Hemolytic Performance in Two Generations of the Sputnik Left Ventricular Assist Device: A Combined Numerical and Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design Features
2.2. CFD Simulations
2.2.1. Meshing
2.2.2. Hemolysis Estimation
2.2.3. Blood Damage Evaluation
2.2.4. Boundary and Initial Conditions
2.3. Experimental Set-Up
2.3.1. Donor Blood Preparation and the SCL Filling
2.3.2. Blood Sampling and Hemolysis Assessment
3. Results
3.1. CFD Predictions
3.2. Experimental Hemolysis Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khush, K.K.; Potena, L.; Cherikh, W.S.; Chamber, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Sadavarte, A.; Singh, T.P.; Zuckermann, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th Adult Heart Transplantation Report—2020; Focus on Deceased Donor Characteristics. J. Heart Lung Transplant. 2020, 39, 1003–1015. [Google Scholar] [CrossRef]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K., III; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Netuka, I.; Sood, P.; Pya, Y.; Zimpfer, D.; Krabatsch, T.; Garbade, J.; Rao, V.; Morshuis, M.; Marasco, S.; Beyersdorf, F.; et al. Fully magnetically levitated left ventricular assist system for treating advanced HF: A multicenter study. J. Am. Coll. Cardiol. 2015, 66, 2579–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, A.J.; MacArthur, R.G.G.; Kim, D.; Singh, G.; Buchholz, H.; Chatterley, P.; Klarenbach, S.W. A systematic review of the cost-effectiveness of long-term mechanical circulatory support. Value Health 2016, 19, 494–504. [Google Scholar] [CrossRef] [Green Version]
- Schmier, J.K.; Patel, J.D.; Leonhard, M.J.; Midha, P.A. A systematic review of cost-effectiveness analyses of left ventricular assist devices: Issues and challenges. Appl. Health Econ. Health Policy 2019, 17, 35–46. [Google Scholar] [CrossRef]
- Bludszuweit, C. Model for a general mechanical blood damage prediction. Artif. Organs 1995, 19, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Sekine, K.; Mitoh, A.; Mitamura, Y.; Okamoto, E.; Kim, D.W.; Nishimura, I.; Murabayashi, S.; Yozu, R. An estimation method of hemolysis within an axial flow blood pump by computational fluid dynamics analysis. Artif. Organs 2003, 27, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Throckmorton, A.L.; Wood, H.G.; Antaki, J.F.; Olsen, D.B. Computational fluid dynamics prediction of blood damage in a centrifugal pump. Artif. Organs 2003, 27, 938–941. [Google Scholar] [CrossRef]
- Hentschel, B.; Tedjo, I.; Probst, M.; Wolter, M.; Behr, M.; Bischof, C.; Kuhlen, T. Interactive blood damage analysis for ventricular assist devices. IEEE Trans. Vis. Comput. Graph. 2008, 14, 1515–1522. [Google Scholar] [CrossRef]
