Olive Leaf Extracts for a Green Synthesis of Silver-Functionalized Multi-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Water Extract from Olive Leaves
2.3. Preparation and Characterization of SFMWCNTs
2.3.1. Preparation of SFMWCNTs Films
2.3.2. Characterization of SFMWCNTs Films
2.4. Cell Culture
2.5. Determination of Cytotoxicity Using MTT Assay
3. Results
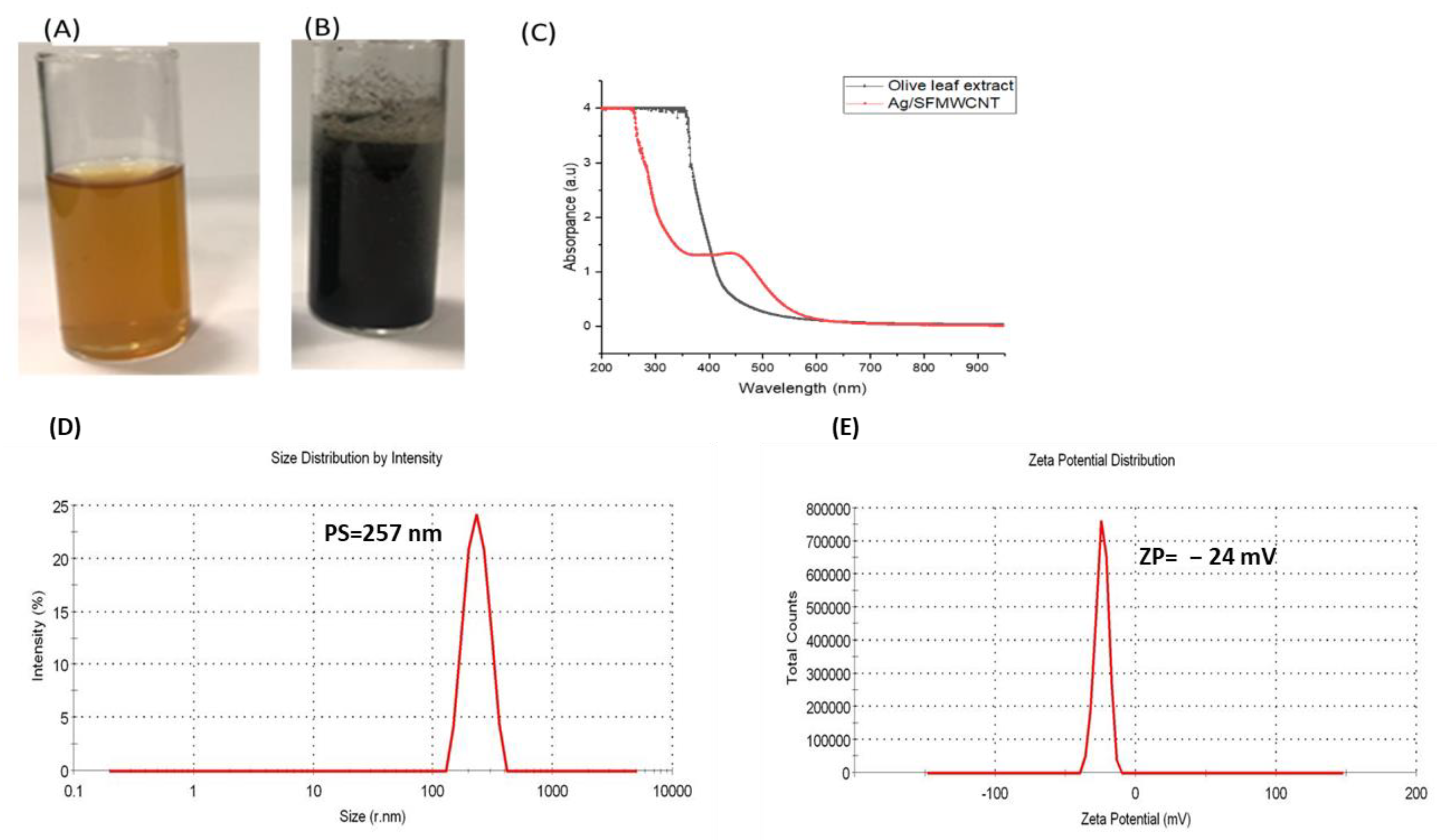
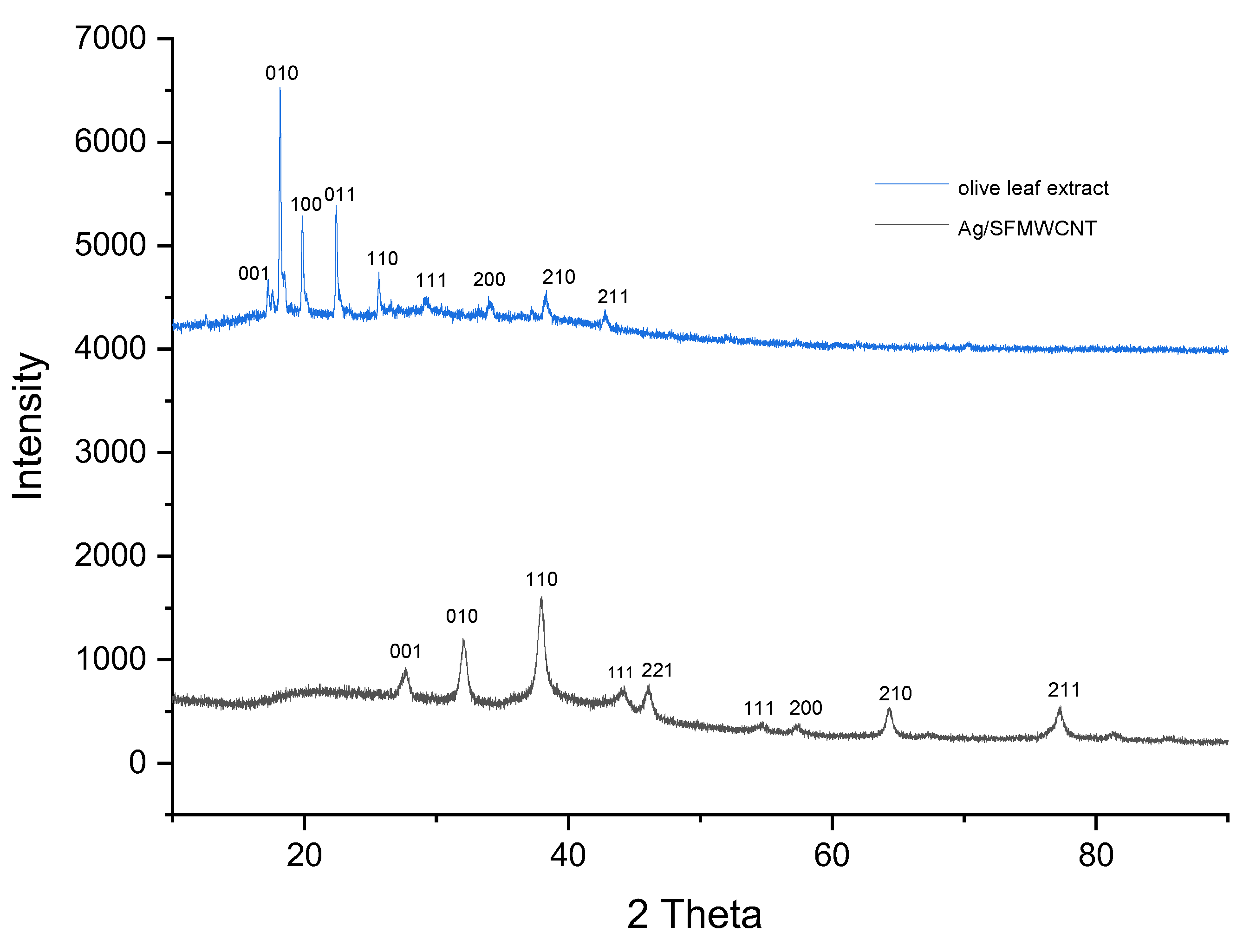
3.1. Preparation and Characterization of SFMWCNTs
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Results
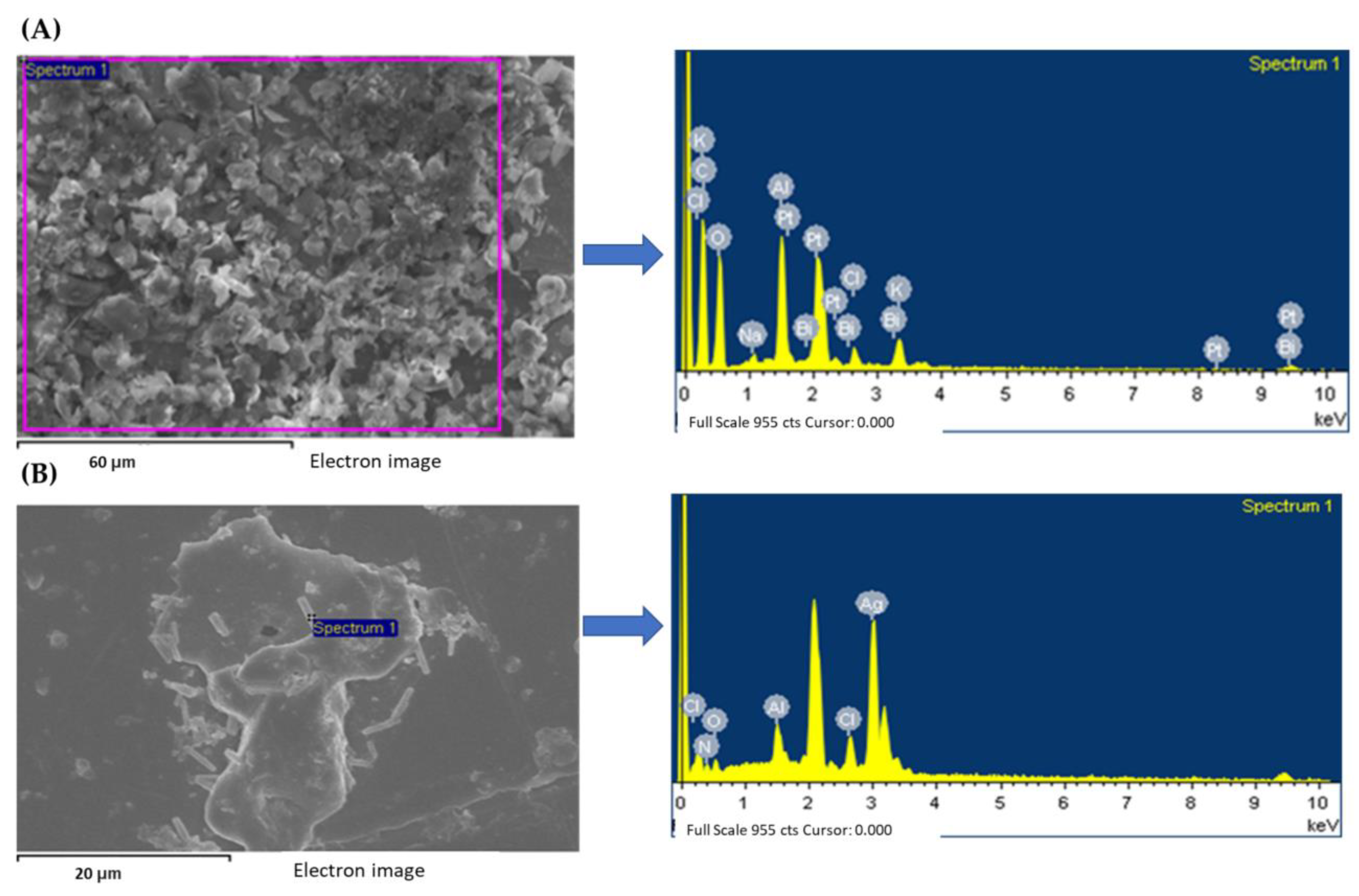
3.3. Morphological Studies: SEM and EDS Measurements
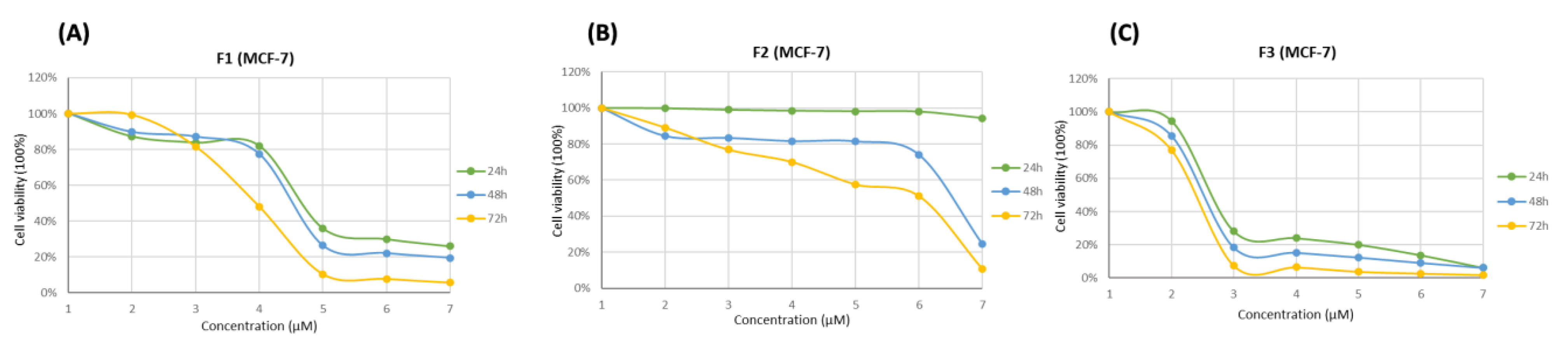
3.4. Cytotoxicity Effects of SFMWCNT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric plant-derived excipients in drug delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef] [PubMed]
- Kukovecz, A.; Kordás, K.; Kiss, J.; Kónya, Z. Atomic scale characterization and surface chemistry of metal modified titanate nanotubes and nanowires. Surf. Sci. Rep. 2016, 71, 473–546. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Mergulhão, F. Carbon Nanotube-Based Antimicrobial and Antifouling Surfaces. In Engineered Antimicrobial Surfaces; Springer: Singapore, 2020; pp. 65–93. [Google Scholar]
- Sedaghat, S. Green Biosynthesis of Silver Functionalized multi-Walled Carbon Nanotubes, using Satureja hortensis L. water extract and its bactericidal activity. J. Nanoanalysis 2017, 4, 59–64. [Google Scholar]
