Surface Modification of Additively Fabricated Titanium-Based Implants by Means of Bioactive Micro-Arc Oxidation Coatings for Bone Replacement
Abstract
:1. Introduction
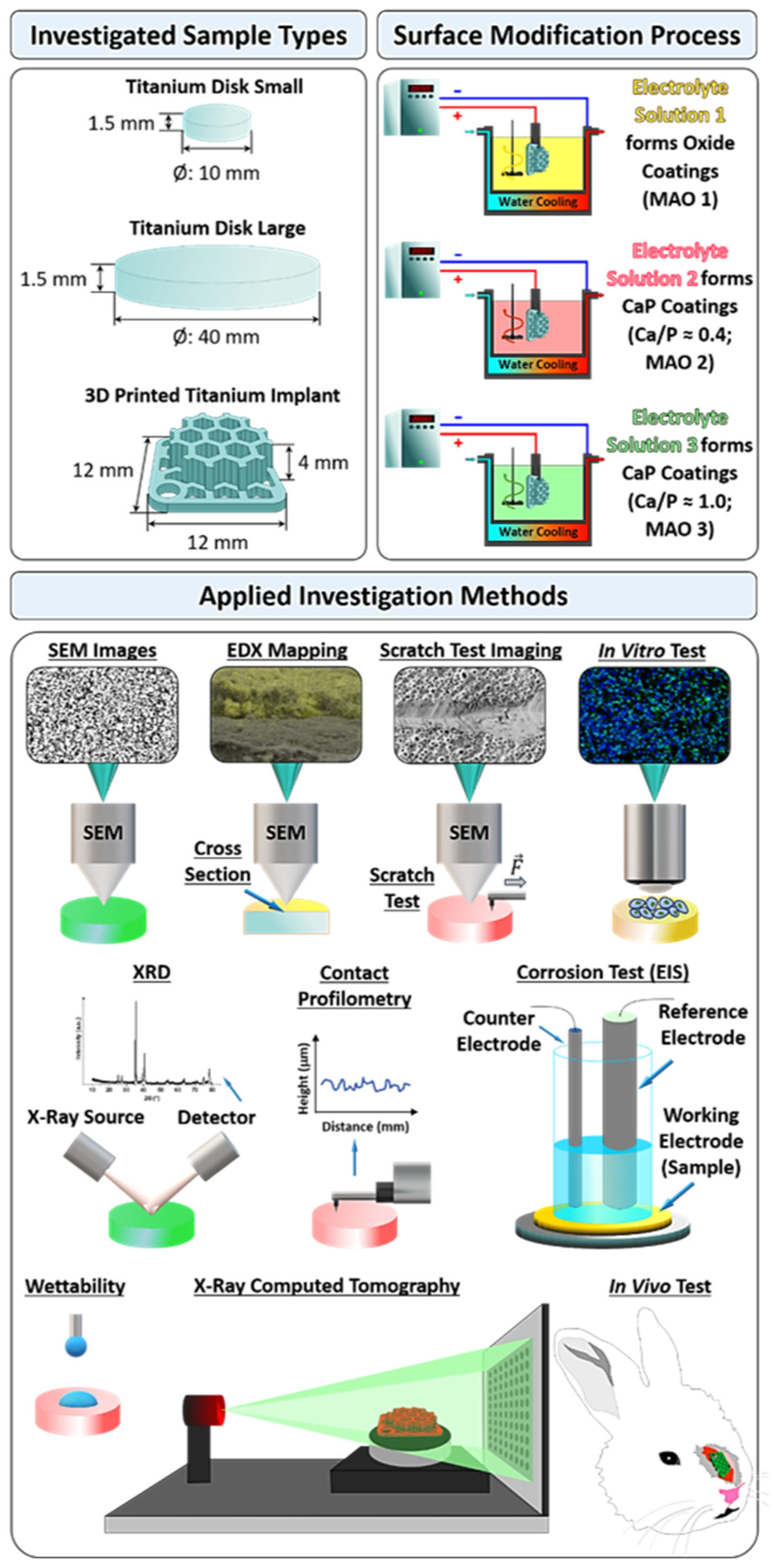
2. Materials and Methods
2.1. Coatings Fabrication
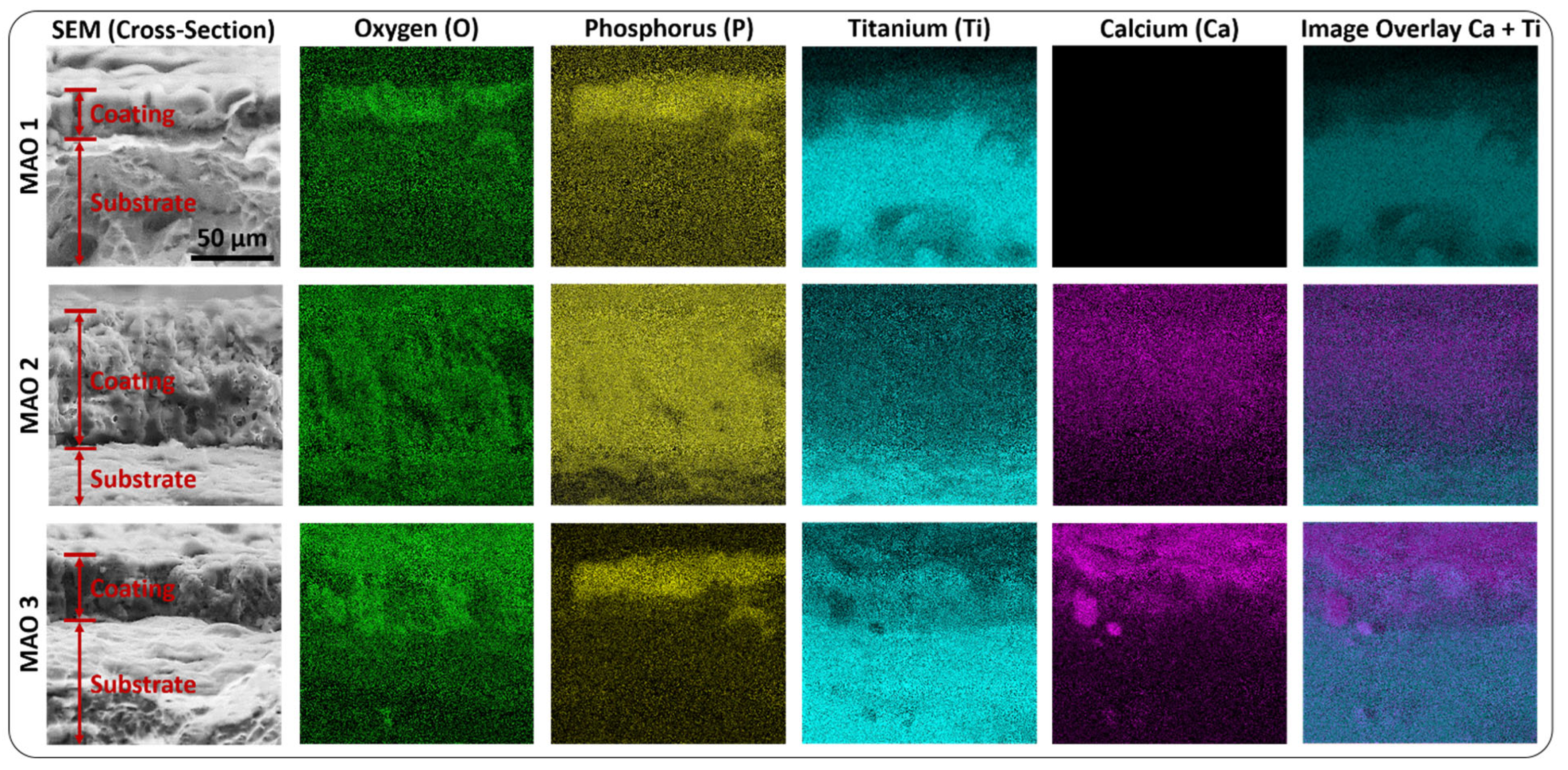
2.2. Coating Characterization
2.3. X-ray Computed Tomography (X-ray CT)
2.4. Cell Line Utilized for In-Vitro Tests
2.5. Cell Viability Test (MTT Assay)
2.6. Cell Adhesion
2.7. Alkaline Phosphatase Detection
2.8. Alizarin Red Staining
2.9. In Vivo Test
- (1)
- first group—rabbits implanted with 3D printed Ti implants surface-modified with MAO 1 coatings (control group);
- (2)
- second group—rabbits implanted with 3D printed Ti implants surface-modified with MAO 2 coatings;
- (3)
- third group—rabbits implanted with 3D printed Ti implants surface-modified with MAO 3 coatings.
