Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application
Abstract
:1. Introduction
2. The Adhesion Process of Oral Bacteria
3. Implant Surface Properties Affecting Bacterial Adhesion
3.1. Roughness and Surface Topography
3.2. Hydrophilicity
3.3. Charge
3.4. Surface Free Energy
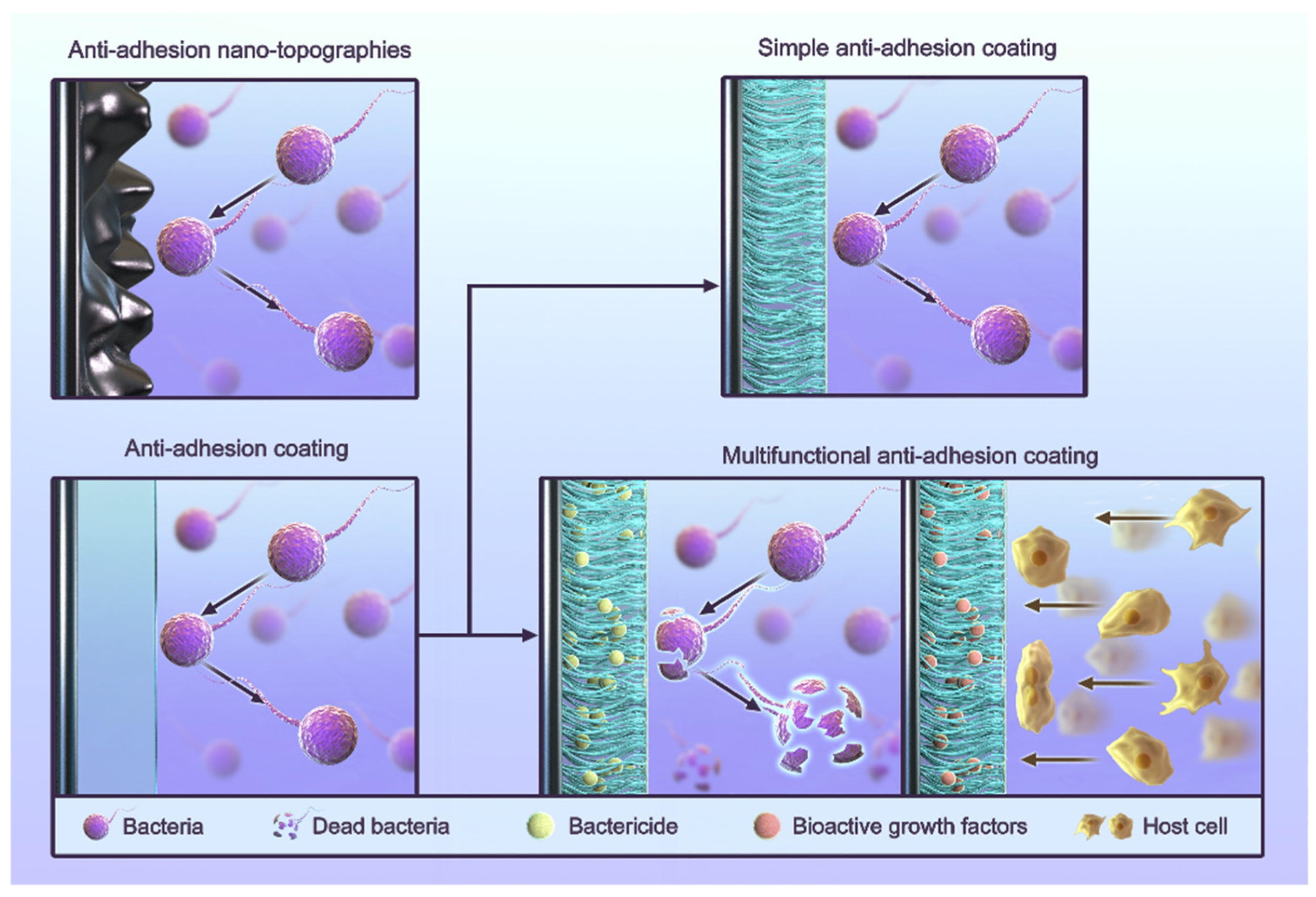
4. Anti-Adhesion Strategies for Titanium Implants
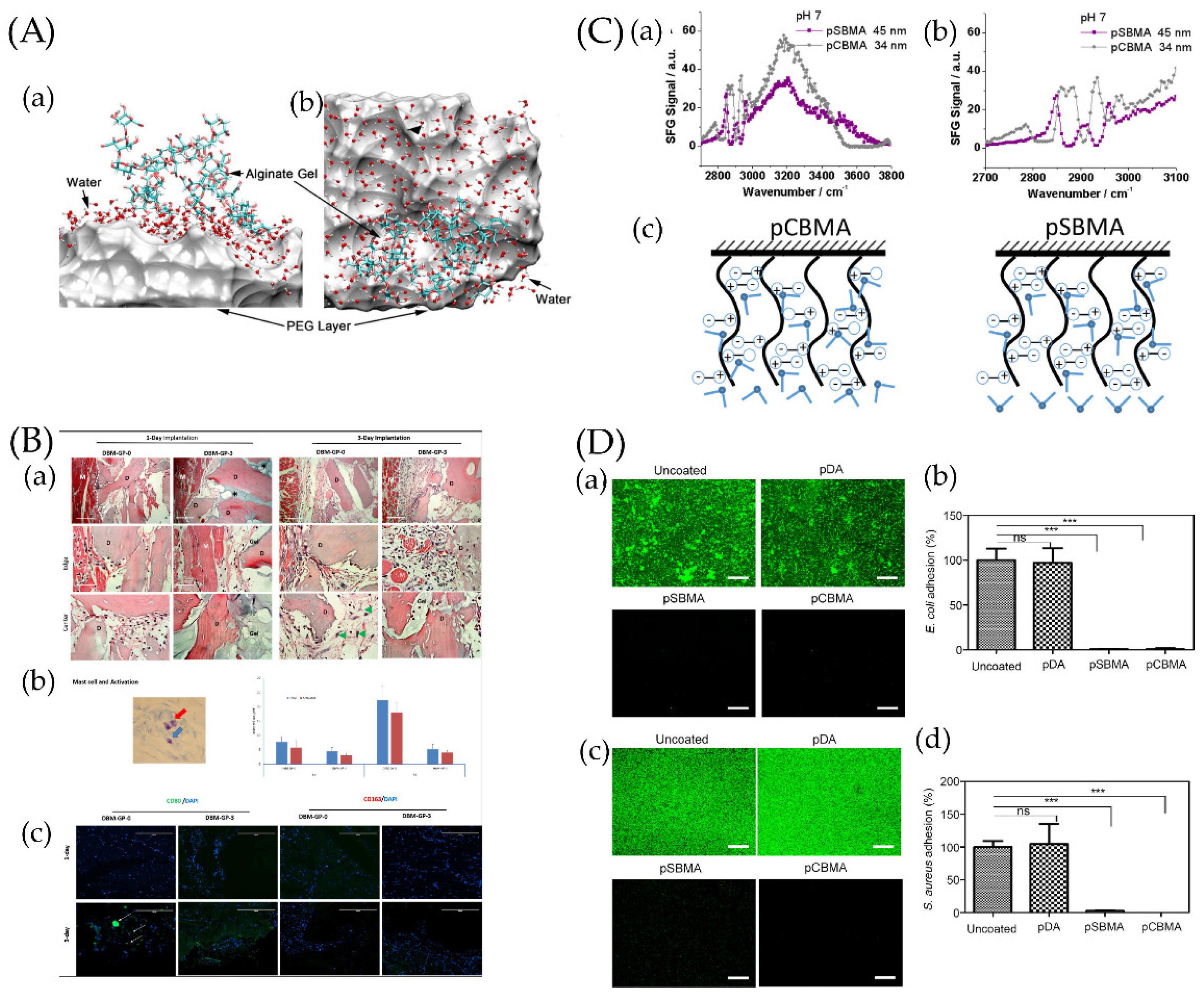
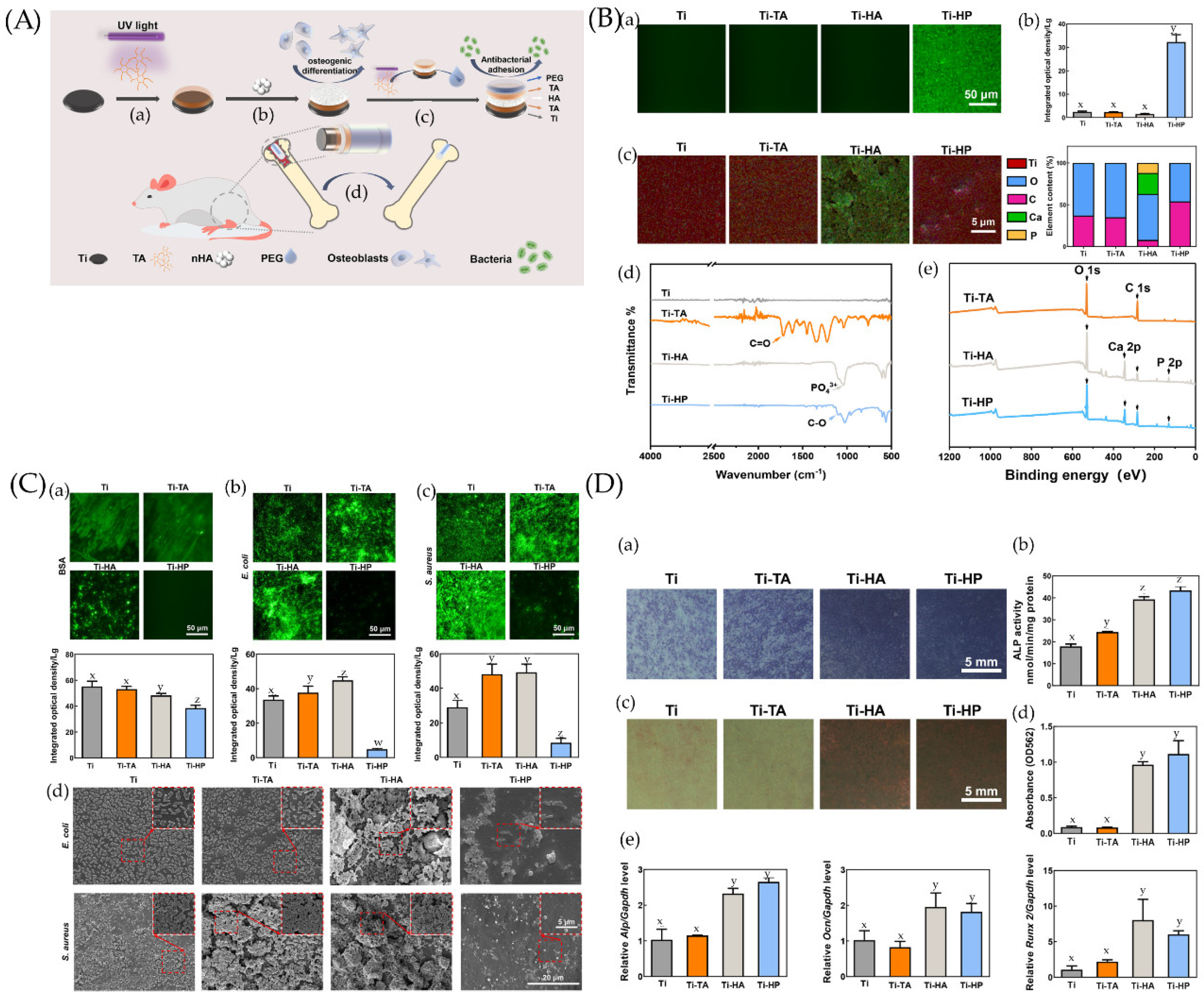
4.1. Anti-Adhesion Coating
4.1.1. Simple Anti-Adhesion Coatings
4.1.2. Composite Anti-Adhesion Coatings
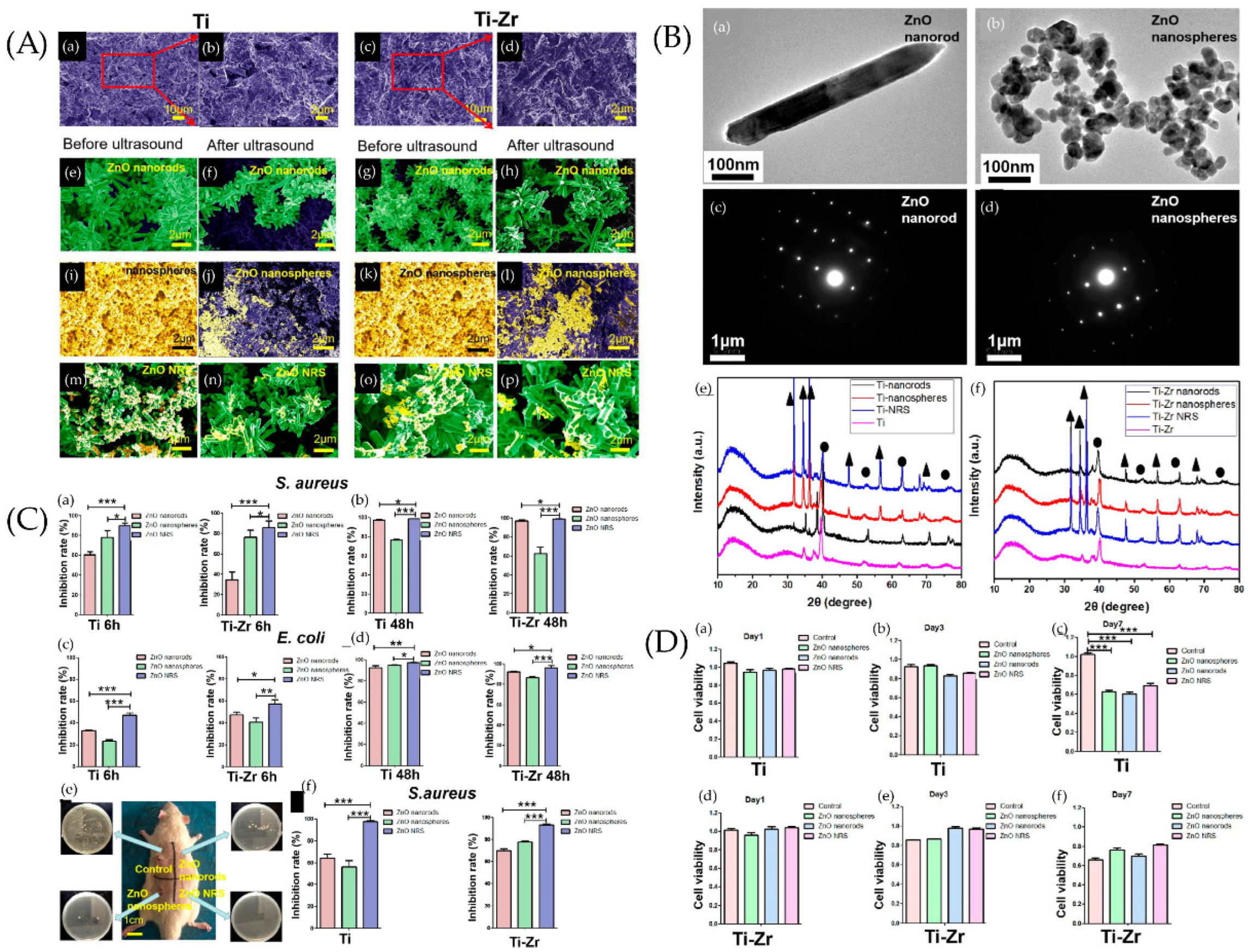
4.2. Anti-Adhesion Nano-Topographies
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross Occlusion in implant dentistry. A review of the literature of prosthetic determinants and current concepts. Aust. Dent. J. 2008, 53, S60–S68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Lu, D.; Dong, W.; Bi, W.; Feng, X.; Wen, L.; Sun, H.; Qi, M. Evaluation of osteogenic and antibacterial properties of strontium/silver-containing porous TiO2 coatings prepared by micro-arc oxidation. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Stone, P.W. Popping the (PICO) question in research and evidence-based practice. Appl. Nurs. Res. 2002, 15, 197–198. [Google Scholar] [CrossRef]
- Charalampakis, G.; Leonhardt, A.; Rabe, P.; Dahlén, G. Clinical and microbiological characteristics of peri-implantitis cases: A retrospective multicentre study. Clin. Oral Implant. Res. 2012, 23, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Newman, M.G.; Nachnani, S.; Holt, R.; Stewart, R.; Flemmig, T. Characterization of the subgingival microbial flora around endosteal sapphire dental implants in partially edentulous patients. Int. J. Oral Maxillofac. Implant. 1990, 5, 247–253. [Google Scholar]
- Heitz-Mayfield, A.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol. 2000 2010, 53, 167–181. [Google Scholar] [CrossRef]
- Gooty, J.R.; Kannam, D.; Guntakala, V.R.; Palaparthi, R. Distribution of dendritic cells and langerhans cells in peri-implant mucosa. Contemp. Clin. Dent. 2018, 9, 548–553. [Google Scholar] [CrossRef]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S6–S22. [Google Scholar] [CrossRef]
- Pascual, M.; Calvo-Rodriguez, M.; Núñez, L.; Villalobos, C.; Ureña, J.; Guerri, C. Toll-like receptors in neuroinflammation, neurodegeneration, and alcohol-induced brain damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Sojod, B.; Chateau, D.; Mueller, C.; Babajko, S.; Berdal, A.; Lézot, F.; Castaneda, B. RANK/RANKL/OPG Signalization Implication in Periodontitis: New Evidence from a RANK Transgenic Mouse Model. Front. Physiol. 2017, 8, 338. [Google Scholar] [CrossRef]
- Lim, W.H.; Liu, B.; Mah, S.-J.; Yin, X.; Helms, J.A. Alveolar Bone Turnover and Periodontal Ligament Width Are Controlled by Wnt. J. Periodontol. 2015, 86, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Ma, L.; Bai, N.; Xu, H. Wnt5a is involved in LOX-1 and TLR4 induced host inflammatory response in peri-implantitis. J. Periodontal Res. 2020, 55, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-Q.; Ming, P.-P.; Qiu, J.; Shao, S.-Y.; Yu, Y.-J.; Chen, J.-X.; Yang, J.; Xu, L.-N.; Zhang, S.-M.; Tang, C.-B. Effect of titanium ions on the Hippo/YAP signaling pathway in regulating biological behaviors of MC3T3-E1 osteoblasts. J. Appl. Toxicol. 2018, 38, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Garaicoa-Pazmino, C.; Nelson, K.; Giannobile, W.V.; Squarize, C.; Larsson, L.; Castilho, R.M. Characterization of macrophages infiltrating peri-implantitis lesions. Clin. Oral Implant. Res. 2020, 31, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Eo, M.Y.; Kim, S.M. Real Appearance of Dendritic Cell on Failed Implant Fixture. J. Craniofacial Surg. 2021, 32, e607–e609. [Google Scholar] [CrossRef]
- Tang, K.; Chen, W.; Tang, Z.; Yu, X.; Zhu, W.; Zhang, S.; Qiu, J. Role of the Hippo-YAP/NF-κB signaling pathway crosstalk in regulating biological behaviors of macrophages under titanium ion exposure. J. Appl. Toxicol. 2021, 41, 561–571. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Heyman, O.; Koren, N.; Mizraji, G.; Capucha, T.; Wald, S.; Nassar, M.; Tabib, Y.; Shapira, L.; Hovav, A.-H.; Wilensky, A. Impaired Differentiation of Langerhans Cells in the Murine Oral Epithelium Adjacent to Titanium Dental Implants. Front. Immunol. 2018, 9, 1712. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.-L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- Cyprus, G.; Overlin, J.; Hotchkiss, K.; Kandalam, S.; Olivares-Navarrete, R. Cigarette smoke increases pro-inflammatory markers and inhibits osteogenic differentiation in experimental exposure model. Acta Biomater. 2018, 76, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Catania, D.; Neiva, K.; Wallet, S.M. Innate Immune Receptor Expression in Peri-Implant Tissues of Patients with Different Susceptibility to Periodontal Diseases. J. Periodontol. 2012, 84, 221–229. [Google Scholar] [CrossRef]