- Chen, L.; Xie, H.; Yu, W. Multi-walled carbon nanotube/silver nanoparticles used for thermal transportation. J. Mater. Sci. 2012, 47, 5590–5595. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Kavosi, A.; Noei, S.H.G.; Madani, S.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Mirzaei, M.; Kalhori, M.R.; Yavarian, M.; Alizadeh, A.M. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci. Rep. 2018, 8, 8375. [Google Scholar] [CrossRef]
- Kamble, R.V.; Bhinge, S.D.; Mohite, S.K.; Randive, D.S.; Bhutkar, M.A. In vitro targeting and selective killing of mcf-7 and colo320dm cells by 5-fluorouracil anchored to carboxylated SWCNTs and MWCNTs. J. Mater. Sci. Mater. Med. 2021, 32, 71. [Google Scholar] [CrossRef]
- Badea, M.A.; Balas, M.; Prodana, M.; Cojocaru, F.G.; Ionita, D.; Dinischiotu, A. Carboxyl-Functionalized Carbon Nanotubes Loaded with Cisplatin Promote the Inhibition of PI3K/Akt Pathway and Suppress the Migration of Breast Cancer Cells. Pharmaceutics 2022, 14, 469. [Google Scholar] [CrossRef]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: Carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963. [Google Scholar]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Doughty, A.; West, C.; Wang, L.; Zhou, F.; Nordquist, R.E.; Chen, W.R. Phototherapy using immunologically modified carbon nanotubes to potentiate checkpoint blockade for metastatic breast cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Wahab, R.; Ahmad, J.; Farshori, N.N.; Musarrat, J.; Al-Khedhairy, A.A. Evaluation of cytotoxic responses of raw and functionalized multi-walled carbon nanotubes in human breast cancer (MCF-7) cells. Vacuum 2017, 146, 578–585. [Google Scholar] [CrossRef]
- Singhai, N.J.; Maheshwari, R.; Ramteke, S. CD44 receptor targeted ‘smart’ multi-walled carbon nanotubes for synergistic therapy of triple-negative breast cancer. Colloid Interface Sci. Commun. 2020, 35, 100235. [Google Scholar] [CrossRef]