- Su, B.; Chua, L.P.; Lim, T.M.; Zhou, T. Evaluation of the impeller shroud performance of an axial flow ventricular assist device using computational fluid dynamics. Artif. Organs 2010, 34, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.H.; Taskin, M.E.; Griffith, B.P.; Wu, Z.J. The use of computational fluid dynamics in the development of ventricular assist devices. Med. Eng. Phys. 2011, 33, 263–280. [Google Scholar] [CrossRef] [Green Version]
- Fraser, K.H.; Zhang, T.; Taskin, M.E.; Griffith, B.P.; Wu, Z.J. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: Shear stress, exposure time and hemolysis index. J. Biomech. Eng. 2012, 134, 081002. [Google Scholar] [CrossRef]
- Chiu, W.C.; Slepian, M.J.; Bluestein, D. Thrombus formation patterns in the HeartMate II ventricular assist device: Clinical observations can be predicted by numerical simulations. ASAIO J. 2014, 60, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thamsen, B.; Blümel, B.; Schaller, J.; Paschereit, C.O.; Affeld, K.; Goubergrits, L.; Kertzscher, U. Numerical analysis of blood damage potential of the HeartMate II and HeartWare HVAD rotary blood pumps. Artif. Organs 2015, 39, 651–659. [Google Scholar] [CrossRef]
- Thamsen, B.; Mevert, R.; Lommel, M.; Preikschat, P.; Gaebler, J.; Krabatsch, T.; Kertzscher, U.; Hennig, E.; Affeld, K. A two-stage rotary blood pump design with potentially lower blood trauma: A computational study. IJAO 2016, 39, 178–183. [Google Scholar] [CrossRef]
- Graefe, R.; Pauli, L. Computational fluid dynamics for mechanical circulatory support device development. In Mechanical Support for Heart Failure; Karimov, J.H., Fukamachi, K., Starling, R.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 399–427. [Google Scholar] [CrossRef]
- Garon, A.; Farinas, M.I. Fast three-dimensional numerical hemolysis approximation. Artif. Organs 2004, 28, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.D.; Stedman, M.R.; Myers, S.L.; Naftel, D.C.; Silver, S.A.; Banerjee, D.; Chang, T.I. Long-term changes in kidney function after left ventricular assist device implant: An analysis of the STS Intermacs database. J. Heart Lung Transplant. 2019, 38, S89–S90. [Google Scholar] [CrossRef]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 121, 1276–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohrabi, S.; Liu, Y. A cellular model of shear-induced hemolysis. Artif. Organs 2017, 41, E80–E91. [Google Scholar] [CrossRef] [PubMed]
- Goubergrits, L.; Osman, J.; Mevert, R.; Kertzscher, U.; Pöthkow, K.; Hege, H.C. Turbulence in blood damage modeling. IJAO 2016, 39, 160–165. [Google Scholar] [CrossRef]
- Arora, D.; Behr, M.; Pasquali, M. A tensor-based measure for estimating blood damage. Artif. Organs 2004, 28, 1002–1015. [Google Scholar] [CrossRef]
- Arora, D.; Behr, M.; Pasquali, M. Hemolysis estimation in a centrifugal blood pump using a tensor-based measure. Artif. Organs 2006, 30, 539–547. [Google Scholar] [CrossRef]
