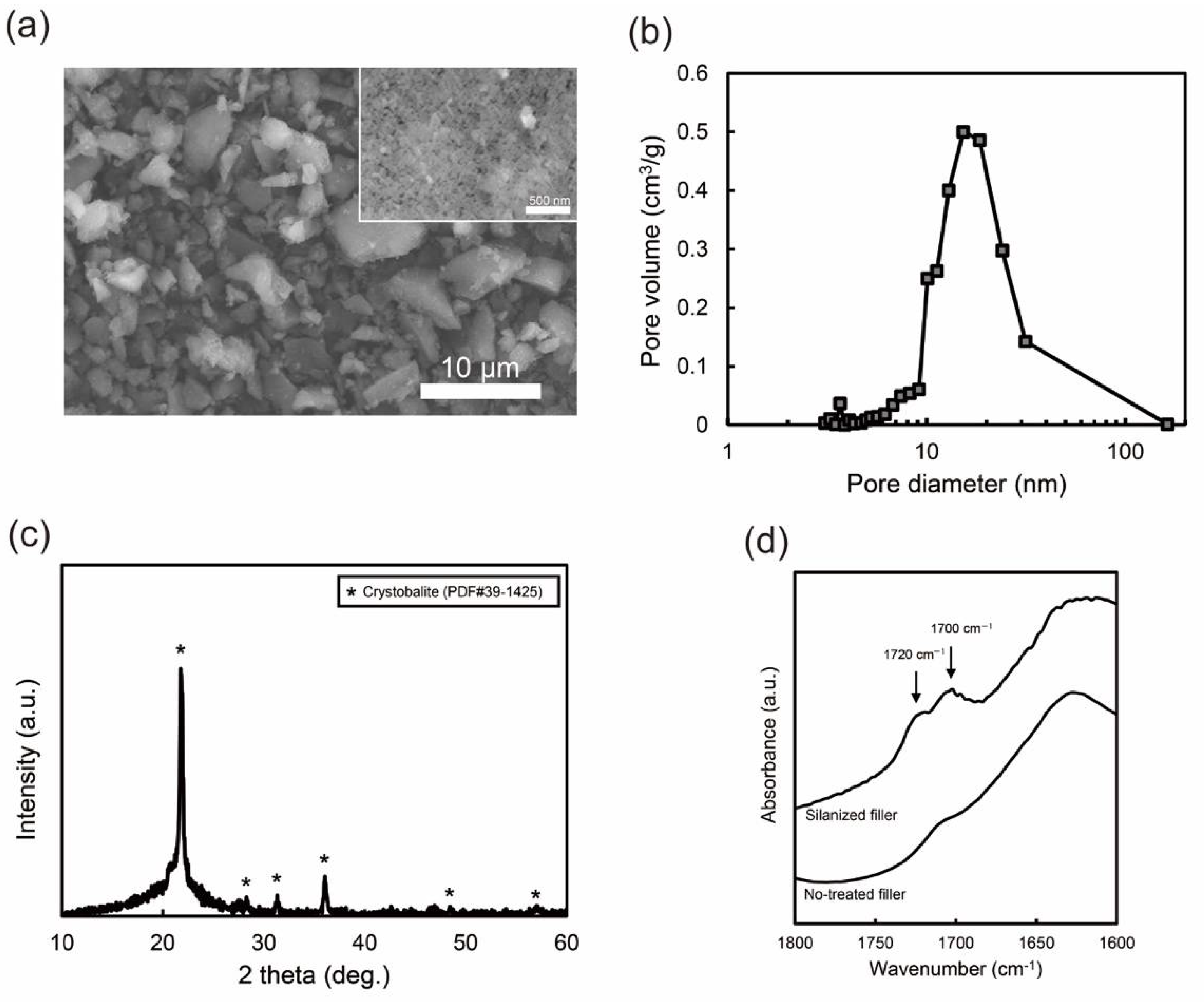
3.1. Nano-Porous Silica Filler
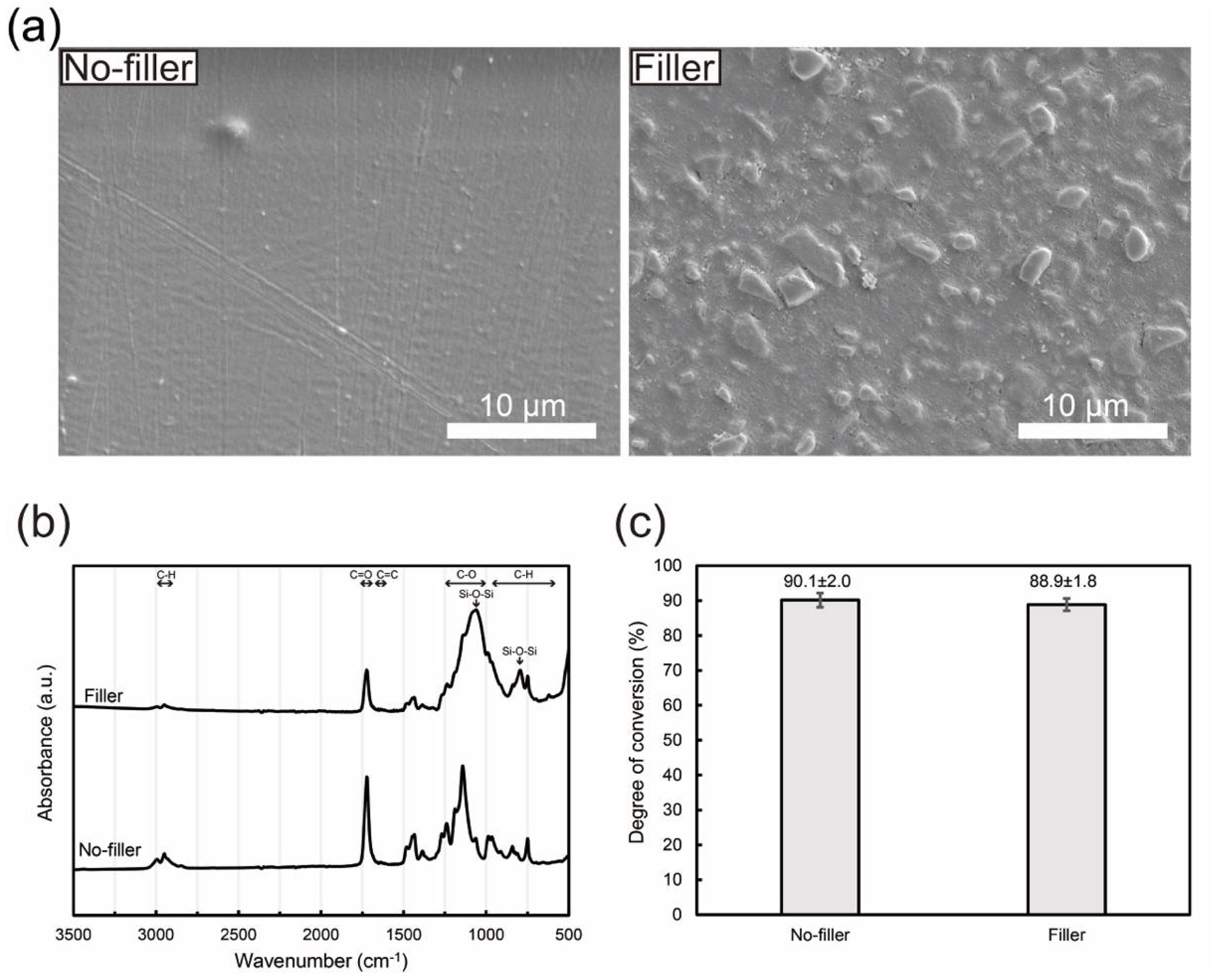
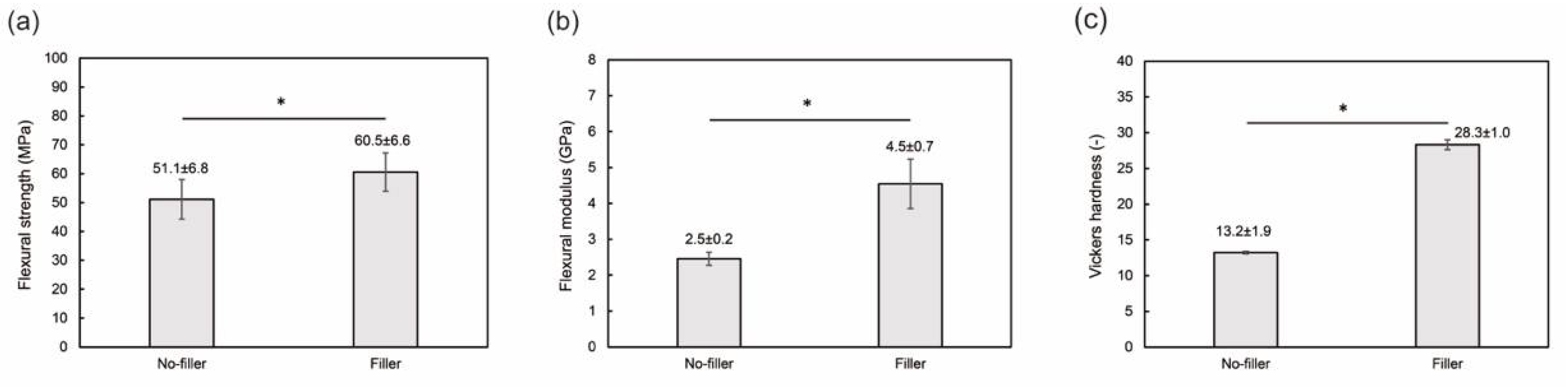
3.2. Filler-Loaded PMMA-Based Resin
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.I.; Ionescu, E. A review on the biocompatibility of PMMA-based dental materials for interim prosthetic restorations with a glimpse into their modern manufacturing techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Assessing fracture toughness and impact strength of PMMA reinforced with nano-particles and fibre as advanced denture base materials. Materials 2021, 14, 4127. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Pellegrino, P.; Albanese, G.; De Giorgi, M.L.; Paladini, F.; Corsalini, M.; Rinaldi, R. Improvement of PMMA dental matrix performance by addition of titanium dioxide nanoparticles and clay nanotubes. Nanomaterials 2021, 11, 2027. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Napankangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldegheishem, A.; AlDeeb, M.; Al-Ahdal, K.; Helmi, M.; Alsagob, E.I. Influence of reinforcing agents on the mechanical properties of denture base resin: A systematic review. Polymers 2021, 13, 3083. [Google Scholar] [CrossRef] [PubMed]
- Rahaman Ali, A.A.A.; John, J.; Mani, S.A.; El-Seedi, H.R. Effect of thermal cycling on flexural properties of microcrystalline cellulose-reinforced denture base acrylic resins. J. Prosthodont. 2020, 29, 611–616. [Google Scholar] [CrossRef]