- Schaudinn, C.; Gorur, A.; Keller, D.; Sedghizadeh, P.P.; Costerton, J.W. Periodontitis: An archetypical biofilm disease. J. Am. Dent. Assoc. 2009, 140, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.-J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Gotz, F. Staphylococcus and biofilms. Mol. Microbiol. 2002, 43, 1367–1378. [Google Scholar] [CrossRef]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Götz, F. The Intercellular Adhesion (ica) Locus Is Present in Staphylococcus aureus and Is Required for Biofilm Formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef]
- Frédéric, L.; Michel, B.; Selena, T. Oral Microbes, Biofilms and Their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alovisi, M.; Carossa, M.; Mandras, N.; Roana, J.; Costalonga, M.; Cavallo, L.; Pira, E.; Putzu, M.G.; Bosio, D.; Roato, I.; et al. Disinfection and Biocompatibility of Titanium Surfaces Treated with Glycine Powder Airflow and Triple Antibiotic Mixture: An In Vitro Study. Materials 2022, 15, 4850. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, Y.; Wang, Y.; Chen, S.; Jiang, S.; Ge, H.; Lei, L.; Huang, X. Antimicrobial effects of photodynamic therapy with antiseptics on Staphylococcus aureus biofilm on titanium surface. Photodiagnosis Photodyn. Ther. 2019, 25, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Mardas, N.; Spratt, D.; Boniface, D.; Dard, M.; Donos, N. Experimental models for contamination of titanium surfaces and disinfection protocols. Clin. Oral Implant. Res. 2016, 27, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- A PhD completed. Prevention and treatment of peri-implant diseases: Cleaning of titanium dental implant surfaces. Ned. Tijdschr. Voor Tandheelkd. 2017, 124, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, K.; Nie, B.; Ji, F.; Zhang, H. Approaches based on passive and active antibacterial coating on titanium to achieve antibacterial activity. J. Biomed. Mater. Res. Part A 2018, 106, 2531–2539. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Ena, J.; Dick, R.W.; Jones, R.N.; Wenzel, R.P. The Epidemiology of Intravenous Vancomycin Usage in a University Hospital. A 10-year study. JAMA 1993, 269, 598–602. [Google Scholar] [CrossRef]
- Omotola, A.M.; Li, Y.; Martin, E.T.; Alshabani, K.; Yadav, D.; Sarkar, M.; Thapa, S.D.; Kumar, V.; Mahabashya, A.; Ahmad, S.; et al. Risk factors for and epidemiology of community-onset vancomycin-resistant Enterococcus faecalis in southeast Michigan. Am. J. Infect. Control 2013, 41, 1244–1248. [Google Scholar] [CrossRef]
- Yang, B.; Liang, J.; Liu, L.; Li, X.; Wang, Q.; Ren, Y. Overview of antibiotic resistance genes database. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2020, 36, 2582–2597. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Chlorhexidine-pharmaco-biological activity and application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Dos Reis, A.C. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Harapanahalli, A.K.; Younes, J.A.; Allan, E.; Van Der Mei, H.C.; Busscher, H.J. Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment. PLoS Pathog. 2015, 11, e1005057. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Straub, H.; Bigger, C.M.; Valentin, J.; Abt, D.; Qin, X.-H.; Eberl, L.; Maniura-Weber, K.; Ren, Q. Bacterial Adhesion on Soft Materials: Passive Physicochemical Interactions or Active Bacterial Mechanosensing? Adv. Health Mater. 2019, 8, e1801323. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Boks, N.P.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154, 3122–3133. [Google Scholar] [CrossRef]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef]
- Vatanyoopaisarn, S.; Nazli, A.; Dodd, C.E.R.; Rees, C.E.D.; Waites, W.M. Effect of Flagella on Initial Attachment of Listeria monocytogenes to Stainless Steel. Appl. Environ. Microbiol. 2000, 66, 860–863. [Google Scholar] [CrossRef]
