Improve Dentin Bonding Performance Using a Hydrolytically Stable, Ether-Based Primer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
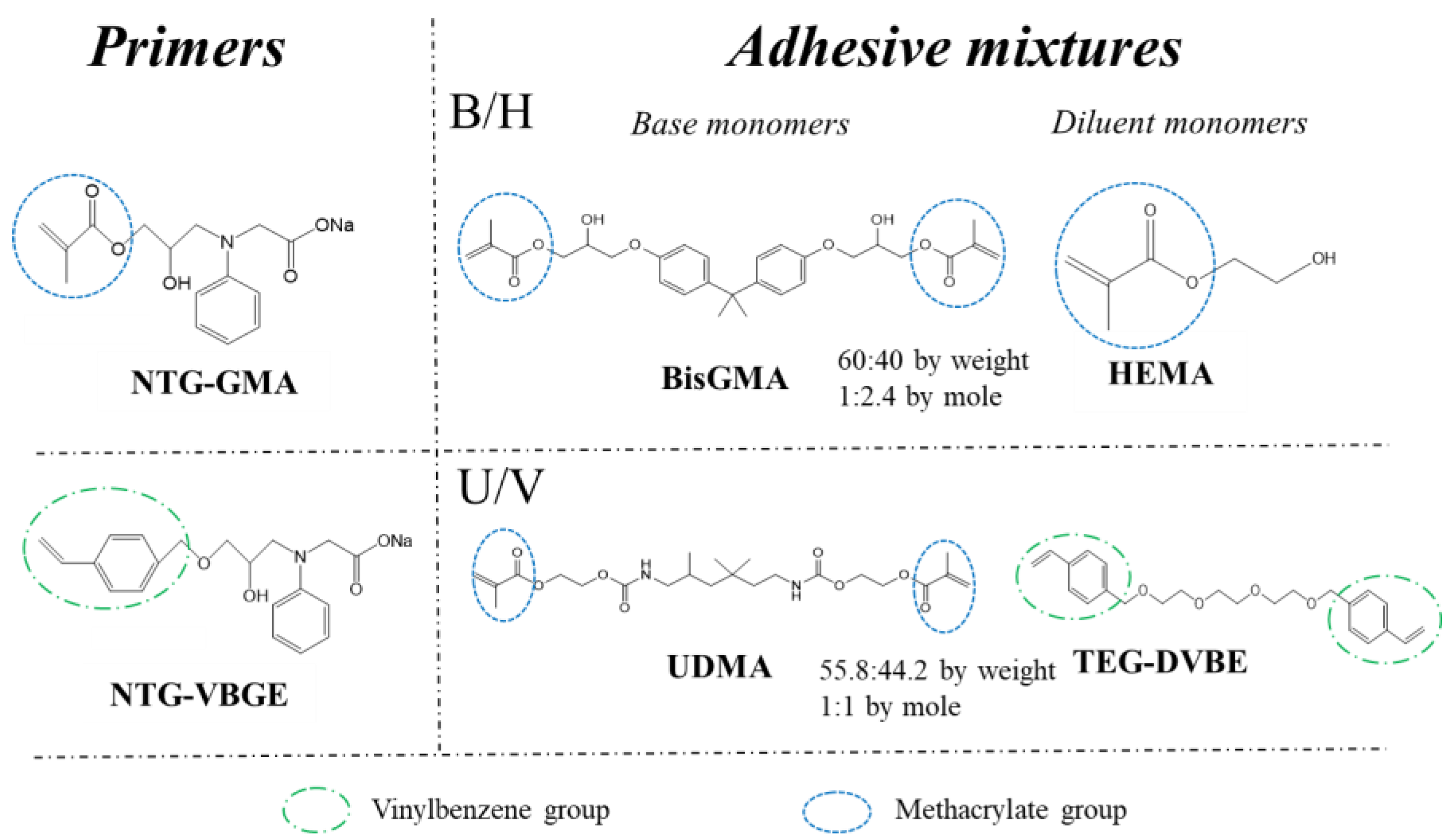
2.2. Preparing Dental Adhesives and Primers
2.3. Dentin Bonding Procedures and Shear Bond Strength (SBS) Evaluation
2.4. Micro-Tensile Bond Strength (μTBS) and Thermal Cycling (TC) for Durability Evaluation
2.5. Hydroxyapatite (HA) Pellets as Model Surfaces to Study the Hydrophilicity and Biostability of Primers
2.6. Contact Angle (CA) Measurements
2.7. Fracture Surfaces Morphological Observation and Failure Mode Analysis
2.8. Statistical Analysis
3. Results
3.1. Hydrophilicity of NTG-VBGE and Its Stability against PCE Challenge
3.2. Dentin Bond Strength Evaluations
3.3. Durability Evaluation by TC
3.4. Failure Mode Analysis
3.5. Resin Spreading Kinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferracane, J.L. Resin composite-State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef]
- Pashley, D.H.; Sano, H.; Ciucchi, B.; Yoshiyama, M.; Carvalho, R.M. Adhesion testing of dentin bonding agents: A review. Dent. Mater. 1995, 11, 117–125. [Google Scholar] [CrossRef]
- Haller, B. Recent developments in dentin bonding. Am. J. Dent. 2000, 13, 44–50. [Google Scholar]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2017, 34, 78–96. [Google Scholar] [CrossRef]
- van Dijken, J.W.; Sunnegårdh-Grönberg, K.; Lindberg, A. Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions a 13 years evaluation. Dent. Mater. 2007, 23, 1101–1107. [Google Scholar] [CrossRef]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A Critical Review of the Durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Nishitani, Y.; Yoshiyama, M.; Donnelly, A.; Agee, K.; Sword, J.; Tay, F.; Pashley, D. Effects of resin hydrophilicity on dentin bond strength. J. Dent. Res. 2006, 85, 1016–1021. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y.; Walker, M.; Wieliczka, D.; Swafford, J. Interfacial chemistry of the dentin/adhesive bond. J. Dent. Res. 2000, 79, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P.; Walker, M.P. Chemical profile of adhesive/caries-affected dentin interfaces using Raman microspectroscopy. J. Biomed. Mater. Res. Part A 2006, 81, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Ohno, H.; Sano, H.; Kaga, M.; Oguchi, H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 2003, 24, 3795–3803. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Pashley, D.H.; Mutluay, M.M. Long-term durability of dental adhesives. Curr. Oral Health Rep. 2015, 2, 174–181. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef]
- Yiu, C.K.Y.; King, N.M.; Pashley, D.H.; Suh, B.I.; Carvalho, R.M.D.; Carrilho, M.R.O.; Tay, F.R. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials 2004, 25, 5789–5796. [Google Scholar] [CrossRef]