- Shakeri, F.; Nodehi, A.; Atai, M. PMMA/double-modified organoclay nanocomposites as fillers for denture base materials with improved mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 90, 11–19. [Google Scholar] [CrossRef]
- Chaijareenont, P.; Takahashi, H.; Nishiyama, N.; Arksornnukit, M. Effect of different amounts of 3-methacryloxypropyltrimethoxysilane on the flexural properties and wear resistance of alumina reinforced PMMA. Dent. Mater. J. 2012, 31, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Wang, X.; Tang, Q.; Guo, M.; Zhao, J. Reinforcement of denture base PMMA with ZrO2 nanotubes. J. Mech. Behav. Biomed. Mater. 2014, 32, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Jiangkongkho, P.; Arksornnukit, M.; Takahashi, H. The synthesis, modification, and application of nanosilica in polymethyl methacrylate denture base. Dent. Mater. J. 2018, 37, 582–591. [Google Scholar] [CrossRef] [Green Version]
- Karci, M.; Demir, N.; Yazman, S. Evaluation of flexural strength of different denture base materials reinforced with different nanoparticles. J. Prosthodont. 2019, 28, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Cevik, P.; Yildirim-Bicer, A.Z. The effect of silica and prepolymer nanoparticles on the mechanical properties of denture base acrylic resin. J. Prosthodont. 