Benzyldimethyldodecyl Ammonium Chloride Doped Dental Adhesive: Impact on Core’s Properties, Biosafety, and Antibacterial/Bonding Performance after Aging
Abstract
1. Introduction
2. Materials and Methods
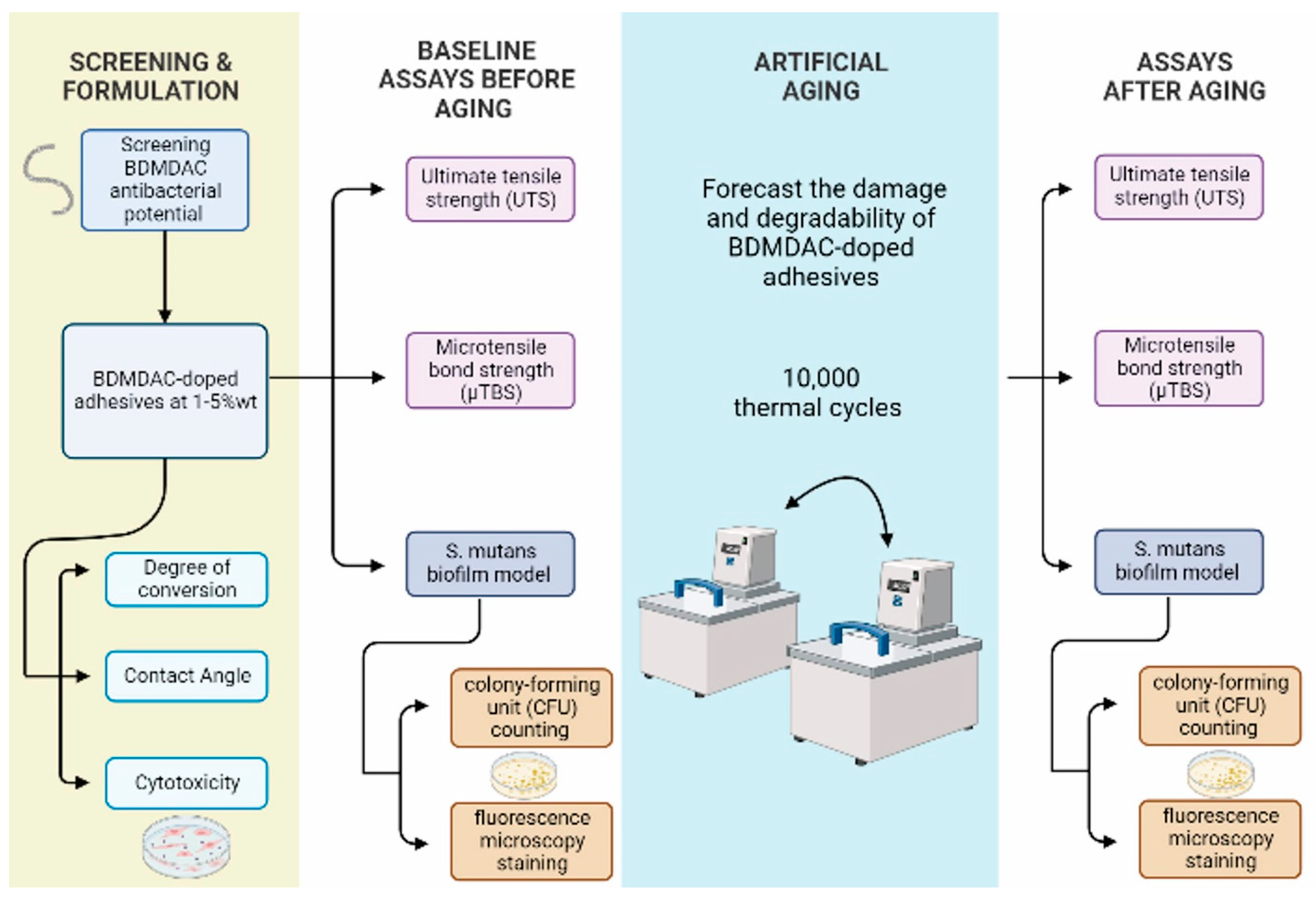
2.1. Experimental Design
2.2. Chemicals and Reagents
2.3. Determination of the Antibacterial Activity of Isolated BDMDAC Compound against S. mutans in Planktonic Cultures
2.4. Formulation of Base Dental Adhesive Resins
2.5. Degree of Conversion
2.6. Ultimate Tensile Strength (UTS)
2.7. Microtensile Bond Strength (µTBS)
2.8. Contact Angle Assays
2.9. Sample Preparation for Microbiological Assays
2.10. Streptococcus mutans Biofilm Model
2.11. Colony-Forming Unit (CFU) Counting Assay
2.12. Fluorescence Microscopy Staining
2.13. Cytotoxicity Assay Using Human Gingival Fibroblasts
2.14. Artificial Aging by Thermocycling
2.15. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Featherstone, J.D.B. Dental Caries: A Dynamic Disease Process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial Activity and Bonding Characteristics of an Adhesive Resin Containing Antibacterial Monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Spałek, J.; Ociepa, P.; Deptuła, P.; Piktel, E.; Daniluk, T.; Król, G.; Góźdź, S.; Bucki, R.; Okła, S. Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties. Int. J. Mol. Sci. 2022, 23, 2575. [Google Scholar] [CrossRef]
- de Melo, M.A.S. (Ed.) Designing Bioactive Polymeric Materials for Restorative Dentistry; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-11328-4. [Google Scholar]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717. [Google Scholar] [CrossRef]
- Garcia, I.M.; Souza, V.S.; Souza, J.D.; Visioli, F.; Leitune, V.C.B.; Scholten, J.D.; Collares, F.M. Zinc-Based Particle with Ionic Liquid as a Hybrid Filler for Dental Adhesive Resin. J. Dent. 2020, 102, 103477. [Google Scholar] [CrossRef]
- Montoya, C.; Jain, A.; Londoño, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional Dental Composite with Piezoelectric Nanofillers for Combined Antibacterial and Mineralization Effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef]
- Collares, F.M.; Garcia, I.M.; Klein, M.; Parolo, C.F.; Sánchez, F.A.L.; Takimi, A.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Leitune, V.C. Exploring Needle-Like Zinc Oxide Nanostructures for Improving Dental Resin Sealers: Design and Evaluation of Antibacterial, Physical and Chemical Properties. Polymers 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yao, S.; Gu, L.; Huang, Z.; Mai, S. Antibacterial Effect and Bond Strength of a Modified Dental Adhesive Containing the Peptide Nisin. Peptides 2018, 99, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Antibacterial Response of Oral Microcosm Biofilm to Nano-Zinc Oxide in Adhesive Resin. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef] [PubMed]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Chwała, P. Synthesis and Anti-Microbial Activities of Choline-like Quaternary Ammonium Chlorides. Eur. J. Med. Chem. 2003, 38, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Syguda, A.; Mirska, I.; Pernak, A.; Nawrot, J.; Pradzyńska, A.; Griffin, S.T.; Rogers, R.D. Choline-Derivative-Based Ionic Liquids. Chemistry 2007, 13, 6817–6827. [Google Scholar] [CrossRef]