- Armstrong, S.; Keller, J.; Boyer, D. The influence of water storage and C-factor on the dentin–resin composite microtensile bond strength and debond pathway utilizing a filled and unfilled adhesive resin. Dent. Mater. 2001, 17, 268–276. [Google Scholar] [CrossRef]
- Bowen, R.L.; Marjenhoff, W.A. Development of an adhesive bonding system. Oper. Dent. 1992, (Suppl. S5), 75–80. [Google Scholar]
- Jemt, T.; Stålblad, P.A.; Øilo, G. Adhesion of polycarboxylate-based dental cements to enamel: An in vivo study. J. Dent. Res. 1986, 65, 885–887. [Google Scholar] [CrossRef]
- Bowen, R.; Bennett, P.; Groh, R.; Farahani, M.; Eichmiller, F.C. New Surface-active comonomer for adhesive bonding. J. Dent. Res. 1996, 75, 606–610. [Google Scholar] [CrossRef]
- Gonzalez-Bonet, A.; Kaufman, G.; Yang, Y.; Wong, C.; Jackson, A.; Huyang, G.; Bowen, R.; Sun, J. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015, 16, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Urbas, A.; Gonzalez-Bonet, A.; Sheridan, R.J.; Seppala, J.E.; Beers, K.L.; Sun, J. A composition-controlled cross-linking resin network through rapid visible-light photo-copolymerization. Polym. Chem. 2016, 7, 5023–5030. [Google Scholar] [CrossRef]
- Ge, X.; Ye, Q.; Song, L.; Misra, A.; Spencer, P. Synthesis and evaluation of novel siloxane-methacrylate monomers used as dentin adhesives. Dent. Mater. 2014, 30, 1073–1087. [Google Scholar] [CrossRef]
- Podgórski, M.; Becka, E.; Claudino, M.; Flores, A.; Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Ester-free thiol-ene dental restoratives-Part A: Resin development. Dent. Mater. 2015, 31, 1255–1262. [Google Scholar] [CrossRef]
- Podgórski, M.; Becka, E.; Claudino, M.; Flores, A.; Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Ester-free thiol–ene dental restoratives—Part B: Resin development. Dent. Mater. 2015, 31, 1263–1270. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. Recent developments of new components for dental adhesives and composites. Macromol. Mater. Eng. 2007, 292, 245–271. [Google Scholar] [CrossRef]
- Song, L.; Sarikaya, R.; Ye, Q.; Misra, A.; Tamerler, C.; Spencer, P. Multifunctional monomer acts as co-initiator and crosslinker to provide autonomous strengthening with enhanced hydrolytic stability in dental adhesives. Dent. Mater. 2020, 36, 284–295. [Google Scholar] [CrossRef]
- Song, L.; Ye, Q.; Ge, X.; Misra, A.; Tamerler, C.; Spencer, P. Fabrication of hybrid crosslinked network with buffering capabilities and autonomous strengthening characteristics for dental adhesives. Acta Biomater. 2018, 67, 111–121. [Google Scholar] [CrossRef]
- Song, L.; Ye, Q.; Ge, X.; Misra, A.; Tamerler, C.; Spencer, P. New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives. Acta Biomater. 2018, 83, 130–139. [Google Scholar] [CrossRef]