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Dulimi, Z.; Wallis, M.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing technology as innovative solutions for biomedical applications. Drug Discov. Today 2021, 26, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Ni, R.; Wang, J.; Lin, X.; Fan, D.; Wei, Q.; Zhang, T.; Zheng, Y.; Cai, H.; Liu, Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.D.; Bose, S.; Bandyopadhyay, A. Additive manufacturing of titanium and titanium alloys for biomedical applications. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–343. [Google Scholar]
- Du, R.; Su, Y.-X.; Yan, Y.; Choi, W.S.; Yang, W.-F.; Zhang, C.; Chen, X.; Curtin, J.P.; Ouyang, J.; Zhang, B. A Systematic Approach for Making 3D-Printed Patient-Specific Implants for Craniomaxillofacial Reconstruction. Engineering 2020, 6, 1291–1301. [Google Scholar] [CrossRef]
- Melville, J.C.; Mañón, V.A.; Arribas, A.R.; Wong, M.E. Custom 3D printed titanium reconstruction plate with in-situ tissue engineering for the reconstruction and dental rehabilitation of a severely infected atrophic mandible. A review of technique. Dent. Rev. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Liebsch, C.; Aleinikov, V.; Kerimbayev, T.; Akshulakov, S.; Kocak, T.; Vogt, M.; Jansen, J.U.; Wilke, H.-J. In vitro comparison of personalized 3D printed versus standard expandable titanium vertebral body replacement implants in the mid-thoracic spine using entire rib cage specimens. Clin. Biomech. 2020, 78, 105070. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.G. Anatomics 3D-printed titanium implants from head to heel. In Titanium in Medical and Dental Applications; Froes, F.H., Qian, M., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 225–237. [Google Scholar]
- Wang, S.; Zhao, S.; Yu, J.; Gu, Z.; Zhang, Y. Advances in Translational 3D Printing for Cartilage, Bone, and Osteochondral Tissue Engineering. Small 2022, 18, 2201869. [Google Scholar] [CrossRef]
- Wang, L.; Rutkowski, S.; Si, T.; Hashem, T.; Guo, B.; Xu, J.; Kozelskaya, A.I.; Tverdokhlebov, S.I.; Frueh, J. Shape stabilization and laser triggered shape transformation of magnetic particle functionalized liquid metal motors. Colloid Interface Sci. Commun. 2022, 47, 100600. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Aufa, A.N.; Hassan, M.Z.; Ismail, Z. Recent advances in Ti-6Al-4V additively manufactured by selective laser melting for biomedical implants: Prospect development. J. Alloys Compd. 2022, 896, 163072. [Google Scholar] [CrossRef]
- Myakinin, A.; Turlybekuly, A.; Pogrebnjak, A.; Mirek, A.; Bechelany, M.; Liubchak, I.; Oleshko, O.; Husak, Y.; Korniienko, V.; Leśniak-Ziółkowska, K.; et al. In vitro evaluation of electrochemically bioactivated Ti6Al4V 3D porous scaffolds. Mater. Sci. Eng. C 2021, 121, 111870. [Google Scholar] [CrossRef]
- Singh, V.; Katyal, P.; Kumar, K.; Kumar, R. Surface Integrity and Biological Response of Ti-Alloy Implants after Surface Modification. Mater. Today Proc. 2021, 56, 2451–2468. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, Y.; Ma, Y.; Liu, Z.; Sun, H.; Zhou, X.; Kang, R.; Cristino, V.A.M.; Wang, Q. Surface treatment of 3D printed Cu-bearing Ti alloy scaffolds for application in tissue engineering. Mater. Des. 2022, 213, 110350. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, D.; Zhou, R.; Zhang, Y. 3D X-ray micro-computed tomography imaging for the microarchitecture evaluation of porous metallic implants and scaffolds. Micron 2021, 142, 102994. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C 2021, 118, 111505. [Google Scholar] [CrossRef]