2018, 27, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Al-Bakri, I.A.; Swain, M.V.; Naoum, S.J.; Al-Omari, W.M.; Martin, E.; Ellakwa, A. Fluoride release, recharge and flexural properties of polymethylmethacrylate containing fluoridated glass fillers. Aust. Dent. J. 2014, 59, 208–214. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Sun, H.; Liu, Y.; Liu, W.; Su, B.; Li, S. The development of filler morphology in dental resin composites: A review. Materials 2021, 14, 5612. [Google Scholar] [CrossRef]
- Zandinejad, A.A.; Atai, M.; Pahlevan, A. The effect of ceramic and porous fillers on the mechanical properties of experimental dental composites. Dent. Mater. 2006, 22, 382–387. [Google Scholar] [CrossRef]
- Samuel, S.P.; Li, S.; Mukherjee, I.; Guo, Y.; Patel, A.C.; Baran, G.; Wei, Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent. Mater. 2009, 25, 296–301. [Google Scholar] [CrossRef]
- Atai, M.; Pahlavan, A.; Moin, N. Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent. Mater. 2012, 28, 133–145. [Google Scholar] [CrossRef]
- Wang, R.; Habib, E.; Zhu, X.X. Synthesis of wrinkled mesoporous silica and its reinforcing effect for dental resin composites. Dent. Mater. 2017, 33, 1139–1148. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Wang, R.; Jiang, X.; Zhu, M. Size-controllable synthesis of dendritic porous silica as reinforcing fillers for dental composites. Dent. Mater. 2021, 37, 961–971. [Google Scholar] [CrossRef] [PubMed]
- ISO 10477: 2018; Dentistry—Polymer-Based Crown and Veneering Materials. International Organization for Standarization: Geneva, Switzerland, 2020.
