Do Human iPSC-Derived Cardiomyocytes Cultured on PLA Scaffolds Induce Expression of CD28/CTLA-4 by T Lymphocytes?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Cardiomyocytes
2.2. Production of Polymer Matrices and Seeding
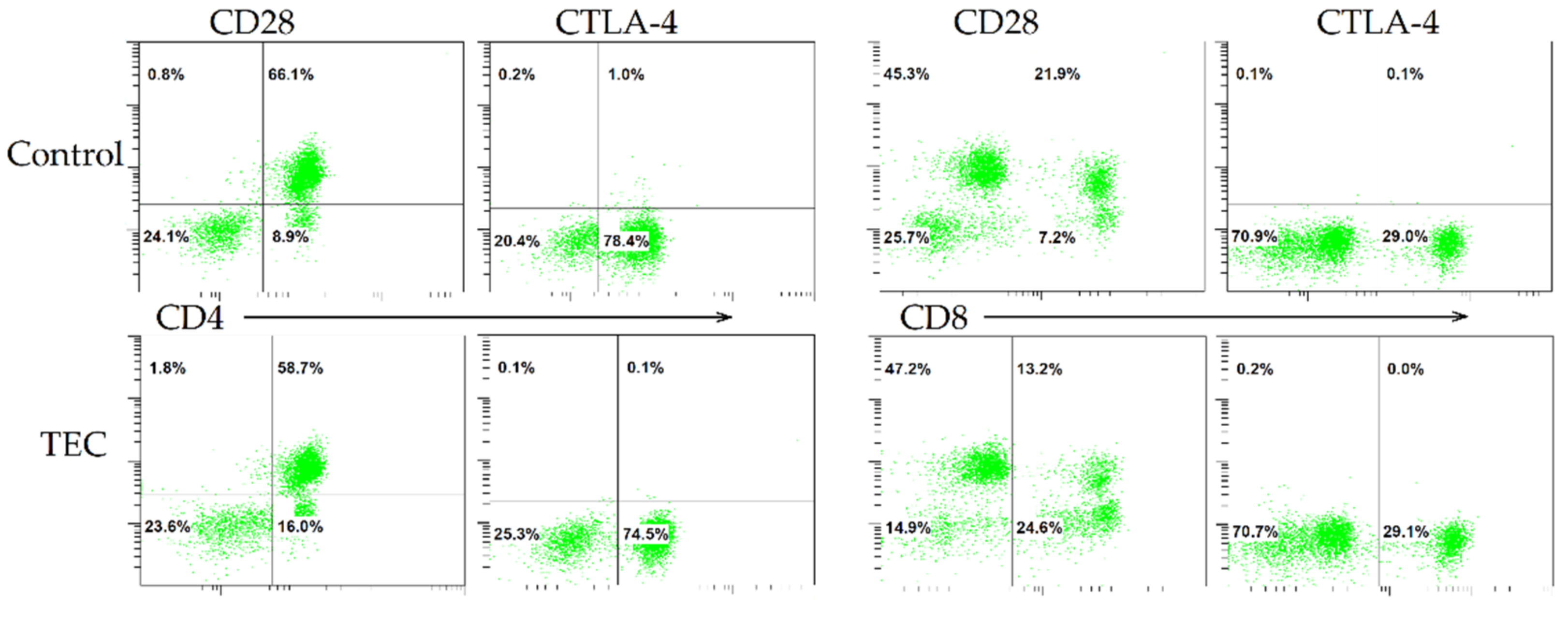
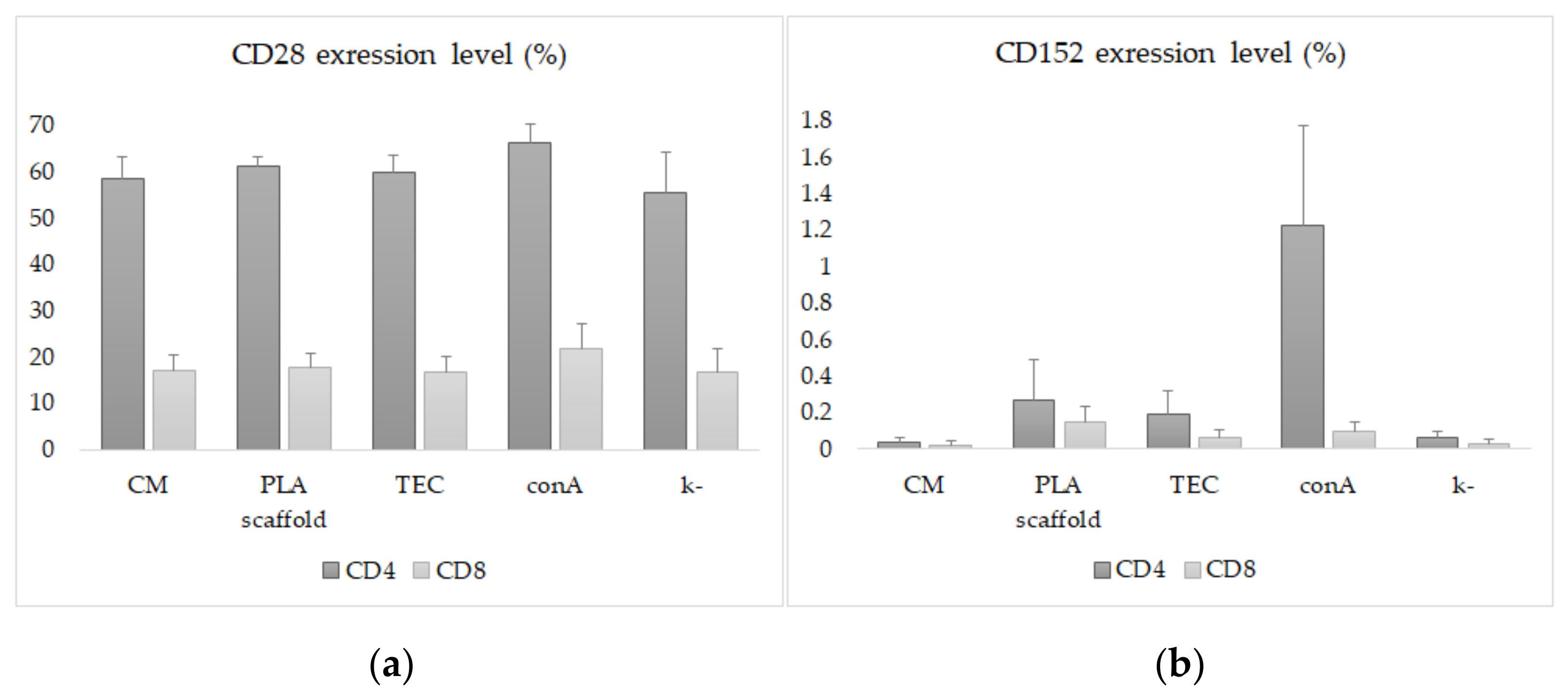
2.3. Immunological Studies and Flow Cytometry
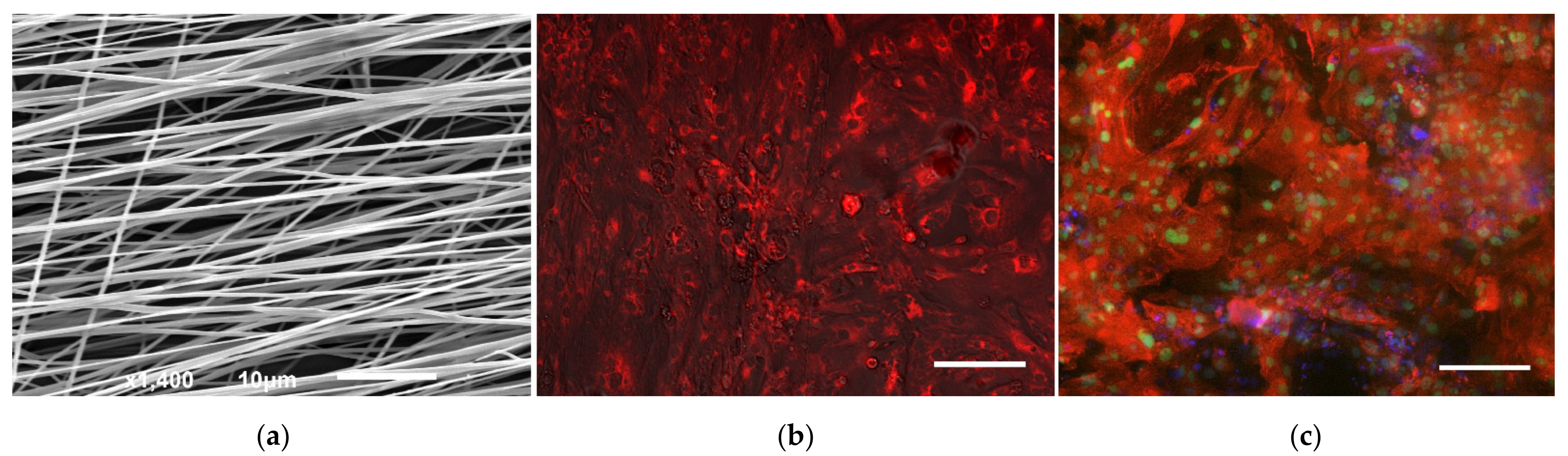
2.4. Electronic and Fluorescence Microscopic Examinations
2.5. Ethical Statement
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Benjamin, L.; Maddox, T. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Barbash, I.; Chouraqui, P.; Baron, J.; Feinberg, M.; Etzion, S. Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef]
- Qasim, M.; Pala, A.; Powell, H.; Khan, M. Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci. 2019, 229, 233–250. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Boheler, K.; Czyz, J.; Tweedie, D.; Yang, H.; Anisimov, S.; Wobus, A. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. 2002, 91, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Zhang, J.; Azarin, S.; Zhu, K.; Hazeltine, L. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Lau, D.; Shlapakova, I.; Zhao, X.; Danilo, P.; Robinson, R.; Cohen, I.; Qu, D.; Xu, Z.; Rosen, M. Implantation of sinoatrial node cells into canine right ventricle: Biological pacing appears limited by the substrate. Cell. Transplant. 2011, 20, 1907–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, A.; Moroncini, F.; Mazzoni, S. Polyglycolic acid-polylactic acid scaffold response to different progenitor cell in vitro cultures: A demonstrative and comparative X-ray synchrotron radiation phase-contrast microtomography study. Tissue Eng. Part C Methods 2014, 20, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Ordoño, J.; Armelin, E.; Pérez-Amodio, S.; Baldissera, A.F. Electrospun conducting and biocompatible uniaxial and Core–Shell fibers having poly(lactic acid), poly(ethylene glycol), and polyaniline for cardiac tissue engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [Green Version]