- Bodenmiller, D.; Toh, E.; Brun, Y.V. Development of Surface Adhesion in Caulobacter crescentus. J. Bacteriol. 2004, 186, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, T.; Koser, H. Direct Upstream Motility in Escherichia coli. Biophys. J. 2012, 102, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Gordon, V.D.; Wang, L. Bacterial mechanosensing: The force will be with you, always. J. Cell Sci. 2019, 132, jcs227694. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, J.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers. mBio 2019, 10, e01908-19. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; Chen, Z.; Allan, E.; van der Mei, H.C.; Busscher, H.J. Emergent heterogeneous microenvironments in biofilms: Substratum surface heterogeneity and bacterial adhesion force-sensing. FEMS Microbiol. Rev. 2018, 42, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Persat, A. Mechanomicrobiology: How bacteria sense and respond to forces. Nat. Rev. Microbiol. 2020, 18, 227–240. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- van Loosdrecht, M.; Lyklema, J.; Norde, W.; Zehnder, A.J.B. Bacterial adhesion: A physicochemical approach. Microb. Ecol. 1989, 17, 1–15. [Google Scholar] [CrossRef]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Futur. Microbiol. 2018, 13, 915–932. [Google Scholar] [CrossRef]
- Viljoen, A.; Mignolet, J.; Viela, F.; Mathelié-Guinlet, M.; Dufrêne, Y.F. How Microbes Use Force to Control Adhesion. J. Bacteriol. 2020, 202, e00125-20. [Google Scholar] [CrossRef] [PubMed]
- Camesano, T.A.; Logan, B.E. Probing Bacterial Electrosteric Interactions Using Atomic Force Microscopy. Environ. Sci. Technol. 2000, 34, 3354–3362. [Google Scholar] [CrossRef]
- Dunne, W.M., Jr. Bacterial Adhesion: Seen Any Good Biofilms Lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol. Spectr. 2015, 3, 163–199. [Google Scholar] [CrossRef]
- Ellison, C.K.; Kan, J.; Dillard, R.S.; Kysela, D.T.; Ducret, A.; Berne, C.; Hampton, C.M.; Ke, Z.; Wright, E.R.; Biais, N.; et al. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 2017, 358, 535–538. [Google Scholar] [CrossRef]
- Chagnot, C.; Zorgani, M.A.; Astruc, T.; Desvaux, M. Proteinaceous determinants of surface colonization in bacteria: Bacterial adhesion and biofilm formation from a protein secretion perspective. Front. Microbiol. 2013, 4, 303. [Google Scholar] [CrossRef]
- Stoodley, P.; Ehrlich, G.; Sedghizadeh, P.; Hall-Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; Costerton, J.W.; DeMeo, P. Orthopaedic biofilm infections. Curr. Orthop. Pract. 2011, 22, 558–563. [Google Scholar] [CrossRef]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. Biomolecular mechanisms of staphylococcal biofilm formation. Futur. Microbiol. 2013, 8, 509–524. [Google Scholar] [CrossRef]
- Gristina, A.G.; Costerton, J.W. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J. Bone Jt. Surg. 1985, 67, 264–273. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Le, K.Y.; Dastgheyb, S.; Ho, T.V.; Otto, M. Molecular determinants of staphylococcal biofilm dispersal and structuring. Front. Cell Infect. Microbiol. 2014, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. European. J. Of. Clinical. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Barão, V.A.; Yoon, C.J.; Mathew, M.T.; Yuan, J.C.-C.; Wu, C.D.; Sukotjo, C. Attachment of Porphyromonas gingivalis to Corroded Commercially Pure Titanium and Titanium-Aluminum-Vanadium Alloy. J. Periodontol. 2014, 85, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Faust, J.; Bächle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm formation and composition on different implant materials in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 101–109. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-Implant Infections of Oral Biofilm Etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; Van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implants 1996, 11, 169–178. [Google Scholar]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- de Avila, E.D.; Vergani, C.; Junior, F.A.M.; Jr, M.J.; Shi, W.; Lux, R. Effect of titanium and zirconia dental implant abutments on a cultivable polymicrobial saliva community. J. Prosthet. Dent. 2017, 118, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Traini, T.; Sinjari, B.; Nostro, A.; Caputi, S.; Cellini, L. Porphyromonas gingivalis biofilm formation in different titanium surfaces, an in vitro study. Clin. Oral Implant. Res. 2016, 27, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef]
- Gu, H.; Ren, D. Materials and surface engineering to control bacterial adhesion and biofilm formation: A review of recent advances. Front. Chem. Sci. Eng. 2014, 8, 20–33. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Watson, G.; Zheng, Y.; Watson, J.; Liang, A. Wetting properties on nanostructured surfaces of cicada wings. J. Exp. Biol. 2009, 212, 3148–3155. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada Wing Surface Topography: An Investigation into the Bactericidal Properties of Nanostructural Features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas aeruginosa Cells by Cicada Wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.; et al. Biophysical Model of Bacterial Cell Interactions with Nanopatterned Cicada Wing Surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