- Viljanen, E.K.; Skrifvars, M.; Vallittu, P.K. Degree of conversion of a copolymer of an experimental monomer and methyl methacrylate for dental applications. J. Appl. Polym. Sci. 2004, 93, 1908–1912. [Google Scholar] [CrossRef]
- Hata, K.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. Development of dental poly(methyl methacrylate)-based resin for stereolithography additive manufacturing. Polymers 2021, 13, 4435. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Ishida, H. Quantitative monomolecular coverage of inorganic particulates by methacryl-functional silanes. Surf. Sci. 1984, 148, 601–622. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, J.; Chambers, D.E.; Debnath, S.; Wunder, S.L.; Baran, G.R. Filler-coupling agent-matrix interactions in silica/polymethylmethacrylate composites. J. Biomed. Mater. Res. 2001, 57, 384–393. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Karabela, M.M. Effect of the amount of 3-methacyloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites. Dent. Mater. 2009, 25, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Wnuczek, K.; Puszka, A.; Klapiszewski, L.; Podkoscielna, B. Preparation, thermal, and thermo-mechanical characterization of polymeric blends based on di(meth)acrylate monomers. Polymers 2021, 13, 878. [Google Scholar] [CrossRef]
- Uchino, T.; Aboshi, A.; Kohara, S.; Ohishi, Y.; Sakashita, M.; Aoki, K. Microscopic structure of nanometer-sized silica particles. Phys. Rev. B 2004, 69, 155409. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Nakajima, M.; Suemoto, T.; Uchino, T. Formation and photoluminescence characterization of transparent silica glass prepared by solid-phase reaction of nanometer-sized silica particles. J. Phys. Chem. C 2007, 111, 12973–12979. [Google Scholar] [CrossRef]
- Ikeda, H.; Nagamatsu, Y.; Shimizu, H. Preparation of silica-poly(methyl methacrylate) composite with a nanoscale dual-network structure and hardness comparable to human enamel. Dent. Mater. 2019, 35, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic fillers for dental resin composites: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Hickel, R.; van Landuyt, K.L.; Reichl, F.X. Nanoparticles in dentistry. Dent. Mater. 2017, 33, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Saen-Isara, T.; Dechkunakorn, S.; Anuwongnukroh, N.; Srikhirin, T.; Tanodekaew, S.; Wichai, W. Influence of the cross-linking agent on mechanical properties of PMMA powder with compromised particle morphology. Int. Orthod. 2017, 15, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Nateghi, M.; Negahdari, R.; Molaei, S.; Barzegar, A.; Bohlouli, S. Comparison of the accuracy of fixture-level implant impression making with different splinting techniques. Int. J. Dent. 2021, 2021, 2959055. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-soluble photoinitiators in biomedical applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Leao, R.S.; de Moraes, S.L.D.; Aquino, K.; Isolan, C.P.; Casado, B.; Montes, M. Effect of pressure, post-pressing time, and polymerization cycle on the degree of conversion of thermoactivated acrylic resin. Int. J. Dent. 2018, 2018, 5743840. [Google Scholar] [CrossRef] [PubMed]
- Zorzin, J.; Maier, E.; Harre, S.; Fey, T.; Belli, R.; Lohbauer, U.; Petschelt, A.; Taschner, M. Bulk-fill resin composites: Polymerization properties and extended light curing. Dent. Mater. 2015, 31, 293–301. [Google Scholar] [CrossRef]