- Guarino, V.; Cirillo, V.; Altobelli, R.; Ambrosio, L. Polymer-based platforms by electric field-assisted techniques for tissue engineering and cancer therapy. Expert Rev. Med. Devices 2015, 12, 113–129. [Google Scholar] [CrossRef]
- Chepeleva, E.; Balashov, V.; Dokuchaeva, A.; Korobejnikov, A.; Strelnikov, A. Analyis of biological compatibility of polylactide nanofibrous matrix vitalized with cardiac fibroblasts in a porcine model. Genes Cells 2017, 12, 62–68. [Google Scholar] [CrossRef]
- Alsughayyir, J.; Chhabra, M.; Qureshi, M.; Mallik, M.; Ali, J. Relative frequencies of alloantigen-specific helper CD4 T cells and B cells determine mode of antibody-mediated allograft rejection. Front. Immunol. 2019, 22, 3039. [Google Scholar] [CrossRef] [Green Version]
- Sergeevichev, D.; Sergeevicheva, V.; Subbotovskaya, A.; Vasilyeva, M.; Dokuchaeva, A.; Karaskov, A.; Kozlov, V. Decellularization as a way to prevent activation of the immune response to allogeneic pulmonary heart valves. Genes Cells 2013, 8, 55–60. [Google Scholar]
- Zhang, T.; Azimzadeh, A.; Sun, W.; O’Neill, N.; Sievert, E. Selective CD28 Inhibition Modulates Alloimmunity and Cardiac Allograft Vasculopathy in Anti–CD154-Treated Monkeys. Transplantation 2018, 102, e90–e100. [Google Scholar] [CrossRef]
- Grigor’eva, E.; Malankhanova, T.; Surumbayeva, A.; Minina, J.; Kizilova, E.; Lebedev, I.; Zakian, S. Generation and characterization of iPSCs from human embryonic dermal fibroblasts of a healthy donor from Siberian population. BioRxiv 2018, 455535. [Google Scholar] [CrossRef]
- Burridge, P.; Matsa, E.; Shukla, P.; Lin, Z.; Churko, J. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balashov, V.; Efimov, A.; Agapova, O.; Pogorelov, A.; Agapov, I.; Agladze, K. High resolution 3D microscopy study of cardiomyocytes on polymer scaffold nanofibers reveals formation of unusual sheathed structure. Acta Biomater. 2018, 68, 214–222. [Google Scholar] [CrossRef]