- Sousa-Silva, M.; Simões, M.; Melo, L.; Machado, I. Pseudomonas Fluorescens Tolerance to Benzyldimethyldodecyl Ammonium Chloride: Altered Phenotype and Cross-Resistance. J. Glob. Antimicrob. Resist. 2018, 15, 188–195. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Kist, T.L.; Takimi, A.; Samuel, S.M.W.; Collares, F.M. Quantum Dots as Nonagglomerated Nanofillers for Adhesive Resins. J. Dent. Res. 2016, 95, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Magnetic Motion of Superparamagnetic Iron Oxide Nanoparticles- Loaded Dental Adhesives: Physicochemical/Biological Properties, and Dentin Bonding Performance Studied through the Tooth Pulpal Pressure Model. Acta Biomater. 2021, 134, 337–347. [Google Scholar] [CrossRef]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.B.; Samuel, S.M.W. Discrepancies in Degree of Conversion Measurements by FTIR. Braz. Oral Res. 2013, 27, 453–454. [Google Scholar] [CrossRef]
- Stürmer, M.; Garcia, I.M.; Souza, V.S.; Visioli, F.; Scholten, J.D.; Samuel, S.M.W.; Leitune, V.C.B.; Collares, F.M. Titanium Dioxide Nanotubes with Triazine-Methacrylate Monomer to Improve Physicochemical and Biological Properties of Adhesives. Dent. Mater. 2021, 37, 223–235. [Google Scholar] [CrossRef]
- Tian, F.-C.; Wang, X.-Y.; Huang, Q.; Niu, L.-N.; Mitchell, J.; Zhang, Z.-Y.; Prananik, C.; Zhang, L.; Chen, J.-H.; Breschi, L.; et al. Effect of Nanolayering of Calcium Salts of Phosphoric Acid Ester Monomers on the Durability of Resin-Dentin Bonds. Acta Biomater. 2016, 38, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymer. J. Appl. Polym. Sci. 1969, 13, 1741–1747. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.1969.070130815 (accessed on 10 March 2022). [CrossRef]
- Tani, C.; Manabe, A.; Itoh, K.; Hisamitsu, H.; Wakumoto, S. Contact Angle of Dentin Bonding Agents on the Dentin Surface. Dent. Mater. J. 1996, 15, 39–44. [Google Scholar] [CrossRef][Green Version]
- Katyal, D.; Subramanian, A.K.; Venugopal, A.; Marya, A. Assessment of Wettability and Contact Angle of Bonding Agent with Enamel Surface Etched by Five Commercially Available Etchants: An In Vitro Study. Int. J. Dent. 2021, 2021, 9457553. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ibrahim, A.S.; Balhaddad, A.A.; Weir, M.D.; Lin, N.J.; Tay, F.R.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. A Novel Dental Sealant Containing Dimethylaminohexadecyl Methacrylate Suppresses the Cariogenic Pathogenicity of Streptococcus Mutans Biofilms. Int. J. Mol. Sci. 2019, 20, 3491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.-X.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on the Antibacterial and Remineralizing Effects of a Calcium Phosphate Nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Development of a Multifunctional Adhesive System for Prevention of Root Caries and Secondary Caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef]