- Tajabadi, M. Application of carbon nanotubes in breast cancer therapy. Drug Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Saliev, T. The advances in biomedical applications of carbon nanotubes. C 2019, 5, 29. [Google Scholar] [CrossRef]
- Singhai, N.J.; Ramteke, S. Functionalized carbon nanotubes: Emerging applications in the diverse biomedical arena. Curr. Nanosci. 2020, 16, 170–186. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnology 2021, 19, 423. [Google Scholar] [CrossRef]
- Al-Ruqaie, I.; Al-Khalifah, N.; Shanavaskhan, A. Morphological cladistic analysis of eight popular Olive (Olea europaea L.) cultivars grown in Saudi Arabia using Numerical Taxonomic System for personal computer to detect phyletic relationship and their proximate fruit composition. Saudi J. Biol. Sci. 2016, 23, 115–121. [Google Scholar] [CrossRef]
- Tripathi, N.; Pavelyev, V.; Islam, S. Synthesis of carbon nanotubes using green plant extract as catalyst: Unconventional concept and its realization. Appl. Nanosci. 2017, 7, 557–566. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Di Stefano, M.; Poli, G.; Tuccinardi, T.; Bader, A.; Vassallo, A.; Abdallah, M.E.; El-Readi, M.Z.; Refaat, B.; Algarni, A.S. Co-Inhibition of P-gp and Hsp90 by an Isatin-Derived Compound Contributes to the Increase of the Chemosensitivity of MCF7/ADR-Resistant Cells to Doxorubicin. Molecules 2021, 27, 90. [Google Scholar] [CrossRef]