- Qi, D.; Hu, W.; Xin, K.; Zeng, Q.; Xi, L.; Tao, R.; Liao, H.; Deng, Y.; Liao, B.; Wu, W. In-situ synchrotron X-ray tomography investigation of micro lattice manufactured with the projection micro-stereolithography (PμSL) 3D printing technique: Defects characterization and in-situ shear test. Compos. Struct. 2020, 252, 112710. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Rutkowski, S.; Gogolev, A.S.; Chistyakov, S.G.; Krasovsky, I.B.; Zheravin, A.A.; Tverdokhlebov, S.I. Bioactive coatings on 3D printed titanium implants with a complex internal structure for bone replacement. J. Phys. Conf. Ser. 2021, 2144, 12015. [Google Scholar] [CrossRef]
- du Plessis, A.; Yadroitsev, I.; Yadroitsava, I.; Le Roux, S.G. X-Ray Microcomputed Tomography in Additive Manufacturing: A Review of the Current Technology and Applications. 3D Print. Addit. Manuf. 2018, 5, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Zanini, F.; Pagani, L.; Savio, E.; Carmignato, S. Characterisation of additively manufactured metal surfaces by means of X-ray computed tomography and generalised surface texture parameters. CIRP Ann. 2019, 68, 515–518. [Google Scholar] [CrossRef]
- Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Lo Vecchio, G.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Tolkacheva, T.V.; Uvarkin, P.V.; Chebodaeva, V.V.; Prosolov, K.A.; Bakina, O.V.; Kashin, A.D.; Shcheglova, N.A.; Panchenko, A.A.; et al. Additively manufactured porous titanium 3D–scaffolds with antibacterial Zn-, Ag- calcium phosphate biocoatings. Mater. Charact. 2022, 186, 111782. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cheng, Y.; Zhang, K.; Song, C.; Leng, H.; Zheng, Y.; Cai, H.; et al. Tailored Surface Treatment of 3D Printed Porous Ti6Al4V by Microarc Oxidation for Enhanced Osseointegration via Optimized Bone In-Growth Patterns and Interlocked Bone/Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 17964–17975. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-M.; Kim, H.-J.; Ahn, S.-G.; Choe, H.-C. Plasma electrolytic oxidation of Ti-6Al-4V alloy in electrolytes containing bone formation ions. Appl. Surf. Sci. 2020, 513, 145776. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.-C. Triggering the hydroxyapatite deposition on the surface of PEO-coated Ti–6Al–4V alloy via the dual incorporation of Zn and Mg ions. J. Alloy. Compd. 2020, 819, 153038. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Muhaffel, F.; Kaba, M.; Cempura, G.; Derin, B.; Kruk, A.; Atar, E.; Cimenoglu, H. Influence of alumina and zirconia incorporations on the structure and wear resistance of titania-based MAO coatings. Surf. Coat. Technol. 2019, 377, 124900. [Google Scholar] [CrossRef]
- Jamali, R.; Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M.; Kolahi, A. Effects of co-incorporated ternary elements on biocorrosion stability, antibacterial efficacy, and cytotoxicity of plasma electrolytic oxidized titanium for implant dentistry. Mater. Chem. Phys. 2022, 276, 125436. [Google Scholar] [CrossRef]
