Heparin-Loaded Composite Coatings on Porous Stent from Pure Magnesium for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cathodic Polarization Examinations and Accumulation
2.3. Coating Characterization
2.4. Degradation Evaluation
2.5. Drug Loading and Release
2.6. Cell Tests
2.7. Statistical Analysis
3. Results
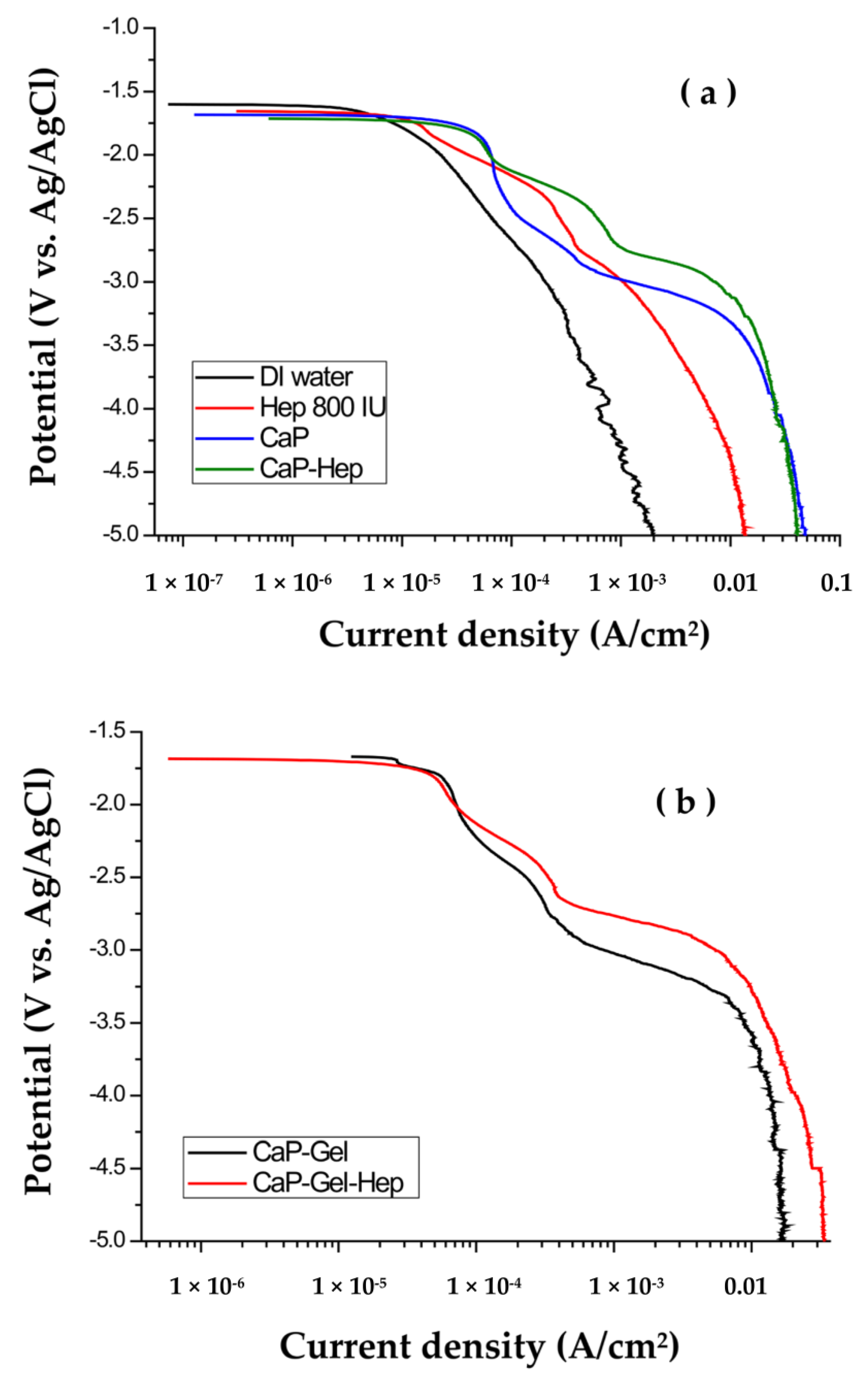
3.1. Polarization
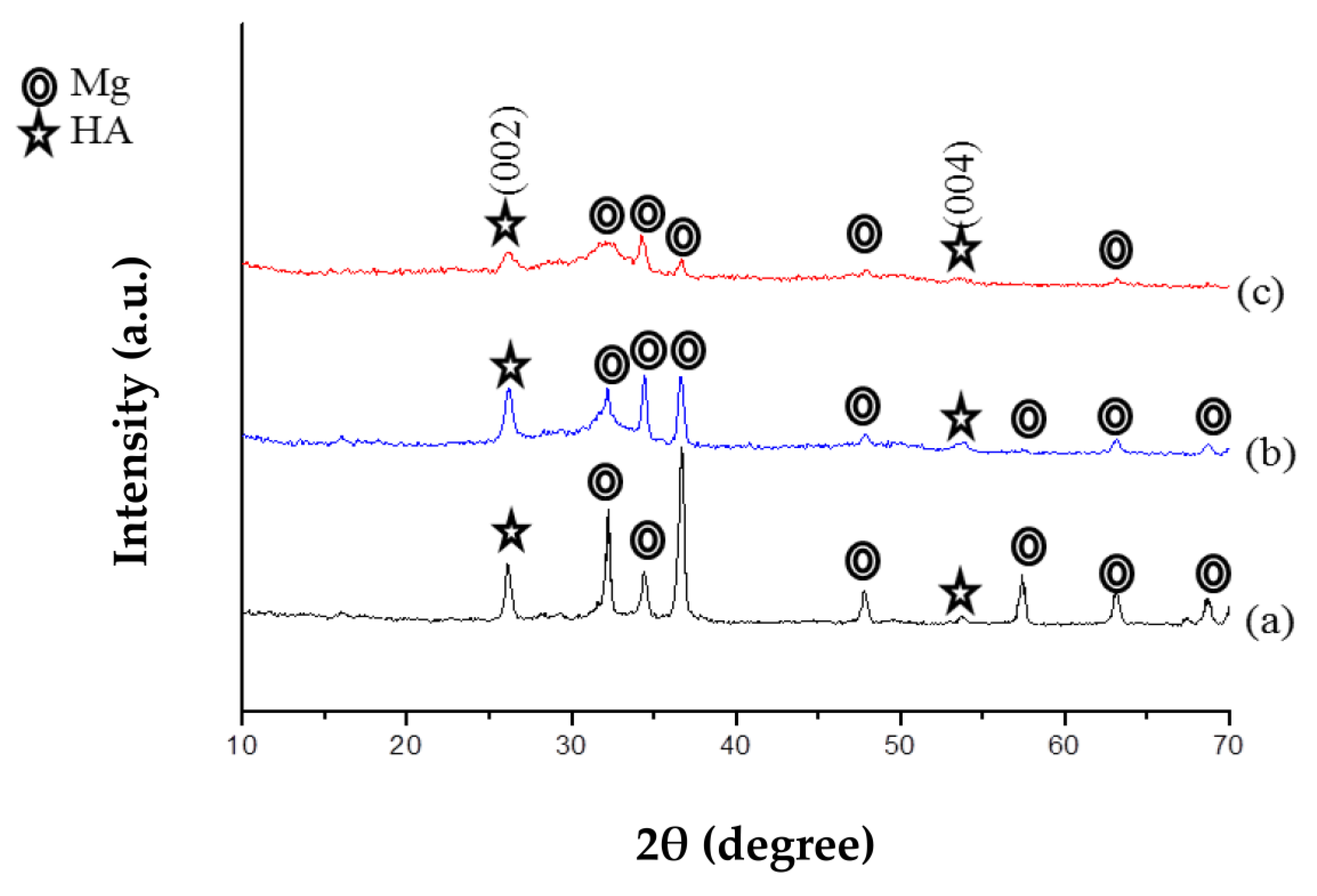
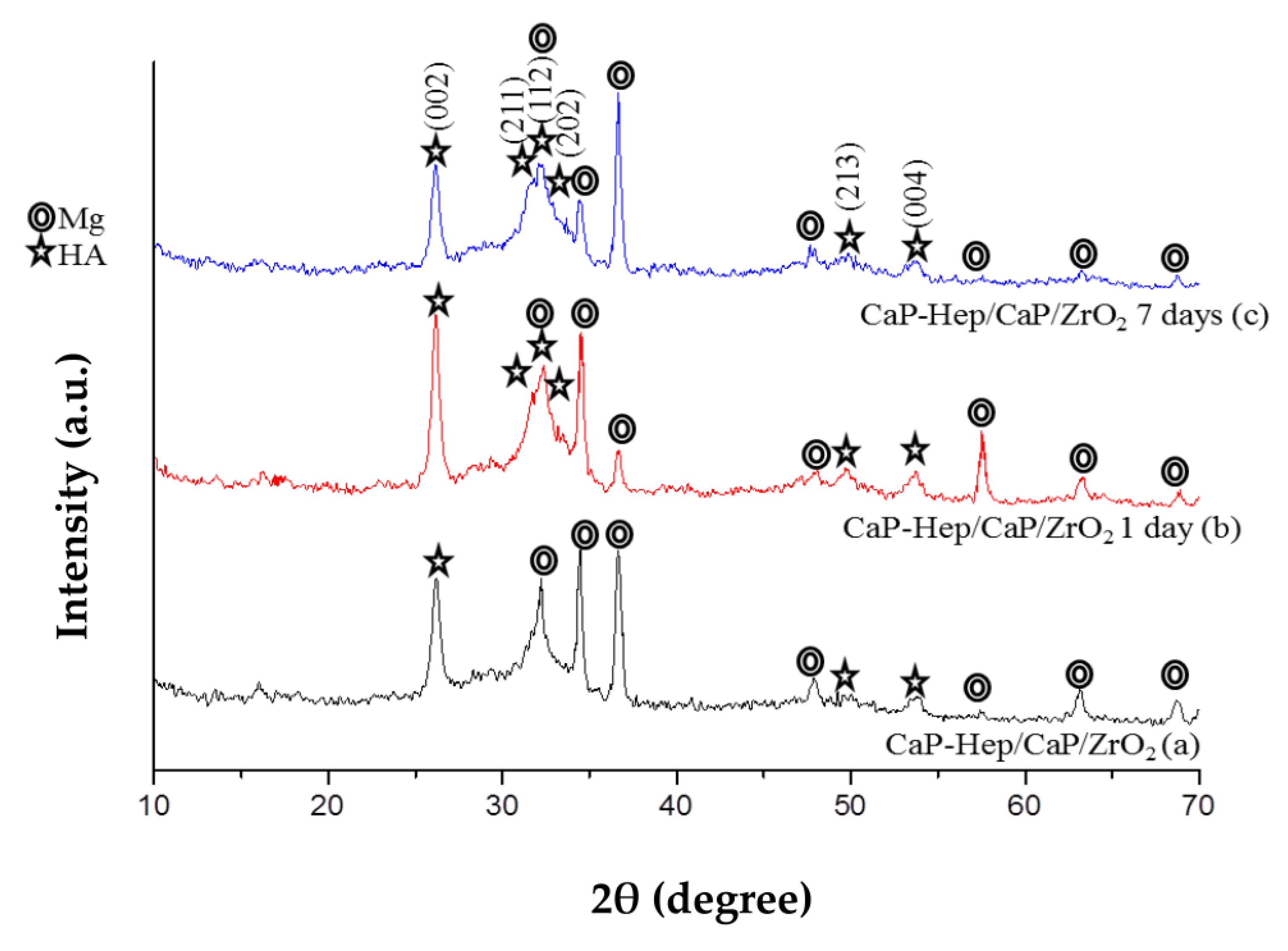
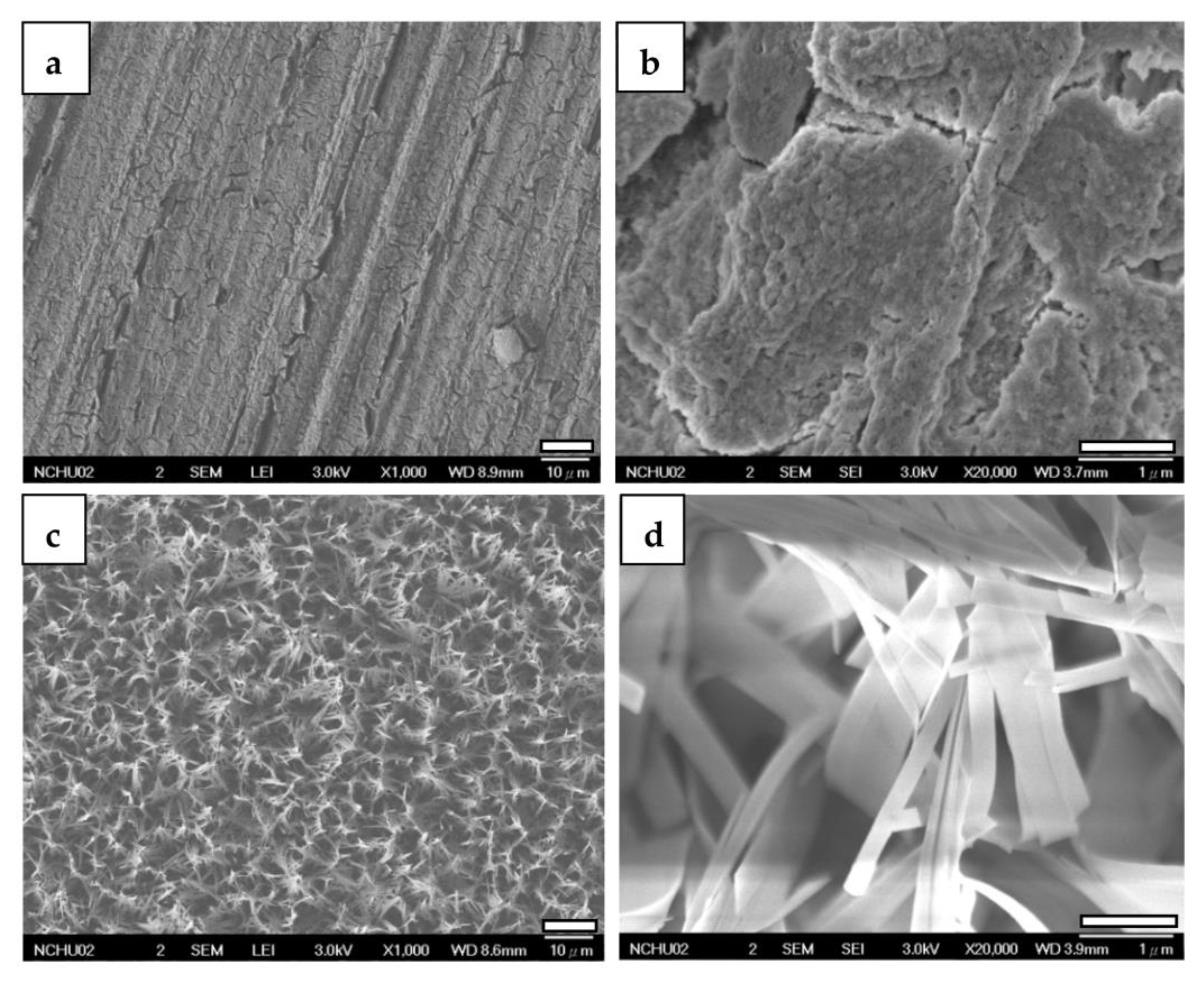
3.2. Characterization of the Coatings
3.2.1. X-ray Diffraction
3.2.2. FESEM
3.2.3. Cross-Section Observations by Focused Ion Beam (FIB) System
3.3. Corrosion Evaluation
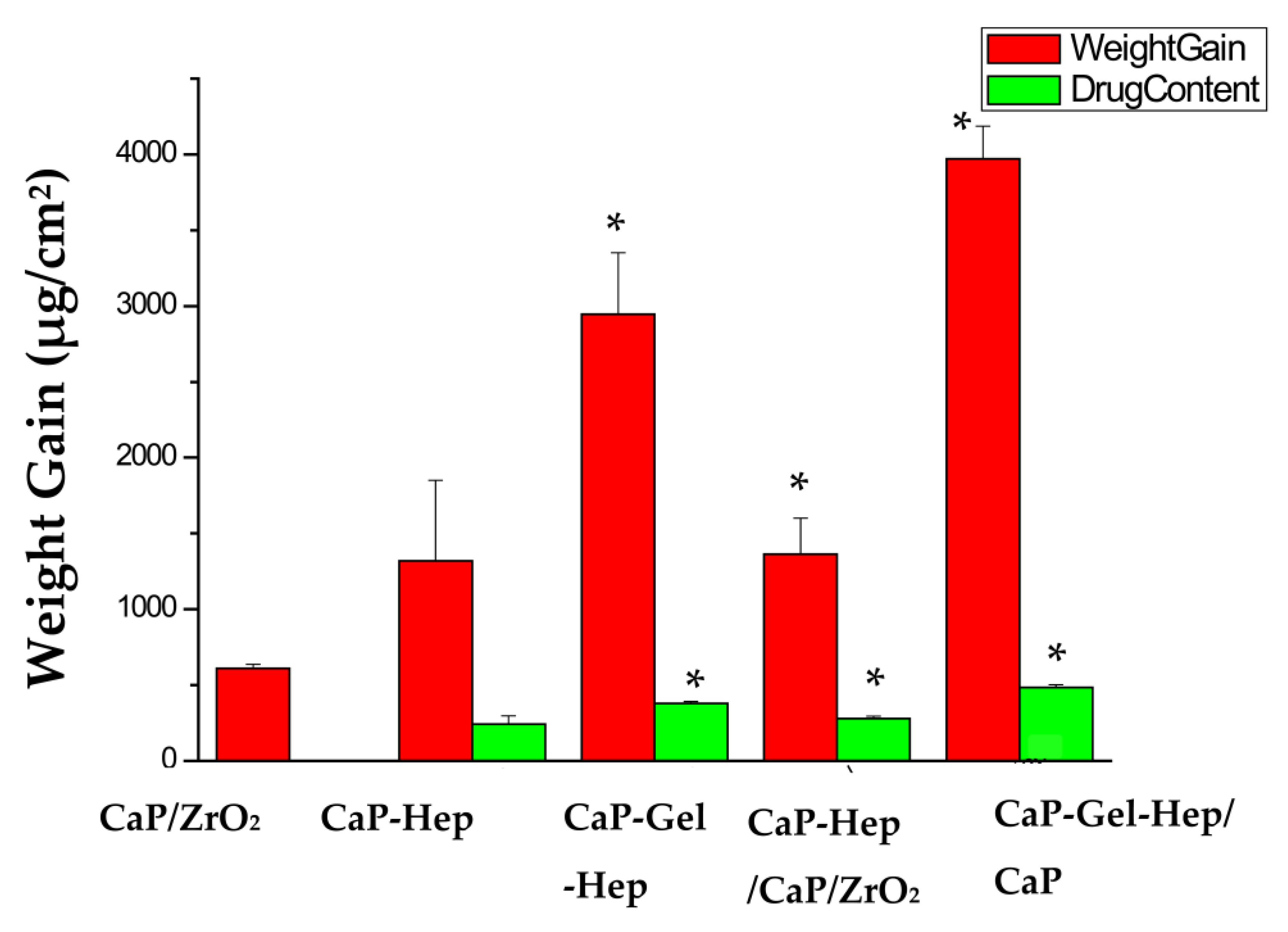
3.4. Weight Increase and Medication Encapsulation
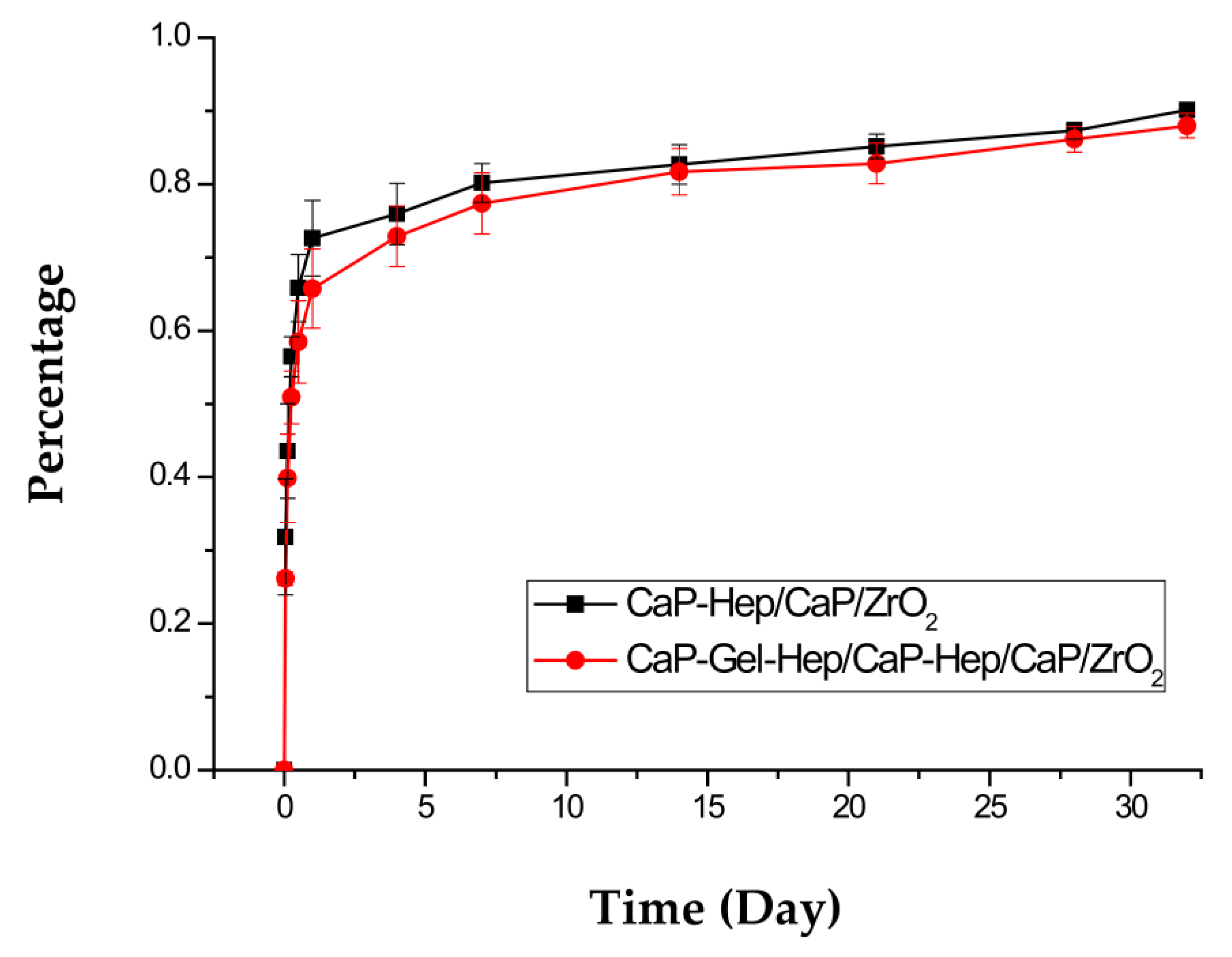
3.5. Heparin Release In Vitro
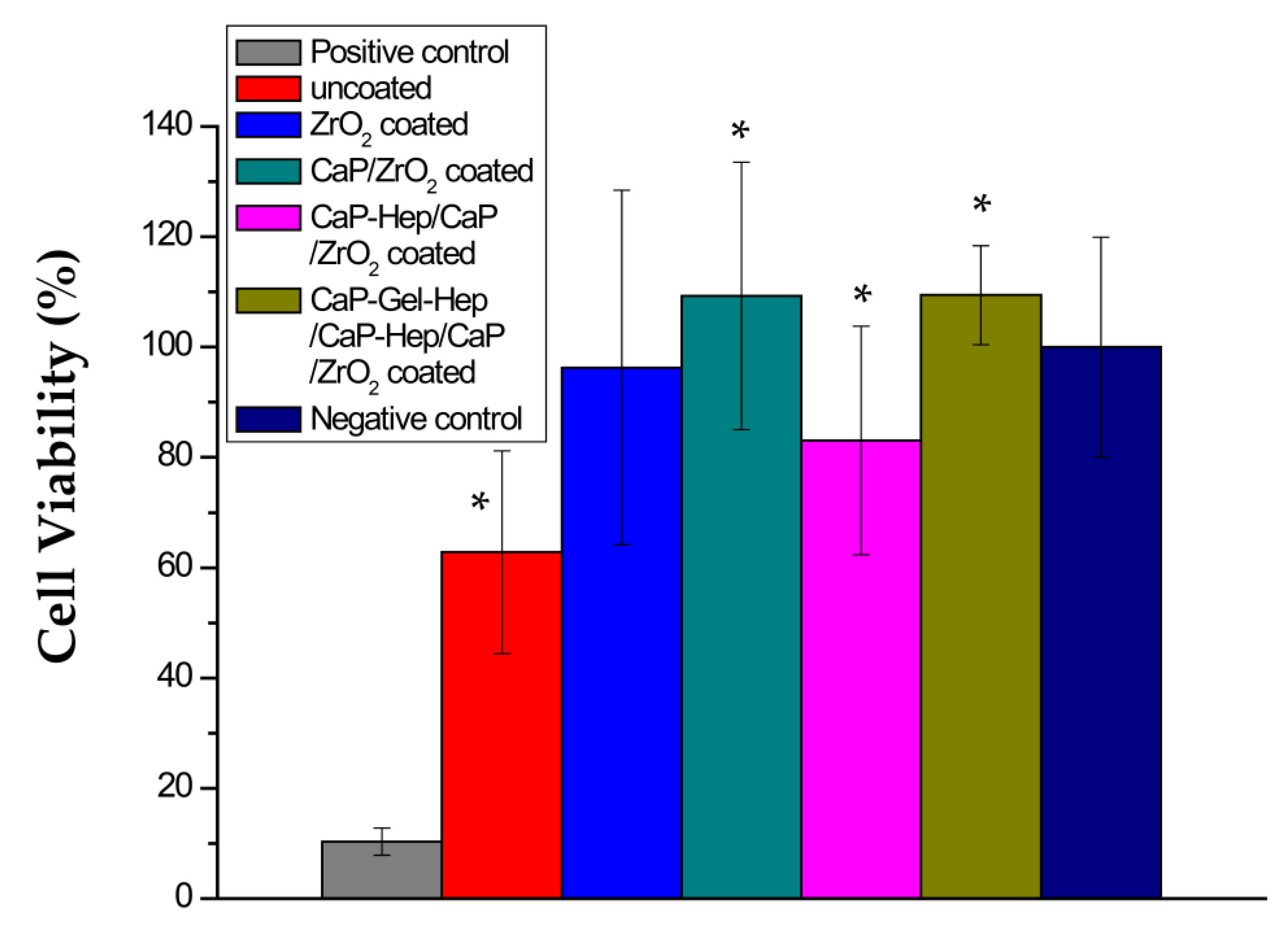
3.6. MTT Test