- Zhang, C.; McAdams, D.A.; Grunlan, J.C. Nano/Micro-Manufacturing of Bioinspired Materials: A Review of Methods to Mimic Natural Structures. Adv. Mater. 2016, 28, 6292–6321. [Google Scholar] [CrossRef]
- Cho, W.K.; Choi, I.S. Fabrication of Hairy Polymeric Films Inspired by Geckos: Wetting and High Adhesion Properties. Adv. Funct. Mater. 2008, 18, 1089–1096. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Llama-Palacios, A.; Fernández, E.; Figuero, E.; Marin, M.J.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. An in vitro biofilm model associated to dental implants: Structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent. Mater. 2014, 30, 1161–1171. [Google Scholar] [CrossRef]
- Yang, Y.; Ao, H.; Wang, Y.; Lin, W.; Yang, S.; Zhang, S.; Yu, Z.; Tang, T. Cytocompatibility with osteogenic cells and enhanced in vivo anti-infection potential of quaternized chitosan-loaded titania nanotubes. Bone Res. 2016, 4, 16027. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Xia, H.; Kim, E.; Sun, H.-B. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217–11231. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, L.; Yang, Y.; Hu, R.; Vogler, E.A.; Lin, C. Role of trapped air in the formation of cell-and-protein micropatterns on superhydrophobic/superhydrophilic microtemplated surfaces. Biomaterials 2012, 33, 8213–8220. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Rzhepishevska, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef]
- Mishra, S.; Horswill, A.R. Heparin Mimics Extracellular DNA in Binding to Cell Surface-Localized Proteins and Promoting Staphylococcus aureus Biofilm Formation. mSphere 2017, 2, e00135-17. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ebi, N.; Takahashi, Y.; Kaneko, T.; Ebisu, S.; Russell, R.R.B. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials 2003, 24, 3605–3609. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2013, 30, 182–191. [Google Scholar] [CrossRef]
- Hauslich, L.B.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The adhesion of oral bacteria to modified titanium surfaces: Role of plasma proteins and electrostatic forces. Clin. Oral Implant. Res. 2013, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kwon, J.-S.; Lee, J.-H.; Uhm, S.-H.; Choi, E.H.; Kim, K.-M. Bacterial attachment on titanium surfaces is dependent on topography and chemical changes induced by nonthermal atmospheric pressure plasma. Biomed. Mater. 2017, 12, 045015. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ren, D. Stiffness of Cross-Linked Poly(Dimethylsiloxane) Affects Bacterial Adhesion and Antibiotic Susceptibility of Attached Cells. Langmuir 2014, 30, 10354–10362. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Shiels, S.M.; Mangum, L.H.; Wenke, J.C. Revisiting the "race for the surface" in a pre-clinical model of implant infection. Eur. Cells Mater. 2020, 39, 77–95. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Health Mater. 2019, 8, e1801103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies —A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Nanda, A.; Walsh, L.J.; Xu, C. Microbial Decontamination and Antibacterial Activity of Nanostructured Titanium Dental Implants: A Narrative Review. Nanomaterials 2021, 11, 2336. [Google Scholar] [CrossRef]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barão, V.A.; Souza, J.G.S. Fitting pieces into the puzzle: The impact of titanium-based dental implant surface modifications on bacterial accumulation and polymicrobial infections. Adv. Colloid Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cai, D.; Hu, J.; Nie, J.; Chen, D.; Qin, G.; Zhang, E. A high-hydrophilic Cu2O-TiO2/Ti2O3/TiO coating on Ti-5Cu alloy: Perfect antibacterial property and rapid endothelialization potential. Biomater. Adv. 2022, 140, 213044. [Google Scholar] [CrossRef]
- Atrian, M.; Kharaziha, M.; Javidan, H.; Alihosseini, F.; Emadi, R. Zwitterionic keratin coating on silk-Laponite fibrous membranes for guided bone regeneration. J. Tissue Eng. Regen. Med. 2022; early view. [Google Scholar] [CrossRef]