- Gesenhues, L.; Pauli, L.; Behr, M. Strain-based blood damage estimation for computational design of ventricular assist devices. IJAO 2016, 39, 166–170. [Google Scholar] [CrossRef]
- Treichler, J.; Rosenow, S.E.; Damm, G.; Naito, K.; Ohara, Y.; Mizuguchi, K.; Makinouchi, K.; Takatani, S.; Nosé, Y. A fluid dynamic analysis of a rotary blood pump for design improvement. Artif. Organs 1993, 17, 797–808. [Google Scholar] [CrossRef]
- Yeleswarapu, K.K.; Antaki, J.F.; Kameneva, M.V.; Rajagopal, K.R. A mathematical model for shear-induced hemolysis. Artif. Organs 1995, 19, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, X. Hydrodynamic and hemolysis analysis on distance and clearance between impeller and diffuser of axial blood pump. J. Mech. Med. Biol. 2016, 16, 1650014. [Google Scholar] [CrossRef]
- Wiegmann, L.; Boës, S.; De Zélicourt, D.; Thamsen, B.; Daners, S.M.; Meboldt, M.; Kurtcuoglu, V. Blood pump design variations and their influence on hydraulic performance and indicators of hemocompatibility. Ann. Biomed. Eng. 2017, 46, 417–428. [Google Scholar] [CrossRef]
- Liu, G.M.; Jin, D.H.; Zhou, J.Y.; Zhang, Y.; Chen, H.; Sun, H.S.; Hu, S.; Gui, X.M. Numerical investigation of the influence of blade radial gap flow on axial blood pump performance. ASAIO J. 2019, 65, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Untaroiu, A.; Throckmorton, A.L.; Patel, S.M.; Wood, H.G.; Allaire, P.E.; Olsen, D.B. Numerical and experimental analysis of an axial flow left ventricular assist device: The influence of the diffuser on overall pump performance. Artif. Organs 2005, 29, 581–591. [Google Scholar] [CrossRef]
- Su, B.; Chua, L.P.; Zhong, L. Numerical studies of an axial flow blood pump with different diffuser designs. J. Mech. Med. Biol. 2013, 13, 1350029. [Google Scholar] [CrossRef]
- Telyshev, D.; Denisov, M.; Selishchev, S. The effect of rotor geometry on the H−Q curves of the sputnik implantable pediatric rotary blood pump. Biomed. Eng. 2017, 50, 420–424. [Google Scholar] [CrossRef]
- Chan, W.K.; Wong, Y.W.; Ong, W.; Koh, S.Y.; Chong, V. Numerical investigation of the effects of the clearance gap between the inducer and impeller of an axial blood pump. Artif. Organs 2005, 29, 250–258. [Google Scholar] [CrossRef]
- Korakianitis, T.; Rezaienia, M.A.; Paul, G.M.; Avital, E.J.; Rothman, M.T.; Mozafari, S. Optimization of axial pump characteristic dimensions and induced hemolysis for mechanical circulatory support devices. ASAIO J. 2018, 64, 727–734. [Google Scholar] [CrossRef]
- Korakianitis, T.; Rezaienia, M.A.; Paul, G.M.; Rahideh, A.; Rothman, M.T.; Mozafari, S. Optimization of centrifugal pump characteristic dimensions for mechanical circulatory support devices. ASAIO J. 2016, 62, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, Z.; Zhu, L.; Zhang, X. Hemolysis analysis of axial blood pump with various structure impellers. J. Mech. Med. Biol. 2013, 13, 1350054. [Google Scholar] [CrossRef]