- Daood, D.; Yiu, C.K.Y.; Burrow, M.F.; Niu, L.-N.; Tay, F.R. Effect of a Novel Quaternary Ammonium Silane Cavity Disinfectant on Durability of Resin-Dentine Bond. J. Dent. 2017, 60, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Zhou, C.-C.; Weir, M.D.; Zhou, X.-D.; Xu, H.H.K. One-Year Water-Ageing of Calcium Phosphate Composite Containing Nano-Silver and Quaternary Ammonium to Inhibit Biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.-H.; Xing, X.-D.; Li, F.; Ma, S.; Qi, L.-L.; Chen, J.-H. Antibacterial Activity and Cytotoxicity of Two Novel Cross-Linking Antibacterial Monomers on Oral Pathogens. Arch. Oral Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Comparison of Quaternary Ammonium-Containing with Nano-Silver-Containing Adhesive in Antibacterial Properties and Cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef]
- Association for the Advancement of Medical Instrumentation. Biological Evaluation of Medical Devices, Part 12: Sample Preparation and Reference Materials; ANSI/AAMI/ISO 10993-12: 2007; AAMI: Arlington, VA, USA, 2007. [Google Scholar]
- Sigusch, B.W.; Pflaum, T.; Völpel, A.; Gretsch, K.; Hoy, S.; Watts, D.C.; Jandt, K.D. Resin-Composite Cytotoxicity Varies with Shade and Irradiance. Dent. Mater. 2012, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials Guidance on In Vitro Testing of Dental Composite Bonding Effectiveness to Dentin/Enamel Using Micro-Tensile Bond Strength (ΜTBS) Approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Baracco, B.; Fuentes, M.; Garrido, M.A.; Gonzalez-Lopez, S.; Ceballos, L. ISO/TS 11405: Dental materials-testing of adhesion to tooth structure. ISO/TS 11405: Dental materials-testing of adhesion to tooth structure, 2003. Odontology 2013, 101, 177–185. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal Cycling Procedures for Laboratory Testing of Dental Restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Daood, U.; Sauro, S.; Pichika, M.R.; Omar, H.; Liang Lin, S.; Fawzy, A.S. Novel Riboflavin/VE-TPGS Modified Universal Dentine Adhesive with Superior Dentine Bond Strength and Self-Crosslinking Potential. Dent. Mater. 2020, 36, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Omar, H.; Qasim, S.; Nogueira, L.P.; Pichika, M.R.; Mak, K.-K.; Steier, L.; Cky, Y.; Lin, S.L.; Fawzy, A.S. New Antimicrobial and Collagen Crosslinking Formulated Dentin Adhesive with Improved Bond Durability. J. Mech. Behav. Biomed. Mater. 2020, 110, 103927. [Google Scholar] [CrossRef]
- Liang, X.; Söderling, E.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Optimizing the Concentration of Quaternary Ammonium Dimethacrylate Monomer in Bis-GMA/TEGDMA Dental Resin System for Antibacterial Activity and Mechanical Properties. J. Mater. Sci. Mater. Med. 2014, 25, 1387–1393. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Simão, L.C.; Esmerino, L.A.; Gomes, O.M.M.; Gomes, J.C. Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives. Int. J. Mol. Sci. 2014, 15, 8998–9015. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of Conversion and Permeability of Dental Adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]