- Martim, G.C.; Pfeifer, C.S.; Girotto, E.M. Novel urethane-based polymer for dental applications with decreased monomer leaching. Mater. Sci. Eng. C 2017, 72, 192–201. [Google Scholar] [CrossRef]
- Yamauchi, S.; Wang, X.; Egusa, H.; Sun, J. High-performance dental adhesives containing an ether-based monomer. J. Dent. Res. 2019, 99, 189–195. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Eichmiller, F.C.; Smith, D.T.; Schumacher, G.E.; Giuseppetti, A.A.; Antonucci, J.M. Effect of thermal cycling on whisker-reinforced dental resin composites. J. Mater. Sci. Mater. Med. 2002, 13, 875–883. [Google Scholar] [CrossRef]
- Sawyer, C.H. Hydrolysis of choline esters by liver. Science 1945, 101, 385–386. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J. Salivary esterase activity and its association with the biodegradation of dental composites. J. Dent. Res. 2004, 83, 22–26. [Google Scholar] [CrossRef]
- Wang, X.; Song, S.; Chen, L.; Stafford, C.M.; Sun, J. Short-time dental resin biostability and kinetics of enzymatic degradation. Acta Biomater. 2018, 74, 326–333. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Sano, H.; Burrow, M.; Tagami, J.; Pashley, D. Effects of dentin depth and cavity configuration on bond strength. J. Dent. Res. 1999, 78, 898–905. [Google Scholar] [CrossRef]
- Scherrer, S.S.; Cesar, P.F.; Swain, M.V. Direct comparison of the bond strength results of the different test methods: A critical literature review. Dent. Mater. 2010, 26, e78–e93. [Google Scholar] [CrossRef]
- Francois, P.; Vennat, E.; Le Goff, S.; Ruscassier, N.; Attal, J.-P.; Dursun, E. Shear bond strength and interface analysis between a resin composite and a recent high-viscous glass ionomer cement bonded with various adhesive systems. Clin. Oral Investig. 2018, 23, 2599–2608. [Google Scholar] [CrossRef]
- Tani, C.; Manabe, A.; Itoh, K.; Hisamitsu, H.; Wakumoto, S. Contact angle of dentin bonding agents on the dentin surface. Dent. Mater. J. 1996, 15, 39–44, 72. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Tjäderhane, L. Dentin bonding: Can we make it last? Oper. Dent. 2015, 40, 4–18. [Google Scholar] [CrossRef]
- Good, R.J. Contact angle, wetting, and adhesion: A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Arola, D.D.; Gao, S.; Zhang, H.; Masri, R. The tooth: Its structure and properties. Dent. Clin. 2017, 61, 651–668. [Google Scholar]
- Mischo, J.; Faidt, T.; McMillan, R.B.; Dudek, J.; Gunaratnam, G.; Bayenat, P.; Holtsch, A.; Spengler, C.; Müller, F.; Hähl, H.; et al. Hydroxyapatite Pellets as Versatile Model Surfaces for Systematic Adhesion Studies on Enamel: A Force Spectroscopy Case Study. ACS Biomater. Sci. Eng. 2022, 8, 1476–1485. [Google Scholar] [CrossRef]