- Jin, S.; Ma, X.; Wu, R.; Wang, G.; Zhang, J.; Krit, B.; Betsofen, S.; Liu, B. Advances in micro-arc oxidation coatings on Mg-Li alloys. Appl. Surf. Sci. Adv. 2022, 8, 100219. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Munir, K.; Wen, C. Calcium Phosphate-Based Composite Coating by Micro-Arc Oxidation (MAO) for Biomedical Application: A Review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 392–416. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, O.; Gan, Y.; Li, Q.; Zhou, T.; Xi, W. Effect of titanium implants with coatings of different pore sizes on adhesion and osteogenic differentiation of BMSCs. Artif. Cells Nanomed. Biotechnol. 2019, 47, 290–299. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Student, S.; Brzychczy-Włoch, M.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W. Physico-chemical and biological evaluation of doxycycline loaded into hybrid oxide-polymer layer on Ti–Mo alloy. Bioact. Mater. 2020, 5, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mora Sánchez, H.; Moreno, L.; Arrabal, R.; Mohedano, M.; Gallardo, A.; Rodríguez-Hernández, J.; Matykina, E. Hybrid functionalized coatings on Metallic Biomaterials for Tissue Engineering. Surf. Coat. Technol. 2021, 422, 127508. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Y.; Gao, B.; Liu, X.; Zhao, X.; Sun, W.; Ren, H. In vivo evaluation of an antibacterial coating containing halogenated furanone compound-loaded poly(L-lactic acid) nanoparticles on microarc-oxidized titanium implants. Int. J. Nanomed. 2016, 2016, 1337–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Liu, X.; Wang, Y.; Zhang, Q.; Li, D.; Liu, X.; Su, H.; Zhang, Y.; Yu, S.; Shen, D. Role of the electrolyte composition in establishing plasma discharges and coating growth process during a micro-arc oxidation. Surf. Coat. Technol. 2020, 402, 126349. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Feng, R.; Cui, H.; Gong, B.; Zhang, L.; Gao, Z.; Cui, X.; Zhang, H.; Jia, Z. Effect of electrolyte composition on the microstructure and bio-corrosion behavior of micro-arc oxidized coatings on biomedical Ti6Al4V alloy. J. Mater. Res. Technol. 2020, 9, 1477–1490. [Google Scholar] [CrossRef]
- Tverdokhlebov, S.I.; Bolbasov, E.N.; Soldatova, E.A.; Markov, A.B.; Myagkov, A.S.; Petrov, V.I.; Makeev, V.A. Impul’snyy Generator [The Pulse Generator]. RF Patent 2718420, 2019. [Google Scholar]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Egorkin, V.S.; Ustinov, A.Y.; Sergienko, V.I.; Gnedenkov, S.V. The detailed corrosion performance of bioresorbable Mg-0.8Ca alloy in physiological solutions. J. Magnes. Alloy. 2022, 10, 1326–1350. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Shi, Z.; Atrens, A. An innovative specimen configuration for the study of Mg corrosion. Corros. Sci. 2011, 53, 226–246. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Hofstetter, J.; Uggowitzer, P.J.; Song, G.; Liu, M.; Atrens, A. Corrosion of ultra-high-purity Mg in 3.5% NaCl solution saturated with Mg(OH)2. Corros. Sci. 2013, 75, 78–99. [Google Scholar] [CrossRef]
- ASTM G59-97(2009); Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM: West Conshohocken, PA, USA, 2009; In Annual Book of ASTM Standards. Volume 97, 4p.
- Microfocus X-ray Source L9181-02. Available online: https://www.hamamatsu.com/eu/en/product/type/L9181-02/index.html (accessed on 13 June 2022).
- Dynamic High Resolution X-ray Flat Panels Prodis.Mark (CMOS)-PRODIS.NDT. Available online: https://prodis-tech.ru/en/dynamic-high-resolution-x-ray-flat-panels-prodis-mark/ (accessed on 2 October 2022).
- VGSTUDIO MAX. Available online: https://www.volumegraphics.com/en/products/vgsm.html (accessed on 2 October 2022).