- Yasmin, S.; Nouren, S.; Bhatti, H.N.; Iqbal, D.N.; Iftikhar, S.; Majeed, J.; Mustafa, R.; Nisar, N.; Nisar, J.; Nazir, A. Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus. Green Processing Synth. 2020, 9, 87–96. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hussein, G.; Brza, M.; Mohammed, S.J.; Abdulwahid, R.T.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of interconnected plasmonic spherical silver nanoparticles with enhanced localized surface plasmon resonance (LSPR) peaks using quince leaf extract solution. Nanomaterials 2019, 9, 1557. [Google Scholar] [CrossRef]
- do Amaral Montanheiro, T.L.; Cristóvan, F.H.; Machado, J.P.B.; Tada, D.B.; Durán, N.; Lemes, A.P. Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J. Mater. Res. 2015, 30, 55–65. [Google Scholar] [CrossRef]
- Li, Y.; Gan, G.; Huang, Y.; Yu, X.; Cheng, J.; Liu, C. Ag-NPs/MWCNT composite-modified silver-epoxy paste with improved thermal conductivity. RSC Adv. 2019, 9, 20663–20669. [Google Scholar] [CrossRef] [PubMed]
- Maleszewski, A.A. The Functionalization and Characterization of Adherent Carbon Nanotubes with Silver Nanoparticles for Biological Applications. Master’s Thesis, Wright State University, Dayton, OH, USA, 2011. [Google Scholar]
- Hasnain, M.S.; Javed, M.N.; Alam, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K.; Beg, S. Purple heart plant leaves extract-mediated silver nanoparticle synthesis: Optimization by Box-Behnken design. Mater. Sci. Eng. C 2019, 99, 1105–1114. [Google Scholar] [CrossRef]
- Jabbar, A.H.; Al-Janabi, H.S.O.; Hamzah, M.; Mezan, S.; Tumah, A.; Ameruddin, A. Green Synthesis and Characterization of Silver Nanoparticle (AgNPs) using Pandanus Atrocarpus Extract. Int. J. Adv. Sci. Technol. 2020, 29, 4913–4922. [Google Scholar]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef]
- Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Pavunraj, M.; Annadurai, G. Green synthesis of silver nanoparticle and silver based chitosan bionanocomposite using stem extract of Saccharum officinarum and assessment of its antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035019. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, S336–S343. [Google Scholar] [CrossRef]
- Alhajri, H.; Alterary, S.; Alrfaei, B.M.; Al-Qahtani, W.S. Therapeutic potential evaluation of green synthesized silver nanoparticles derived from olive (Olea europaea L.) leaf extracts against breast cancer cells. J. Nanophotonics 2021, 15, 036003. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Rasaee, I.; Ghannadnia, M.; Baghshahi, S. Biosynthesis of silver nanoparticles using leaf extract of Satureja hortensis treated with NaCl and its antibacterial properties. Microporous Mesoporous Mater. 2018, 264, 240–247. [Google Scholar] [CrossRef]
- Dizaji, B.F.; Farboudi, A.; Rahbar, A.; Azarbaijan, M.H.; Asgary, M.R. The role of single-and multi-walled carbon nanotube in breast cancer treatment. Ther. Deliv. 2020, 11, 653–672. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Elgebaly, H.A.; Mosa, N.M.; Allach, M.; El-Massry, K.F.; El-Ghorab, A.H.; Al Hroob, A.M.; Mahmoud, A.M. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2018, 98, 446–453. [Google Scholar] [CrossRef]
- Erdogan, I.; Demir, M.; Bayraktar, O. Olive leaf extract as a crosslinking agent for the preparation of electrospun zein fibers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green synthesis of silver nanoparticles using leaf extracts of Clitoria ternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J. Nanosci. 2015, 2015, 928204. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Attari, K.; Amhaimar, L.; Asselman, A.; Bassou, M. The design and optimization of GaAs single solar cells using the genetic algorithm and Silvaco ATLAS. Int. J. Photoenergy 2017, 2017, 8269358. [Google Scholar] [CrossRef]