- Ozturk, C.; Aka, I.B.; Lazoglu, I. Effect of blade curvature on the hemolytic and hydraulic characteristics of a centrifugal blood pump. IJAO 2018, 41, 730–737. [Google Scholar] [CrossRef]
- Kim, N.J.; Diao, C.; Ahn, K.H.; Lee, S.J.; Kameneva, M.V.; Antaki, J.F. Parametric study of blade tip clearance, flow rate, and impeller speed on blood damage in rotary blood pump. Artif. Organs 2009, 33, 468–474. [Google Scholar] [CrossRef]
- Kannojiya, V.; Das, A.K.; Das, P.K. Proposal of hemodynamically improved design of an axial flow blood pump for LVAD. Med. Biol. Eng. Comput. 2020, 58, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Selishchev, S.V.; Telyshev, D.V. Optimisation of the Sputnik-VAD design. IJAO 2016, 39, 407–414. [Google Scholar] [CrossRef]
- Gautier, S.V.; Shevchenko, A.O.; Itkin, G.P.; Zakharevich, V.M.; Poptsov, V.N.; Drobyshev, A.A.; Telyshev, D.V. Artificial heart in Russia: Past, present and future. Artif. Organs 2021, 45, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Pugovkin, A.A.; Markov, A.G.; Selishchev, S.V.; Korn, L.; Walter, M.; Leonhardt, S.; Bockeria, L.A.; Bockeria, O.L.; Telyshev, D.V. Advances in hemodynamic analysis in cardiovascular diseases Investigation of energetic characteristics of adult and pediatric Sputnik left ventricular assist devices during mock circulation support. Cardiol. Res. Pract. 2019, 2019, 4593174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giersiepen, M.; Wurzinger, L.J.; Opitz, R.; Reul, H. Estimation of shear stress-related blood trauma in heart valve prostheses—In vitro comparison of 25 aortic valves. IJAO 1990, 13, 300–306. [Google Scholar] [CrossRef]
- Heuser, G.; Opitz, R. A Couette viscometer for short time shearing of blood. Biorheology 1980, 17, 17–24. [Google Scholar] [CrossRef]
- Taskin, M.E.; Fraser, K.H.; Zhang, T.; Wu, C.; Griffith, B.P.; Wu, Z.J. Evaluation of Eulerian and Lagrangian models for hemolysis estimation. ASAIO J. 2012, 58, 363–372. [Google Scholar] [CrossRef]
- Mitoh, A.; Yano, T.; Sekine, K.; Mitamura, Y.; Okamoto, E.; Kim, D.W.; Yozu, R.; Kawada, S. Computational fluid dynamics analysis of an intra-cardiac axial flow pump. Artif. Organs 2003, 27, 34–40. [Google Scholar] [CrossRef]
- Bludszuweit, C. Three-dimensional numerical prediction of stress loading of blood particles in a centrifugal pump. Artif. Organs 1995, 19, 590–596. [Google Scholar] [CrossRef] [PubMed]
- ASTM International F-1841-97(2013). Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps. In Annual Book of ASTM Standards, vol. 13.01. Medical and Surgical Materials and Devices (I): E667–F2477; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM International F-1830-97(2013). Standard Practice for Selection of Blood for in vitro Evaluation of Blood Pumps. In Annual Book of ASTM Standards, vol. 13.01. Medical and Surgical Materials and Devices (I): E667–F2477; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Malinauskas, R.A. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif. Organs 1997, 21, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Nosé, Y. Design and development strategy for the rotary blood pump. Artif. Organs 1998, 22, 438–446. [Google Scholar] [CrossRef]