- Francis, M.P.; Sachs, P.C.; Elmore, L.W.; Holt, S.E. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 2010, 6, 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Kang, T.-Y.; Shin, H.; Kim, K.-M.; Hong, M.-H.; Kwon, J.-S. Osteogenic Properties of Novel Methylsulfonylmethane-Coated Hydroxyapatite Scaffold. Int. J. Mol. Sci. 2020, 21, 8501. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xia, Y.; Liu, L.; Si, N. Preparation and performance of MAO coatings obtained on AZ91D Mg alloy under unipolar and bipolar modes in a novel dual electrolyte. Ceram. Int. 2014, 40, 93–99. [Google Scholar] [CrossRef]
- Lou, B.-S.; Yen, C.-A.; Chen, Y.-Y.; Lee, J.-W. Effects of processing parameters on the adhesion and corrosion resistance of oxide coatings grown by plasma electrolytic oxidation on AZ31 magnesium alloys. J. Mater. Res. Technol. 2021, 10, 1355–1371. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Babaei, K. Effect of particles addition to solution of plasma electrolytic oxidation (PEO) on the properties of PEO coatings formed on magnesium and its alloys: A review. J. Magnes. Alloy. 2020, 8, 799–818. [Google Scholar] [CrossRef]
- Liu, W.; Pu, Y.; Liao, H.; Lin, Y.; He, W. Corrosion and Wear Behavior of PEO Coatings on D16T Aluminum Alloy with Different Concentrations of Graphene. Coatings 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef]
- Pede, D.; Li, M.; Virovac, L.; Poleske, T.; Balle, F.; Müller, C.; Mozaffari-Jovein, H. Microstructure and corrosion resistance of novel β-type titanium alloys manufactured by selective laser melting. J. Mater. Res. Technol. 2022, 19, 4598–4612. [Google Scholar] [CrossRef]
- Yoneda, Y. Anomalous Surface Reflection of X Rays. Phys. Rev. 1963, 131, 2010–2013. [Google Scholar] [CrossRef]
- Dietrich, S.; Haase, A. Scattering of X-rays and neutrons at interfaces. Phys. Rep. 1995, 260, 1–138. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Han, R.; Ren, J.; Li, L.; Han, N.; Xing, F.; Zhu, J. A review on the mechanical properties for thin film and block structure characterised by using nanoscratch test. Nanotechnol. Rev. 2019, 8, 628–644. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Jiang, B.L.; Wang, Y.M. Plasma electrolytic oxidation treatment of aluminium and titanium alloys. In Surface Engineering of Light Alloys; Dong, H., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 110–154. [Google Scholar]
- Sun, Z.; Khlusov, I.A.; Evdokimov, K.E.; Konishchev, M.E.; Kuzmin, O.S.; Khaziakhmatova, O.G.; Malashchenko, V.V.; Litvinova, L.S.; Rutkowski, S.; Frueh, J.; et al. Nitrogen-doped titanium dioxide films fabricated via magnetron sputtering for vascular stent biocompatibility improvement. J. Colloid Interface Sci. 2022, 626, 101–112. [Google Scholar] [CrossRef]
- O’Grady, S.; Morgan, M.P. Deposition of calcium in an in vitro model of human breast tumour calcification reveals functional role for ALP activity, altered expression of osteogenic genes and dysregulation of the TRPM7 ion channel. Sci. Rep. 2019, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Gai, M.; Frueh, J.; Tao, T.; Petrov, A.V.; Petrov, V.V.; Shesterikov, E.V.; Tverdokhlebov, S.I.; Sukhorukov, G.B. Polylactic acid nano- and microchamber arrays for encapsulation of small hydrophilic molecules featuring drug release via high intensity focused ultrasound. Nanoscale 2017, 9, 7063–7070. [Google Scholar] [CrossRef] [Green Version]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Schurov, I.; Sedykh, N.; Goriainov, S.; Karamyan, A.; Mordovina, E.A.; Inozemtseva, O.A.; Kudryavtseva, V.; Shchesnyak, L.E.; et al. Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics 2021, 13, 1437. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Kudryavtseva, V.L.; Mao, R.; Kiryukhin, M.V.; Sukhorukov, G.B. Patterned Microstructure Fabrication: Polyelectrolyte Complexes vs Polyelectrolyte Multilayers. Sci. Rep. 2016, 6, 37000. [Google Scholar] [CrossRef]