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Prabu, H.G. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Devanesan, S.; Jayamala, M.; AlSalhi, M.S.; Umamaheshwari, S.; Ranjitsingh, A.J.A. Antimicrobial and Anticancer properties of Carica papaya leaves derived di-methyl flubendazole mediated silver nanoparticles. J. Infect. Public Health 2021, 14, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Bura, C.; Mocan, T.; Grapa, C.; Mocan, L. Carbon Nanotubes-Based Assays for Cancer Detection and Screening. Pharmaceutics 2022, 14, 781. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Grasa, L.; Frontiñán-Rubio, J.; Abás, E.; Domínguez-Alfaro, A.; Mesonero, J.E.; Criado, A.; Ansón-Casaos, A. Intrinsic and selective activity of functionalized carbon nanotube/nanocellulose platforms against colon cancer cells. Colloids Surf. B Biointerfaces 2022, 212, 112363. [Google Scholar] [CrossRef] [PubMed]
- Sabanis, C.D.; Kannan, B.R.; Joga, R.; Simran; Kumar, S.; Kumar, N. Potential of novel self-assembled functionalized carbon nanotubes for selective tumor targeting. Pharm. Pat. Anal. 2022, 11, 111–117. [Google Scholar] [CrossRef]



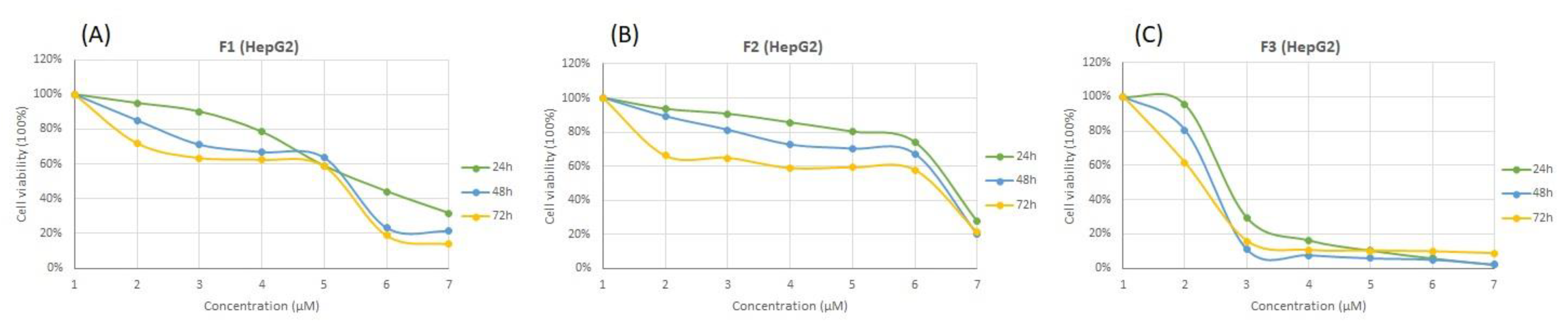
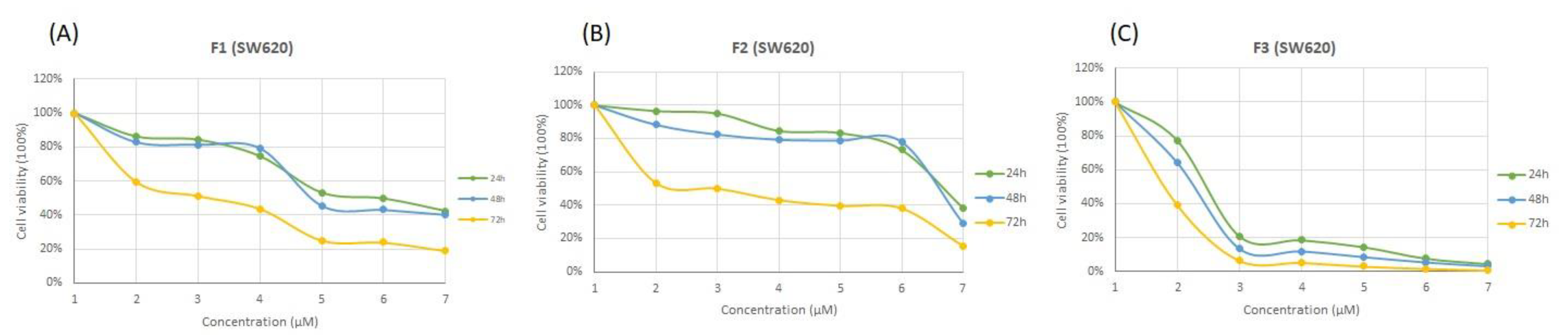
| Sample | MCF7 | HepG2 | SW620 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| F1 | 169.35 ± 19 | 151.20 ± 18 | 93.97 ± 2.29 | 261.2 ± 26 | 142.55 ± 14 | 69.49 ± 7.55 | 126.30 ± 26 | 121.25 ± 15 | 2.74 ± 0.24 |