- Berk, Z.B.K.; Zhang, J.; Chen, Z.; Tran, D.; Griffith, B.P.; Wu, Z.J. Evaluation of in vitro hemolysis and platelet activation of a newly developed maglev LVAD and two clinically used LVADs with human blood. Artif. Organs 2019, 43, 870–879. [Google Scholar] [CrossRef]
- Bourque, K.; Cotter, C.; Dague, C.; Harjes, D.; Dur, O.; Duhamel, J.; Spink, K.; Walsh, K.; Burke, E. Design rationale and preclinical evaluation of the HeartMate 3 left ventricular assist system for hemocompatibility. ASAIO J. 2016, 62, 375–383. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of blood coagulation by hemocompatible material surfaces—A review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef]
- Bird, R.B.; Armstrong, R.C.; Hassager, O. Dynamics of Polymeric Liquids, Volume 1: Fluid Mechanics; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Kim, S. A Study of Non-Newtonian Viscosity and Yield Stress of Blood in a Scanning Capillary-Tube Rheometer. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2002. [Google Scholar]
- Kim, S.; Namgung, B.; Ong, P.K.; Cho, Y.I.; Chun, K.J.; Lim, D. Determination of rheological properties of whole blood with a scanning capillary-tube rheometer using constitutive models. J. Mech. Sci. Technol. 2009, 23, 1718–1726. [Google Scholar] [CrossRef]
- Papathanasopoulou, P.; Zhao, S.; Köhler, U.; Robertson, M.B.; Long, Q.; Hoskins, P.; Xu, X.Y.; Marshall, I. MRI measurement of time-resolved wall shear stress vectors in a carotid bifurcation model, and comparison with CFD predictions. J. Magn. Reson. Imaging 2003, 17, 153–162. [Google Scholar] [CrossRef]
- Long, Q.; Xu, X.Y.; Ariff, B.; Thom, S.A.; Hughes, A.D.; Stanton, A.V. Reconstruction of blood flow patterns in a humancarotid bifurcation: A combined CFD and MRI study. J. Magn. Reson. Imaging 2000, 11, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Song, S.M.; Napel, S.; Glover, G.H.; Pelc, N.J. Noise reduction in three-dimensional phase contrast MR velocity measurements. J. Magn. Reson. Imaging 1993, 3, 587–596. [Google Scholar] [CrossRef]
- Liu, L.; Funamoto, K.; Hayase, T. Numerical experiment for ultrasonic-measurement-integrated simulation of developed laminar pipe flow using axisymmetric model. J. Biomech. Sci. Eng. 2008, 3, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Steinman, D.A.; Thomas, J.B.; Ladak, H.M.; Milner, J.S.; Rutt, B.K.; Spence, J.D. Reconstruction of carotid bifurcation hemodynamics and wall thickness using computational fluid dynamics and MRI. Magn. Reson. Med. 2002, 47, 149–159. [Google Scholar] [CrossRef]