- Fox, J.L.; Iyer, B.V.; Higuchi, W.I.; Hefferren, J.J. Solution activity product (KFAP) and simultaneous demineralization–remineralization in bovine tooth enamel and hydroxyapatite pellets. J. Pharm. Sci. 1983, 72, 1252–1255. [Google Scholar] [CrossRef]
- Carpenter, G.H.; Pramanik, R.; Proctor, G.B. An in vitro model of chlorhexidine-induced tooth staining. J. Periodontal Res. 2005, 40, 225–230. [Google Scholar] [CrossRef]
- Zeitz, C.; Faidt, T.; Grandthyll, S.; Hähl, H.; Thewes, N.; Spengler, C.; Schmauch, J.; Deckarm, M.J.; Gachot, C.; Natter, H.; et al. Synthesis of hydroxyapatite substrates: Bridging the gap between model surfaces and enamel. ACS Appl. Mater. Interfaces 2016, 8, 25848–25855. [Google Scholar] [CrossRef]
- Della Bona, A.; Van Noort, R. Shear vs. Tensile Bond Strength of Resin Composite Bonded to Ceramic. J. Dent. Res. 1995, 74, 1591–1596. [Google Scholar] [CrossRef]
- Kitasako, Y.; Burrow, M.F.; Nikaido, T.; Harada, N.; Inokoshi, S.; Yamada, T.; Takatsu, T. Shear and tensile bond testing for resin cement evaluation. Dent. Mater. 1995, 11, 298–304. [Google Scholar] [CrossRef]
- Rasmussen, S. Analysis of dental shear bond strength tests, shear or tensile? Int. J. Adhes. Adhes. 1996, 16, 147–154. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal cycling for restorative materials: Does a standardized protocol exist in laboratory testing? A literature review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef]



| 0-TC | 10,000-TC | |||
|---|---|---|---|---|
| Group | Mean (MPa) | Number of Failures by Mode (C/AD/M) | Mean (MPa) | Number of Failures by Mode (C/AD/M) |
| Scotchbond | 40.4 ± 8.4 a | 9/1/5 | 28.4 ± 11.6 * | 6/7/2 ** |
| NTG-GMA + B/H | 39.3 ± 12.3 a | 7/8/0 | 22.8 ± 14.0 * | 4/10/1 ** |
| NTG-GMA + U/V | 38.5 ± 8.7 a | 9/3/3 | 41.4 ± 13.4 | 7/7/1 ** |
| NTG-VBGE + B/H | 34.4 ± 11.5 a | 8/7/0 | 26.4 ± 8.4 * | 5/10/0 ** |
| NTG-VBGE + U/V | 48.7 ± 12.4 b | 11/2/2 | 46.4 ± 12.1 | 10/2/3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yamauchi, S.; Sun, J. Improve Dentin Bonding Performance Using a Hydrolytically Stable, Ether-Based Primer. J. Funct. Biomater. 2022, 13, 128. https://doi.org/10.3390/jfb13030128
Wang X, Yamauchi S, Sun J. Improve Dentin Bonding Performance Using a Hydrolytically Stable, Ether-Based Primer. Journal of Functional Biomaterials. 2022; 13(3):128. https://doi.org/10.3390/jfb13030128
Chicago/Turabian StyleWang, Xiaohong, Shinobu Yamauchi, and Jirun Sun. 2022. "Improve Dentin Bonding Performance Using a Hydrolytically Stable, Ether-Based Primer" Journal of Functional Biomaterials 13, no. 3: 128. https://doi.org/10.3390/jfb13030128
APA StyleWang, X., Yamauchi, S., & Sun, J. (2022). Improve Dentin Bonding Performance Using a Hydrolytically Stable, Ether-Based Primer. Journal of Functional Biomaterials, 13(3), 128. https://doi.org/10.3390/jfb13030128