- Bao, X.; Huang, X.; Jin, X.; Hu, Q. Bactericidal Anti-Adhesion Potential Integrated Polyoxazoline/Silver Nanoparticle Composite Multilayer Film with pH Responsiveness. Polymers 2022, 14, 3685. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Chen, H.; Chang, Y.; Chen, Z.; Zheng, J. Probing the Structural Dependence of Carbon Space Lengths of Poly(N-hydroxyalkyl acrylamide)-Based Brushes on Antifouling Performance. Biomacromolecules 2014, 15, 2982–2991. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Sanchez-Salcedo, S.; Colilla, M.; Feito, M.J.; Ramírez-Santillán, C.; Portolés, M.T.; Vallet-Regí, M. Inhibition of bacterial adhesion on biocompatible zwitterionic SBA-15 mesoporous materials. Acta Biomater. 2011, 7, 2977–2985. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Liu, P.; Zhang, X.; Xin, J.; Wang, Y.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to improve bioactive and antibacterial properties of polyetheretherketone (PEEK) for use as orthopedic implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; De Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Aray, Y.; Marquez, M.; Rodríguez, J.; Vega, D.; Simón-Manso, Y.; Coll, S.; Gonzalez, A.C.; Weitz, D.A. Electrostatics for Exploring the Nature of the Hydrogen Bonding in Polyethylene Oxide Hydration. J. Phys. Chem. B 2004, 108, 2418–2424. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Wenande, E.; Kroigaard, M.; Mosbech, H.; Garvey, L.H. Polyethylene Glycols (PEG) and Related Structures: Overlooked allergens in the perioperative setting. A A Case Rep. 2015, 4, 61–64. [Google Scholar] [CrossRef]
- Han, B.; Fang, J.; Yang, Z.; Zhao, S.; Fang, W.; Hoang, B.X. PEGylated Coating Affects DBM Osteoinductivity In Vivo by Changing Inflammatory Responses. ACS Appl. Bio Mater. 2020, 3, 8722–8730. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Jiang, X.; Fan, J.; Peng, W.; Liu, P.; Yang, T.; Chen, H.; Jiang, W.; Yin, G.; et al. Rational design of a zwitterionic–phosphonic copolymer for the surface antifouling modification of multiple biomedical metals. J. Mater. Chem. B 2019, 7, 4055–4065. [Google Scholar] [CrossRef]
- Maddikeri, R.; Tosatti, S.; Schuler, M.; Chessari, S.; Textor, M.; Richards, R.; Harris, L. Reduced medical infection related bacterial strains adhesion on bioactive RGD modified titanium surfaces: A first step toward cell selective surfaces. J. Biomed. Mater. Res. Part A 2008, 84, 425–435. [Google Scholar] [CrossRef]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface Grafted Sulfobetaine Polymers via Atom Transfer Radical Polymerization as Superlow Fouling Coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Murou, M.; Kitano, H.; Ohno, K.; Saruwatari, Y. Silica particles coated with zwitterionic polymer brush: Formation of colloidal crystals and anti-biofouling properties in aqueous medium. Colloids Surf. B Biointerfaces 2011, 84, 111–116. [Google Scholar] [CrossRef]
- Sun, J.-T.; Yu, Z.-Q.; Hong, C.-Y.; Pan, C.-Y. Biocompatible Zwitterionic Sulfobetaine Copolymer-Coated Mesoporous Silica Nanoparticles for Temperature-Responsive Drug Release. Macromol. Rapid Commun. 2012, 33, 811–818. [Google Scholar] [CrossRef]
- Wu, Y.; Raju, C.; Hou, Z.; Si, Z.; Xu, C.; Pranantyo, D.; Marimuthu, K.; De, P.P.; Ng, O.T.; Pethe, K.; et al. Mixed-charge pseudo-zwitterionic copolymer brush as broad spectrum antibiofilm coating. Biomaterials 2021, 273, 120794. [Google Scholar] [CrossRef]
- Leng, C.; Hung, H.-C.; Sun, S.; Wang, D.; Li, Y.; Jiang, S.; Chen, Z. Probing the Surface Hydration of Nonfouling Zwitterionic and PEG Materials in Contact with Proteins. ACS Appl. Mater. Interfaces 2015, 7, 16881–16888. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Xiao, S.; Shen, M.; Chen, F.; Fan, P.; Zhong, M.; Zheng, J. Salt-Responsive Zwitterionic Polymer Brushes with Tunable Friction and Antifouling Properties. Langmuir 2015, 31, 9125–9133. [Google Scholar] [CrossRef]
- Huang, K.-T.; Hsieh, P.-S.; Dai, L.-G.; Huang, C.-J. Complete zwitterionic double network hydrogels with great toughness and resistance against foreign body reaction and thrombus. J. Mater. Chem. B 2020, 8, 7390–7402. [Google Scholar] [CrossRef]
- Zhang, Z.; Chao, T.; Chen, S.; Jiang, S. Superlow Fouling Sulfobetaine and Carboxybetaine Polymers on Glass Slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the Hydration of Nonfouling Material Poly(sulfobetaine methacrylate) by Low-Field Nuclear Magnetic Resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, S. Investigation of the Hydration of Nonfouling Material Poly(ethylene glycol) by Low-Field Nuclear Magnetic Resonance. Langmuir 2012, 28, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hower, J.; Chen, S.; Bernards, M.T.; Chang, Y.; Jiang, S. Molecular Simulation Studies of Protein Interactions with Zwitterionic Phosphorylcholine Self-Assembled Monolayers in the Presence of Water. Langmuir 2008, 24, 10358–10364. [Google Scholar] [CrossRef]
- Nishida, M.; Nakaji-Hirabayashi, T.; Kitano, H.; Saruwatari, Y.; Matsuoka, K. Titanium alloy modified with anti-biofouling zwitterionic polymer to facilitate formation of bio-mineral layer. Colloids Surf. B Biointerfaces 2017, 152, 302–310. [Google Scholar] [CrossRef]