| F2 | 1003.35 ± 126 | 1119 ± 103 | 375.10 ± 9.05 | 1091 ± 70 | 600.9 ± 15 | 54.27 ± 4.20 | 958.90 ± 162 | 922 ± 147 | 3.86 ± 0.04 |
| F3 | 15.78 ± 0.45 | 7.04 ± 2.62 | 2.94 ± 0.54 | 18.75 ± 4.87 | 4.51 ± 0.88 | 1.85 ± 0.09 | 5.80 ± 0.49 | 4.97 ± 1.31 | 0.49 ± 0.01 |
| Dox | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.63 ± 0.07 | 0.26 ± 0.02 | 0.11 ± 0.02 | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhajri, H.M.; Aloqaili, S.S.; Alterary, S.S.; Alqathama, A.; Abdalla, A.N.; Alzhrani, R.M.; Alotaibi, B.S.; Alsaab, H.O. Olive Leaf Extracts for a Green Synthesis of Silver-Functionalized Multi-Walled Carbon Nanotubes. J. Funct. Biomater. 2022, 13, 224. https://doi.org/10.3390/jfb13040224
Alhajri HM, Aloqaili SS, Alterary SS, Alqathama A, Abdalla AN, Alzhrani RM, Alotaibi BS, Alsaab HO. Olive Leaf Extracts for a Green Synthesis of Silver-Functionalized Multi-Walled Carbon Nanotubes. Journal of Functional Biomaterials. 2022; 13(4):224. https://doi.org/10.3390/jfb13040224
Chicago/Turabian StyleAlhajri, Hassna Mohammed, Sadeem Salih Aloqaili, Seham S. Alterary, Aljawharah Alqathama, Ashraf N. Abdalla, Rami M. Alzhrani, Bander S. Alotaibi, and Hashem O. Alsaab. 2022. "Olive Leaf Extracts for a Green Synthesis of Silver-Functionalized Multi-Walled Carbon Nanotubes" Journal of Functional Biomaterials 13, no. 4: 224. https://doi.org/10.3390/jfb13040224
APA StyleAlhajri, H. M., Aloqaili, S. S., Alterary, S. S., Alqathama, A., Abdalla, A. N., Alzhrani, R. M., Alotaibi, B. S., & Alsaab, H. O. (2022). Olive Leaf Extracts for a Green Synthesis of Silver-Functionalized Multi-Walled Carbon Nanotubes. Journal of Functional Biomaterials, 13(4), 224. https://doi.org/10.3390/jfb13040224