- Santiago-Medina, P.; Sundaram, P.A.; Diffoot-Carlo, N. Titanium Oxide: A Bioactive Factor in Osteoblast Differentiation. Int. J. Dent. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, D.; Wu, C.; Deng, S.; Zhang, Y.; Guan, G. The Anatase Phase of Nanotopography Titania with Higher Roughness Has Better Biocompatibility in Osteoblast Cell Morphology and Proliferation. Biomed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Lin, H.-I.; Chung, C.-J.; Tang, C.-H.; He, J.-L. Osseointegrating and Phase-Oriented Micro-Arc-Oxidized Titanium Dioxide Bone Implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 228080002110068. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Nishiwaki, N.; Ueda, K.; Narushima, T.; Kawamura, H.; Takahashi, T. Evaluation of Thin Amorphous Calcium Phosphate Coatings on Titanium Dental Implants Deposited Using Magnetron Sputtering. Implant Dent. 2014, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Nakamura, T.; Kokubo, T.; Tanahashi, M.; Ogawa, M. Differences of Bone Bonding Ability and Degradation Behaviour in Vivo between Amorphous Calcium Phosphate and Highly Crystalline Hydroxyapatite Coating. Biomaterials 1996, 17, 1771–1777. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozelskaya, A.I.; Rutkowski, S.; Frueh, J.; Gogolev, A.S.; Chistyakov, S.G.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Frueh, A.; Egorkin, V.S.; Choynzonov, E.L.; et al. Surface Modification of Additively Fabricated Titanium-Based Implants by Means of Bioactive Micro-Arc Oxidation Coatings for Bone Replacement. J. Funct. Biomater. 2022, 13, 285. https://doi.org/10.3390/jfb13040285
Kozelskaya AI, Rutkowski S, Frueh J, Gogolev AS, Chistyakov SG, Gnedenkov SV, Sinebryukhov SL, Frueh A, Egorkin VS, Choynzonov EL, et al. Surface Modification of Additively Fabricated Titanium-Based Implants by Means of Bioactive Micro-Arc Oxidation Coatings for Bone Replacement. Journal of Functional Biomaterials. 2022; 13(4):285. https://doi.org/10.3390/jfb13040285
Chicago/Turabian StyleKozelskaya, Anna I., Sven Rutkowski, Johannes Frueh, Aleksey S. Gogolev, Sergei G. Chistyakov, Sergey V. Gnedenkov, Sergey L. Sinebryukhov, Andreas Frueh, Vladimir S. Egorkin, Evgeny L. Choynzonov, and et al. 2022. "Surface Modification of Additively Fabricated Titanium-Based Implants by Means of Bioactive Micro-Arc Oxidation Coatings for Bone Replacement" Journal of Functional Biomaterials 13, no. 4: 285. https://doi.org/10.3390/jfb13040285
APA StyleKozelskaya, A. I., Rutkowski, S., Frueh, J., Gogolev, A. S., Chistyakov, S. G., Gnedenkov, S. V., Sinebryukhov, S. L., Frueh, A., Egorkin, V. S., Choynzonov, E. L., Buldakov, M., Kulbakin, D. E., Bolbasov, E. N., Gryaznov, A. P., Verzunova, K. N., Apostolova, M. D., & Tverdokhlebov, S. I. (2022). Surface Modification of Additively Fabricated Titanium-Based Implants by Means of Bioactive Micro-Arc Oxidation Coatings for Bone Replacement. Journal of Functional Biomaterials, 13(4), 285. https://doi.org/10.3390/jfb13040285