- Parrotta, E.; Lucchino, V.; Scaramuzzino, L.; Scalise, S.; Cuda, G. Modeling cardiac disease mechanisms using induced pluripotent stem cell-derived cardiomyocytes: Progress, promises and challenges. Int. J. Mol. Sci. 2020, 21, 4354. [Google Scholar] [CrossRef]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fink, C.; Ergun, S.; Kralisch, D.; Remmers, U.; Weil, J.; Eschenhagen, T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000, 14, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.; Ingber, D. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1267–1279. [Google Scholar] [CrossRef] [Green Version]
- Parrag, I.; Zandstra, P.; Woodhouse, K. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol. Bioeng. 2012, 109, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, J.; Lin, J.; Tsai, W. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011, 7, 3285–3293. [Google Scholar] [CrossRef]
- Batalov, I.; Jallerat, Q.; Kim, S.; Bliley, J.; Feinberg, A. Engineering aligned human cardiac muscle using developmentally inspired fibronectin micropatterns. Sci. Rep. 2021, 11, 11502. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen-Mattila, M.; Häkli, M.; Pölönen, R.-P.; Mansikkala, T.; Junnila, A. Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Materials 2019, 12, 1805. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Kwon, Y.; Lee, T.; Park, G.; Kim, J. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef] [Green Version]
- Medvedev, S.; Shevchenko, A.; Zakian, S. Induced pluripotent stem cells: Problems and advantages when applying them in regenerative medicine. Acta Nat. 2010, 2, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Loupy, A.; Lefaucheu, C. Antibody-mediated rejection of solid-organ allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef]
- Gilani, S.; Vuga, L.; Lindell, K.; Gibson, K.; Xue, J. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS ONE 2010, 5, e8959. [Google Scholar] [CrossRef] [Green Version]
- Dilek, N.; Poirier, N.; Hulin, P.; Coulon, F.; Mary, C. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PLoS ONE 2013, 8, e83139. [Google Scholar] [CrossRef] [Green Version]
- Schildberg, F.; Klein, S.; Freeman, G.; Sharpe, A. Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family. Immunity 2016, 44, 955–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.; Gu, H.; Lin, Q.; Huang, S.; Xing, T. CD8(+)CD28(+)/CD8(+)CD28(−) T cell equilibrium can predict the active stage for patients with inflammatory bowel disease. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 693–702. [Google Scholar] [CrossRef]