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. Biometals 2019, 32, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. New horizon of bioabsorbable stent. Catheter. Cardiovasc. Interv. 2005, 66, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Meratian, M. Microstructure, mechanical properties and bio-corrosion evaluation of biodegradable AZ91-FA nanocomposites for biomedical applications. Mater. Sci. Eng. A 2010, 527, 6938–6944. [Google Scholar] [CrossRef]
- Lu, P.; Fan, H.; Liu, Y.; Cao, L.; Wu, X.; Xu, X. Controllable biodegradability, drug release behavior and hemocompatibility of PTX-eluting magnesium stents. Colloids Surf. B Biointerfaces 2011, 83, 23–28. [Google Scholar] [CrossRef]
- Tomljenovic, L. Aluminum and Alzheimer’s Disease: After a Century of Controversy, Is there a Plausible Link? J. Alzheimer’s Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef]
- Jaguszewski, M.J.; Cortés, C.; Gutiérrez-Chico, J.L. Implantation of magnesium-bioresorbable scaffolds in a bifurcation under optical coherence tomography guidance. Eur. Heart J. 2017, 38, 2017–2018. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Marcus, P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based conversion coatings. Corros. Sci. 2008, 50, 1907–1918. [Google Scholar] [CrossRef]
- Yen, S.K.; Guo, M.J.; Zan, H.Z. Characterization of electrolytic ZrO2 coating on Co–Cr–Mo implant alloys of hip prosthesis. Biomaterials 2001, 22, 125–133. [Google Scholar] [CrossRef]
- Wang, M.J.; Li, C.F.; Yen, S.K. Electrolytic MgO/ZrO2 duplex-layer coating on AZ91D magnesium alloy for corrosion resistance. Corros. Sci. 2013, 76, 142–153. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Aval, N.A.; Islamian, J.P.; Hatamian, M.; Arabfirouzjaei, M.; Javadpour, J.; Rashidi, M.R. Doxorubicin loaded large-pore mesoporous hydroxyapatite coated superparamagnetic Fe3O4 nanoparticles for cancer treatment. Int. J. Pharm. 2016, 509, 159–167. [Google Scholar] [CrossRef]