- Carvalho, J.L.A.; Nielsen, J.F.; Nayak, K.S. Feasibility of in vivo measurement of carotid wall shear rate using spiral Fourier velocity encoded MRI. Magn. Reson. Med. 2010, 63, 1537–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Zhang, M.; Yang, W.; Zhu, X.; Hu, Q. Effects of non-Newtonian fluid on centrifugal blood pump performance. Int. Commun. Heat Mass Transfer 2008, 35, 613–617. [Google Scholar] [CrossRef]
- Shibeshi, S.S.; Collins, W.E. The rheology of blood flow in a branched arterial system. Appl. Rheo. 2005, 15, 398–405. [Google Scholar] [CrossRef]
- Anand, M.; Rajagopal, K.R. A mathematical model to describe the change in the constitutive character of blood due to platelet activation. Comptes Rendus Mecanique 2002, 330, 557–562. [Google Scholar] [CrossRef]
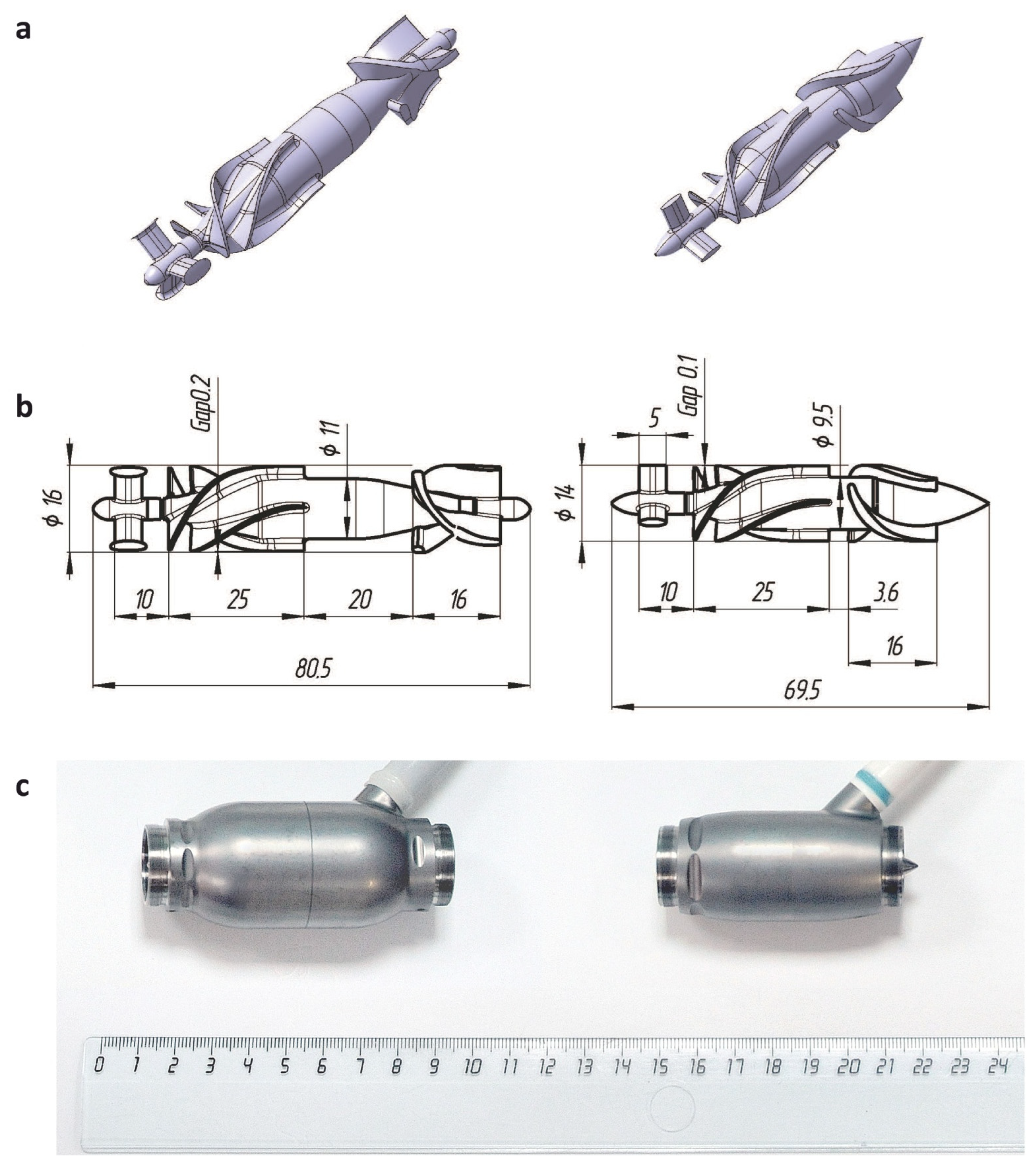





| LVAD Generation | Flow Path Length, mm | Impeller Outer Diameter, mm | Clearance Gap, mm | Distance between Trailing Edge of Impeller Blades and Leading Edge of Diffuser Blades, mm | Overall Pump Weight, g | |
|---|---|---|---|---|---|---|
| Between Impeller Blades and Housing | Between Impeller Hub and Diffuser Blades | |||||
| Sputnik 1 | 80.5 | 15.6 | 0.2 | 0.3 | 20.0 | 246 |
| Sputnik 2 | 69.5 | 13.8 | 0.1 | 0.15 | 3.6 | 205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanova, A.N.; Pugovkin, A.A.; Denisov, M.V.; Ephimov, I.A.; Gusev, D.V.; Walter, M.; Groth, T.; Bockeria, O.L.; Le, T.G.; Satyukova, A.S.; et al. Hemolytic Performance in Two Generations of the Sputnik Left Ventricular Assist Device: A Combined Numerical and Experimental Study. J. Funct. Biomater. 2022, 13, 7. https://doi.org/10.3390/jfb13010007
Romanova AN, Pugovkin AA, Denisov MV, Ephimov IA, Gusev DV, Walter M, Groth T, Bockeria OL, Le TG, Satyukova AS, et al. Hemolytic Performance in Two Generations of the Sputnik Left Ventricular Assist Device: A Combined Numerical and Experimental Study. Journal of Functional Biomaterials. 2022; 13(1):7. https://doi.org/10.3390/jfb13010007
Chicago/Turabian StyleRomanova, Alexandra N., Alexander A. Pugovkin, Maxim V. Denisov, Ivan A. Ephimov, Dmitry V. Gusev, Marian Walter, Thomas Groth, Olga L. Bockeria, Tatyana G. Le, Anna S. Satyukova, and et al. 2022. "Hemolytic Performance in Two Generations of the Sputnik Left Ventricular Assist Device: A Combined Numerical and Experimental Study" Journal of Functional Biomaterials 13, no. 1: 7. https://doi.org/10.3390/jfb13010007
APA StyleRomanova, A. N., Pugovkin, A. A., Denisov, M. V., Ephimov, I. A., Gusev, D. V., Walter, M., Groth, T., Bockeria, O. L., Le, T. G., Satyukova, A. S., Selishchev, S. V., & Telyshev, D. V. (2022). Hemolytic Performance in Two Generations of the Sputnik Left Ventricular Assist Device: A Combined Numerical and Experimental Study. Journal of Functional Biomaterials, 13(1), 7. https://doi.org/10.3390/jfb13010007