- Kucharska, A.; Gorska, E.; Wasik, M.; Demkow, U. Altered expression of T lymphocyte surface markers in children with chronic autoimmune thyroiditis. J. Physiol. Pharmacol. 2008, 59, 375–382. [Google Scholar] [PubMed]
- Halliday, N.; Williams, C.; Kennedy, A.; Waters, E.; Pesenacker, A. CD86 is a selective CD28 ligand supporting FoxP3+ regulatory T cell homeostasis in the presence of high levels of CTLA-4. Front. Immunol. 2020, 11, 600000. [Google Scholar] [CrossRef]
- Ganesan, A.; Moon, T.; Barakat, K. Revealing the atomistic details behind the binding of B7-1 to CD28 and CTLA-4: A comprehensive protein-protein modelling study. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2764–2778. [Google Scholar] [CrossRef] [PubMed]
- Säljö, K.; Barone, A.; Mölne, J.; Rydberg, L.; Teneberg, S.; Breimer, M. HLA and Histo-blood group antigen expression in human pluripotent stem cells and their derivatives. Sci. Rep. 2017, 7, 13072. [Google Scholar] [CrossRef] [Green Version]
- Daar, A.; Fuggle, S.; Fabre, J.; Ting, A.; Morris, P. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation 1984, 38, 293–298. [Google Scholar] [CrossRef]
- Rose, M.; Coles, M.; Griffin, R.; Pomerance, A.; Yacoub, M. Expression of class I and class II major histocompatibility antigens in normal and transplanted human heart. Transplantation 1986, 41, 776–780. [Google Scholar] [CrossRef]
- Delves, P.; Roitt, I. The immune system. First of two parts, N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Miyagawa, S.; Fukushima, S.; Maeda, A.; Kashiyama, N. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Rep. 2016, 6, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Miyagawa, S.; Toyofuku, T.; Satsuki, F.; Kawamura, T. Syngeneic mesenchymal stem cells reduce immune rejection after induced pluripotent stem cell-derived allogeneic cardiomyocyte transplantation. Sci. Rep. 2020, 10, 4593. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Warner, P.; Albers, E.; Kemna, M.; Delaney, M. Donor-specific anti-HLA antibody production following pediatric ABO-incompatible heart transplantation. Pediatr. Transplant. 2019, 23, e13332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Ortuno, S.; Lebrun-Vignes, B.; Johnsond, D.; Moslehid, J. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur. J. Cancer 2021, 148, 36–47. [Google Scholar] [CrossRef]
- Furiasse, N.; Kobashigawa, J.A. Immunosuppression and adult heart transplantation: Emerging therapies and opportunities. Expert Rev. Cardiovasc. Ther. 2017, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Jarrell, D.; Vanderslice, E.; VeDepo, M.; Jacot, J. Engineering Myocardium for Heart Regeneration—Advancements, Considerations, and Future Directions. Front. Cardiovasc. Med. 2020, 15, 586261. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeevichev, D.; Balashov, V.; Kozyreva, V.; Pavlova, S.; Vasiliyeva, M.; Romanov, A.; Chepeleva, E. Do Human iPSC-Derived Cardiomyocytes Cultured on PLA Scaffolds Induce Expression of CD28/CTLA-4 by T Lymphocytes? J. Funct. Biomater. 2022, 13, 6. https://doi.org/10.3390/jfb13010006
Sergeevichev D, Balashov V, Kozyreva V, Pavlova S, Vasiliyeva M, Romanov A, Chepeleva E. Do Human iPSC-Derived Cardiomyocytes Cultured on PLA Scaffolds Induce Expression of CD28/CTLA-4 by T Lymphocytes? Journal of Functional Biomaterials. 2022; 13(1):6. https://doi.org/10.3390/jfb13010006
Chicago/Turabian StyleSergeevichev, David, Victor Balashov, Victoria Kozyreva, Sophia Pavlova, Maria Vasiliyeva, Alexander Romanov, and Elena Chepeleva. 2022. "Do Human iPSC-Derived Cardiomyocytes Cultured on PLA Scaffolds Induce Expression of CD28/CTLA-4 by T Lymphocytes?" Journal of Functional Biomaterials 13, no. 1: 6. https://doi.org/10.3390/jfb13010006
APA StyleSergeevichev, D., Balashov, V., Kozyreva, V., Pavlova, S., Vasiliyeva, M., Romanov, A., & Chepeleva, E. (2022). Do Human iPSC-Derived Cardiomyocytes Cultured on PLA Scaffolds Induce Expression of CD28/CTLA-4 by T Lymphocytes? Journal of Functional Biomaterials, 13(1), 6. https://doi.org/10.3390/jfb13010006