- Alhavaz, A.; Rezaei Dastjerdi, M.; Ghasemi, A.; Ghasemi, A.; Alizadeh Sahraei, A. Effect of untreated zirconium oxide nanofiller on the flexural strength and surface hardness of autopolymerized interim fixed restoration resins. J. Esthet. Restor. Dent. 2017, 29, 264–269. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural strength and hardness of filler-reinforced PMMA targeted for denture base application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Zhu, B.; Lin, K.; Chang, J. Mechanical and thermal properties of denture PMMA reinforced with silanized aluminum borate whiskers. Dent. Mater. J. 2012, 31, 903–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miettinen, V.M.; Vallittu, P.K. Release of residual methyl methacrylate into water from glass fibre-poly(methyl methacrylate) composite used in dentures. Biomaterials 1997, 18, 181–185. [Google Scholar] [CrossRef]
- Kondo, Y.; Takagaki, T.; Okuda, M.; Ikeda, M.; Kadoma, Y.; Yamauchi, J.; Okada, K.; Sadr, A.; Nikaido, T.; Tagami, J. Effect of PMMA filler particles addition on the physical properties of resin composite. Dent. Mater. J. 2010, 29, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Jurado, C.A.; Silikas, N. Behaviour of PMMA resin composites incorporated with nanoparticles or fibre following prolonged water storage. Nanomaterials 2021, 11, 3453. [Google Scholar] [CrossRef] [PubMed]
- Alamoush, R.A.; Kushnerev, E.; Yates, J.M.; Satterthwaite, J.D.; Silikas, N. Response of two gingival cell lines to CAD/CAM composite blocks. Dent. Mater. 2020, 36, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, J.S.; Lee, J.H.; Park, K.J.; Lee, J.J.; Park, H.S. A feasibility study for 3D-printed poly(methyl methacrylate)-resin tracheostomy tube using a hamster cheek pouch model. In Vivo 2020, 34, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Petzel, C.; Bolay, C.; Hiller, K.A.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. Activation of stress-regulated transcription factors by triethylene glycol dimethacrylate monomer. Biomaterials 2011, 32, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Krifka, S.; Hiller, K.A.; Bolay, C.; Petzel, C.; Spagnuolo, G.; Reichl, F.X.; Schmalz, G.; Schweikl, H. Function of MAPK and downstream transcription factors in monomer-induced apoptosis. Biomaterials 2012, 33, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H. Update on dental nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]

| Acronym | Reagent Name | Purpose | Purity (%) | Supplier |
|---|---|---|---|---|
| SiO2 | Fumed silica (Aerosil® 300) | Ceramic | 99.8 | Nippon Aerosil Co., Ltd., Tokyo, Japan |
| PVA | Poly(vinyl alcohol) | Binder | 78–82 * | Fujifilm Wako Pure Chemical Corporation, Osaka, Japan |
| γ-MPTS | 3-Methacryl oxypropyl trimethoxysilane | Silane coupling agent | 99.9 | Shin-Etsu Chemical Co., Ltd., Tokyo, Japan |
| PMMA | Polymethyl methacrylate | Polymer | Mw ≈ 15,000 | Sigma-Aldrich Co. LLC., St. Louis, MS, USA |
| MMA | Methyl methacrylate | Monomer | >98 | Fujifilm Wako Pure Chemical Corporation, Osaka, Japan |
| EGDMA | Ethylene glycol dimethacrylate | Cross-linker | >97 | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
| BAPO | Phenylbis (2,4,6-trimethylbenzoyl) phosphine oxide | Photo-initiator | >96 | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata, K.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. Dental Poly(methyl methacrylate)-Based Resin Containing a Nanoporous Silica Filler. J. Funct. Biomater. 2022, 13, 32. https://doi.org/10.3390/jfb13010032
Hata K, Ikeda H, Nagamatsu Y, Masaki C, Hosokawa R, Shimizu H. Dental Poly(methyl methacrylate)-Based Resin Containing a Nanoporous Silica Filler. Journal of Functional Biomaterials. 2022; 13(1):32. https://doi.org/10.3390/jfb13010032
Chicago/Turabian StyleHata, Kentaro, Hiroshi Ikeda, Yuki Nagamatsu, Chihiro Masaki, Ryuji Hosokawa, and Hiroshi Shimizu. 2022. "Dental Poly(methyl methacrylate)-Based Resin Containing a Nanoporous Silica Filler" Journal of Functional Biomaterials 13, no. 1: 32. https://doi.org/10.3390/jfb13010032
APA StyleHata, K., Ikeda, H., Nagamatsu, Y., Masaki, C., Hosokawa, R., & Shimizu, H. (2022). Dental Poly(methyl methacrylate)-Based Resin Containing a Nanoporous Silica Filler. Journal of Functional Biomaterials, 13(1), 32. https://doi.org/10.3390/jfb13010032