- Xu, R.; Cui, X.; Xin, Q.; Lu, M.; Li, Z.; Li, J.; Chen, X. Zwitterionic PMCP-functionalized titanium surface resists protein adsorption, promotes cell adhesion, and enhances osteogenic activity. Colloids Surf. B Biointerfaces 2021, 206, 111928. [Google Scholar] [CrossRef]
- Topa, S.H.; Palombo, E.A.; Kingshott, P.; Blackall, L.L. Activity of Cinnamaldehyde on Quorum Sensing and Biofilm Susceptibility to Antibiotics in Pseudomonas aeruginosa. Microorganisms 2020, 8, 455. [Google Scholar] [CrossRef]
- Mooney, J.A.; Pridgen, E.M.; Manasherob, R.; Suh, G.; Blackwell, H.E.; Barron, A.E.; Bollyky, P.L.; Goodman, S.B.; Amanatullah, D.F. Periprosthetic bacterial biofilm and quorum sensing. J. Orthop. Res. 2018, 36, 2331–2339. [Google Scholar] [CrossRef]
- Zhang, B.; Skelly, J.D.; Braun, B.M.; Ayers, D.C.; Song, J. Surface-Grafted Zwitterionic Polymers Improve the Efficacy of a Single Antibiotic Injection in Suppressing Staphylococcus aureus Periprosthetic Infections. ACS Appl. Bio Mater. 2020, 3, 5896–5904. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, R.-G.; Leng, Y. Molecular Dynamics Simulations of a Poly(ethylene glycol)-Grafted Polyamide Membrane and Its Interaction with a Calcium Alginate Gel. Langmuir 2016, 32, 4424–4433. [Google Scholar] [CrossRef]
- Han, X.; Leng, C.; Shao, Q.; Jiang, S.; Chen, Z. Absolute Orientations of Water Molecules at Zwitterionic Polymer Interfaces and Interfacial Dynamics after Salt Exposure. Langmuir 2019, 35, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Thuy, L.T.; Kim, Y.; Seong, M.; Cho, W.K.; Choi, J.S.; Kang, S.M. Coordination-Driven Surface Zwitteration for Antibacterial and Antifog Applications. Langmuir 2022, 38, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Suzuki, D.; Omori, S.; Tanaka, R.; Murakami, A.; Kataoka, Y.; Baba, K.; Kamijo, R.; Miyazaki, T. The characteristics of in vitro biological activity of titanium surfaces anodically oxidized in chloride solutions. Biomaterials 2010, 31, 8546–8555. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Park, S.-W.; Lee, K.; Kang, I.-C.; Yun, K.-D.; Kim, H.-S.; Lim, H.-P. Evaluation of antibacterial activity and osteoblast-like cell viability of TiN, ZrN and (Ti1-xZrx)N coating on titanium. J. Adv. Prosthodont. 2015, 7, 166–171. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, S.-H.; Chien, C.-S.; Kung, J.-C.; Shih, C.-J. In Vitro Bioactivity and Antibacterial Effects of a Silver-Containing Mesoporous Bioactive Glass Film on the Surface of Titanium Implants. Int. J. Mol. Sci. 2022, 23, 9291. [Google Scholar] [CrossRef]
- Peyre, J.; Humblot, V.; Méthivier, C.; Berjeaud, J.-M.; Pradier, C.-M. Co-Grafting of Amino–Poly(ethylene glycol) and Magainin I on a TiO2 Surface: Tests of Antifouling and Antibacterial Activities. J. Phys. Chem. B 2012, 116, 13839–13847. [Google Scholar] [CrossRef]
- Harris, L.; Tosatti, S.; Wieland, M.; Textor, M.; Richards, R. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials 2004, 25, 4135–4148. [Google Scholar] [CrossRef]
- Hu, X.; Neoh, K.-G.; Shi, Z.; Kang, E.-T.; Poh, C.; Wang, W. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials 2010, 31, 8854–8863. [Google Scholar] [CrossRef]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.; Losic, D. Advanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloids Surf. B Biointerfaces 2015, 130, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; He, L.; Li, J.; Luo, J.; Liang, K.; Yin, D.; Tao, S.; Yang, J.; Li, J. Mussel-Inspired Organic–Inorganic Implant Coating Based on a Layer-by-Layer Method for Anti-infection and Osteogenesis. Ind. Eng. Chem. Res. 2022, 61, 13040–13051. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, Y.; Gleichauf, K.; Lou, J.; Li, Q. Nanostructure on Taro Leaves Resists Fouling by Colloids and Bacteria under Submerged Conditions. Langmuir 2011, 27, 10035–10040. [Google Scholar] [CrossRef]
- Pu, X.; Li, G.; Liu, Y. Progress and Perspective of Studies on Biomimetic Shark Skin Drag Reduction. ChemBioEng Rev. 2016, 3, 26–40. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Biomimetic structures for fluid drag reduction in laminar and turbulent flows. J. Physics Condens. Matter Inst. Phys. J. 2010, 22, 035104. [Google Scholar] [CrossRef]
- Kim, T.W. Assessment of Hydro/Oleophobicity for Shark Skin Replica with Riblets. J. Nanosci. Nanotechnol. 2014, 14, 7562–7568. [Google Scholar] [CrossRef]
- Autumn, K.J.A.S. How Gecko Toes Stick. Am. Sci. 2006, 94, 124–132. [Google Scholar]
- Tobin, M.J.; Puskar, L.; Hasan, J.; Webb, H.K.; Hirschmugl, C.J.; Nasse, M.J.; Gervinskas, G.; Juodkazis, S.; Watson, G.S.; Watson, J.A.; et al. High-spatial-resolution mapping of superhydrophobic cicada wing surface chemistry using infrared microspectroscopy and infrared imaging at two synchrotron beamlines. J. Synchrotron Radiat. 2013, 20, 482–489. [Google Scholar] [CrossRef]