- Lai, W.; Chen, C.; Ren, X.; Lee, I.S.; Jiang, G.; Kong, X. Hydrothermal fabrication of porous hollow hydroxyapatite microspheres for a drug delivery system. Mater. Sci. Eng. C 2016, 62, 166–172. [Google Scholar] [CrossRef]
- van der Giessen, W.J.; Sorop, O.; Serruys, P.W.; Peters-Krabbendam, I.; van Beusekom, H.M. Lowering the dose of sirolimus, released from a nonpolymeric hydroxyapatite coated coronary stent, reduces signs of delayed healing. JACC Cardiovasc. Interv. 2009, 2, 284–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pezzatini, S.; Solito, R.; Morbidelli, L.; Lamponi, S.; Boanini, E.; Bigi, A.; Ziche, M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J. Biomed. Mater. Res. Part A 2006, 76, 656–663. [Google Scholar] [CrossRef]
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Absolute Ultimate Guide for Lehninger Principles of Biochemistry; Palgrave Macmillan: London, UK, 2008. [Google Scholar]
- Vrolix, M.; Legrand, V.M.; Reiber, J.H.; Grollier, G.; Schalij, M.J.; Brunel, P.; Martinez-Elbal, L.; Gomez-Recio, M.; Bär, F.W.; Bertrand, M.E. Heparin-coated Wiktor stents in human coronary arteries (MENTOR Trial). Am. J. Cardiol. 2000, 86, 385–389. [Google Scholar] [CrossRef]
- Yaylao, M.; Korkusuz, P.; Örs, Ü.; Korkusuz, F.; Hasirci, V. Development of a calcium phosphate–gelatin composite as a bone substitute and its use in drug release. Biomaterials 1999, 20, 711–719. [Google Scholar] [CrossRef]
- Tsung, M.; Burgess, D.J. Preparation and stabilization of heparin/gelatin complex coacervate microCaPsules. J. Pharm. Sci. 1997, 86, 603–607. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Zhao, Y.; Gao, F.; Guo, X.; Yang, M.; Hong, Q.; Yang, Z.; Dai, J.; Pan, C. Incorporation of heparin/BMP2 complex on GOCS-modified magnesium alloy to synergistically improve corrosion resistance, anticoagulation, and osteogenesis. J. Mater. Sci. Mater. Med. 2021, 32, 24. [Google Scholar] [CrossRef]