- Koin, P.J.; Kilislioglu, A.; Zhou, M.; Drummond, J.L.; Hanley, L. Analysis of the Degradation of a Model Dental Composite. J. Dent. Res. 2008, 87, 661–665. [Google Scholar] [CrossRef]
- Sabatini, C.; Pashley, D.H. Mechanisms Regulating the Degradation of Dentin Matrices by Endogenous Dentin Proteases and Their Role in Dental Adhesion. A Review. Am. J. Dent. 2014, 27, 203–214. [Google Scholar] [PubMed]
- Kwaśniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Mokeem, L.S.; Weir, M.D.; Xu, H.; Melo, M.A.S. Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation. Appl. Sci. 2021, 11, 3718. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Zhu, C.G.; Zhou, X.; Wang, H.; Han, Q.; Ren, B.; Cheng, L. Anti-Bacterial and Anti-Microbial Aging Effects of Resin-Based Sealant Modified by Quaternary Ammonium Monomers. J. Dent. 2021, 112, 103767. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C. Correlation of Clinical Performance with ‘In Vitro Tests’ of Restorative Dental Materials That Use Polymer-Based Matrices. Dent. Mater. 2012, 28, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Mena Silva, P.A.; Garcia, I.M.; Nunes, J.; Visioli, F.; Castelo Branco Leitune, V.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus Mutans Grown over Dental Resins: An In Vitro Study. J. Funct. Biomater. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]







| Group # | BDMDAC Concentration |
|---|---|
| Group 1 = Control | Adhesive + 0 wt.% BDMDAC |
| Group 2 | Adhesive + 1 wt.% BDMDAC |
| Group 3 | Adhesive + 3 wt.% BDMDAC |
| Group 4 | Adhesive + 4 wt.% BDMDAC |
| Group 5 | Adhesive + 5 wt.% BDMDAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokeem, L.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Melo, M.A.S. Benzyldimethyldodecyl Ammonium Chloride Doped Dental Adhesive: Impact on Core’s Properties, Biosafety, and Antibacterial/Bonding Performance after Aging. J. Funct. Biomater. 2022, 13, 190. https://doi.org/10.3390/jfb13040190
Mokeem LS, Balhaddad AA, Garcia IM, Collares FM, Melo MAS. Benzyldimethyldodecyl Ammonium Chloride Doped Dental Adhesive: Impact on Core’s Properties, Biosafety, and Antibacterial/Bonding Performance after Aging. Journal of Functional Biomaterials. 2022; 13(4):190. https://doi.org/10.3390/jfb13040190
Chicago/Turabian StyleMokeem, Lamia Sami, Abdulrahman A. Balhaddad, Isadora Martini Garcia, Fabrício Mezzomo Collares, and Mary Anne S. Melo. 2022. "Benzyldimethyldodecyl Ammonium Chloride Doped Dental Adhesive: Impact on Core’s Properties, Biosafety, and Antibacterial/Bonding Performance after Aging" Journal of Functional Biomaterials 13, no. 4: 190. https://doi.org/10.3390/jfb13040190
APA StyleMokeem, L. S., Balhaddad, A. A., Garcia, I. M., Collares, F. M., & Melo, M. A. S. (2022). Benzyldimethyldodecyl Ammonium Chloride Doped Dental Adhesive: Impact on Core’s Properties, Biosafety, and Antibacterial/Bonding Performance after Aging. Journal of Functional Biomaterials, 13(4), 190. https://doi.org/10.3390/jfb13040190