- Selvakumar, R.; Karuppanan, K.K.; Pezhinkattil, R. Analysis on surface nanostructures present in hindwing of dragon fly (Sympetrum vulgatum) using atomic force microscopy. Micron 2012, 43, 1299–1303. [Google Scholar] [CrossRef]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.D.; Bhushan, B. Rice- and butterfly-wing effect inspired self-cleaning and low drag micro/nanopatterned surfaces in water, oil, and air flow. Nanoscale 2014, 6, 76–96. [Google Scholar] [CrossRef] [PubMed]
- Hizal, F.; Rungraeng, N.; Lee, J.; Jun, S.; Busscher, H.J.; Van Der Mei, H.C.; Choi, C.-H. Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9, 12118–12129. [Google Scholar] [CrossRef]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dent. Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Chan, E.P.; Park, J.-H.; Ahn, W.-G.; Jung, H.W.; Hong, S.; Lee, J.S.; Han, J.-Y.; Park, S.; Ko, D.-H.; et al. Nanoscale Pillar-Enhanced Tribological Surfaces as Antifouling Membranes. ACS Appl. Mater. Interfaces 2016, 8, 31433–31441. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Liang, M.N.; Meluleni, G.; Pier, G.; Ingber, A.D.E.; Whitesides, G.M. Self-Assembled Monolayers That Resist the Adsorption of Proteins and the Adhesion of Bacterial and Mammalian Cells. Langmuir 2001, 17, 6336–6343. [Google Scholar] [CrossRef]
- Hardes, J.; Streitbürger, A.; Ahrens, H.; Nusselt, T.; Gebert, C.; Winkelmann, W.; Battmann, A.; Gosheger, G. The Influence of Elementary Silver Versus Titanium on Osteoblasts Behaviour In Vitro Using Human Osteosarcoma Cell Lines. Sarcoma 2007, 2007, 26539. [Google Scholar] [CrossRef]
- Wang, X.; Fan, H.; Zhang, F.; Zhao, S.; Liu, Y.; Xu, Y.; Wu, R.; Li, D.; Yang, Y.; Liao, L.; et al. Antibacterial Properties of Bilayer Biomimetic Nano-ZnO for Dental Implants. ACS Biomater. Sci. Eng. 2020, 6, 1880–1886. [Google Scholar] [CrossRef]
- Holay, M.; Guo, Z.; Pihl, J.; Heo, J.; Park, J.H.; Fang, R.H.; Zhang, L. Bacteria-Inspired Nanomedicine. ACS Appl. Bio Mater. 2021, 4, 3830–3848. [Google Scholar] [CrossRef]
- An, R.; Dong, Y.; Zhu, J.; Rao, C. Adhesion and friction forces in biofouling attachments to nanotube- and PEG- patterned TiO2 surfaces. Colloids Surf. B Biointerfaces 2017, 159, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.; Reis, R.; Santos, I.; Souza, D.; Gonçalves, T.D.F.; Pereira-Da-Silva, M.; Rossi, A.; da Silva, L. In situ impedance spectroscopy study of the electrochemical corrosion of Ti and Ti–6Al–4V in simulated body fluid at 25 °C and 37 °C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Chapter 20—Bone Apposition on Nanoporous Titanium Implants. In Handbook of Nanoceramic and Nanocomposite Coatings and Materials; Makhlouf, A.S.H., Scharnweber, D., Eds.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 427–444. [Google Scholar]
- Hedayati, M.; Salehi, M.; Bagheri, R.; Panjepour, M.; Naeimi, F. Tribological and mechanical properties of amorphous and semi-crystalline PEEK/SiO2 nanocomposite coatings deposited on the plain carbon steel by electrostatic powder spray technique. Prog. Org. Coatings 2012, 74, 50–58. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Di, Y.; Yang, Y.; Yang, Y. Bio-corrosion behavior, antibacterial property and interaction with osteoblast of laser in-situ fabricated Ti–Si–Cu coatings on Ti–6Al–4V alloy. Biomed. Mater. 2021, 16, 065001. [Google Scholar] [CrossRef]
- Wood, J.; Hayles, A.; Bright, R.; Palms, D.; Vasilev, K.; Hasan, J. Nanomechanical tribological characterisation of nanostructured titanium alloy surfaces using AFM: A friction vs velocity study. Colloids Surf. B Biointerfaces 2022, 217, 112600. [Google Scholar] [CrossRef]
- Qin, W.; Ma, J.; Liang, Q.; Li, J.; Tang, B. Tribological, cytotoxicity and antibacterial properties of graphene oxide/carbon fibers/polyetheretherketone composite coatings on Ti–6Al–4V alloy as orthopedic/dental implants. J. Mech. Behav. Biomed. Mater. 2021, 122, 104659. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, Y.; Zhu, Y.; Liu, X.; Zhou, Q. NanoZnO-modified titanium implants for enhanced anti-bacterial activity, osteogenesis and corrosion resistance. J. Nanobiotechnol. 2021, 19, 353. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Belcarz, A.; Przekora, A.; Ginalska, G.; Strugała, G.; Cieślik, B.; Pałubicka, A.; Zieliński, A. The Chemical and Biological Properties of Nanohydroxyapatite Coatings with Antibacterial Nanometals, Obtained in the Electrophoretic Process on the Ti13Zr13Nb Alloy. Int. J. Mol. Sci. 2021, 22, 3172. [Google Scholar] [CrossRef]
- de Avila, E.D.; Nagay, B.E.; Pereira, M.M.; Barão, V.A.; Pavarina, A.C.; van den Beucken, J.J. Race for Applicable Antimicrobial Dental Implant Surfaces to Fight Biofilm-Related Disease: Advancing in Laboratorial Studies vs Stagnation in Clinical Application. ACS Biomater. Sci. Eng. 2022, 8, 3187–3198. [Google Scholar] [CrossRef]