- Ambat, R.; Aung, N.N.; Zhou, W. Studies on the influence of chloride ion and pH on the corrosion and electrochemical behaviour of AZ91D magnesium alloy. J. Appl. Electrochem. 2000, 30, 865–874. [Google Scholar] [CrossRef]
- Hara, N.; Kobayashi, Y.; Kagaya, D.; Akao, N. Formation and breakdown of surface films on magnesium and its alloys in aqueous solutions. Corros. Sci. 2007, 49, 166–175. [Google Scholar] [CrossRef]
- Wang, M.-J.; Chao, S.-C.; Yen, S. Electrolytic calcium phosphate/zirconia composite coating on AZ91D magnesium alloy for enhancing corrosion resistance and bioactivity. Corros. Sci. 2016, 104, 47–60. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In Vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hiromoto, S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater. Sci. Eng. C 2009, 29, 1559–1568. [Google Scholar] [CrossRef]
- Smith, P.; Mallia, A.; Hermanson, G. Colorimetric method for the assay of heparin content in immobilized heparin preparations. Anal. Biochem. 1980, 109, 466–473. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; International Organization for Standardization. Biological Evaluation of Medical Devices Part 5: Tests for in vitro Cytotoxicity: In Vitro Methods. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 1 August 2018).
- Ciapetti, G.; Cenni, E.; Pratelli, L.; Pizzoferrato, A. In Vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14, 359–364. [Google Scholar] [CrossRef]
- Yen, S.K.; Lin, C.M. Cathodic reactions of electrolytic hydroxyapatite coating on pure titanium. Mater. Chem. Phys. 2003, 77, 70–76. [Google Scholar] [CrossRef]
- Li, C.F.; Wang, M.J.; Ho, W.H.; Li, H.N.; Yen, S.K. Effects of electrolytic MgO coating parameters on corrosion resistance of AZ91D magnesium alloy. J. Electrochem. Soc. 2011, 158, C11–C16. [Google Scholar] [CrossRef]
- G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM: Philadelphia, PA, USA, 2010.