- Odatsu, T.; Kuroshima, S.; Sato, M.; Takase, K.; Valanezhad, A.; Naito, M.; Sawase, T. Antibacterial Properties of Nano-Ag Coating on Healing Abutment: An In Vitro and Clinical Study. Antibiotics 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Xu, J.; Zou, L.; Luo, S.; Yao, R.; Zheng, B.; Liang, G.; Wu, D.; Li, Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Jang, Y.-S.; Jang, J.-H.; Bae, T.-S.; Lee, S.-J.; Lee, M.-H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Mei, S.; Kong, X.; Liu, X.; Gao, B.; Chen, B.; Wu, J. Long-term antibacterial activity of a composite coating on titanium for dental implant application. J. Biomater. Appl. 2021, 35, 643–654. [Google Scholar] [CrossRef]
- Shen, X.-T.; Zhang, Y.-Z.; Xiao, F.; Zhu, J.; Zheng, X.-D. Effects on cytotoxicity and antibacterial properties of the incorporations of silver nanoparticles into the surface coating of dental alloys. J. Zhejiang Univ. Sci. B 2017, 18, 615–625. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. [Google Scholar] [CrossRef]
- Ghimire, A.; Song, J. Anti-Periprosthetic Infection Strategies: From Implant Surface Topographical Engineering to Smart Drug-Releasing Coatings. ACS Appl. Mater. Interfaces 2021, 13, 20921–20937. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; LaValle, P. New Smart Antimicrobial Hydrogels, Nanomaterials, and Coatings: Earlier Action, More Specific, Better Dosing? Adv. Health Mater. 2021, 10, e2001199. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Lee, H.R.; Lee, D.; Yu, S.J.; Park, S.A.; Ko, W.-K.; Park, S.W.; Im, S.G.; Moon, J.-H.; et al. Biofunctionalized titanium with anti-fouling resistance by grafting thermo-responsive polymer brushes for the prevention of peri-implantitis. J. Mater. Chem. B 2015, 3, 5161–5165. [Google Scholar] [CrossRef]

| Factors | Methods | Favorable Results | References |
|---|---|---|---|
| Roughness | Increases the adhesive area Provides a barrier against shear forces | Low roughness | [80] |
| Hydrophilicity | Forms a hydration layer | High hydrophilicity | [76,94,95] |
| Charge | Forms electrostatic interactions | Negative charge | [100] |
| Surface free energy | Provides an attractive force | Low surface free energy | [58,82] |
| Surfaces | Characteristics | Nano-Topographies | References |
|---|---|---|---|
| Taro leaves | Anti-biofouling, hydrophobic, and self-cleaning | Microscale elliptical bumps (10–30 µm in diameter) covered by hierarchal, waxy nano-scale epicuticular crystals | [164,165] |
| Lotus leaves | Anti-biofouling, hydrophobic, and self-cleaning | Micro-scale elliptical bumps, covered by nano-scale crystals | [85,165] |
| Shark skin | Self-cleaning, anti-biofouling, hydrophobic, drag-reducing, and aerodynamic | Triangular or placoid micro-structured riblets, some of which have small grooves in the direction of water scales | [166,167,168] |
| Gecko skin | Adhesion properties, anti-wetting properties, and bactericidal ability | A periodic array of hierarchal microscale keratinous hairs, approximately 30–130 µm in length, 5 µm in diameter, and split into hundreds of nano-scale spatula 200–500 nm in diameter | [169] |
| Cicada wing | Hydrophobic and bactericidal ability | Nano-pillar diameter range of 82–148 nm, 44–177 nm pillar spacing, and 159–146 nm in height | [88,170] |
| Dragonfly wing | Hydrophobic, self-cleaning, and bactericidal ability | Irregularly shaped nanostructures between 83.3 and 195 nm | [171] |
| Butterfly wing | Anisotropic flow effects, hydrophobic, low drag, anti-biofouling, and low bacterial adhesion properties | An array of aligned scales covered by hierarchal micro-grooves, approximately 1–2 µm in diameter | [172,173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhou, M.; Zhang, L.; Wei, H. Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application. J. Funct. Biomater. 2022, 13, 169. https://doi.org/10.3390/jfb13040169
Yu J, Zhou M, Zhang L, Wei H. Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application. Journal of Functional Biomaterials. 2022; 13(4):169. https://doi.org/10.3390/jfb13040169
Chicago/Turabian StyleYu, Jingwei, Minghao Zhou, Luxuan Zhang, and Hongbo Wei. 2022. "Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application" Journal of Functional Biomaterials 13, no. 4: 169. https://doi.org/10.3390/jfb13040169
APA StyleYu, J., Zhou, M., Zhang, L., & Wei, H. (2022). Antibacterial Adhesion Strategy for Dental Titanium Implant Surfaces: From Mechanisms to Application. Journal of Functional Biomaterials, 13(4), 169. https://doi.org/10.3390/jfb13040169