- Song, Y.; Shan, D.; Chen, R.; Han, E.H. Corrosion characterization of Mg–8Li alloy in NaCl solution. Corros. Sci. 2009, 51, 1087–1094. [Google Scholar] [CrossRef]
- Yang, Z.; Birkenhauer, P.; Julmy, F.; Chickering, D.; Ranieri, J.P.; Merkle, H.P.; Lüscher, T.F.; Gander, B. Sustained release of heparin from polymeric particles for inhibition of human vascular smooth muscle cell proliferation. J. Control. Release 1999, 60, 269–277. [Google Scholar] [CrossRef] [PubMed]





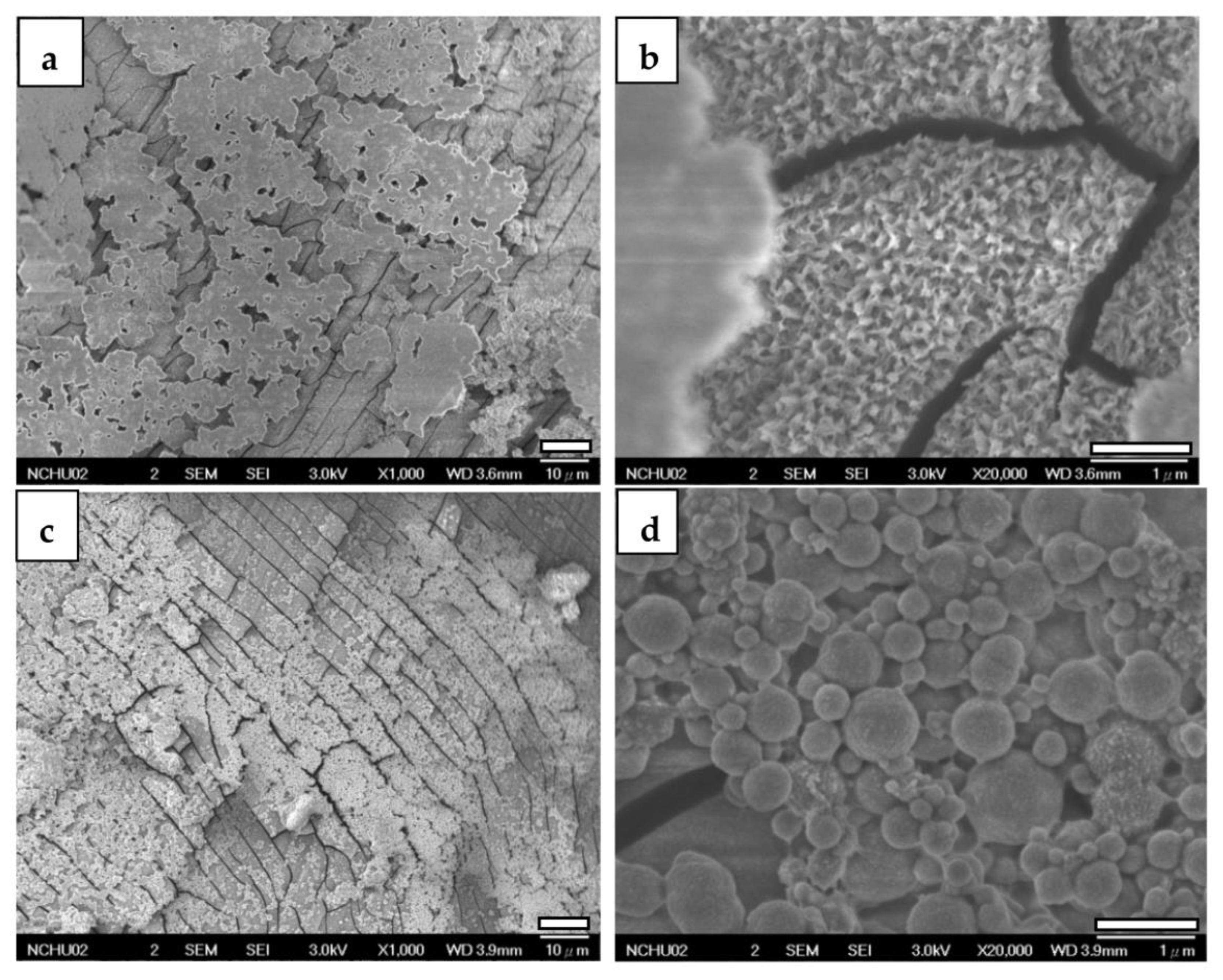

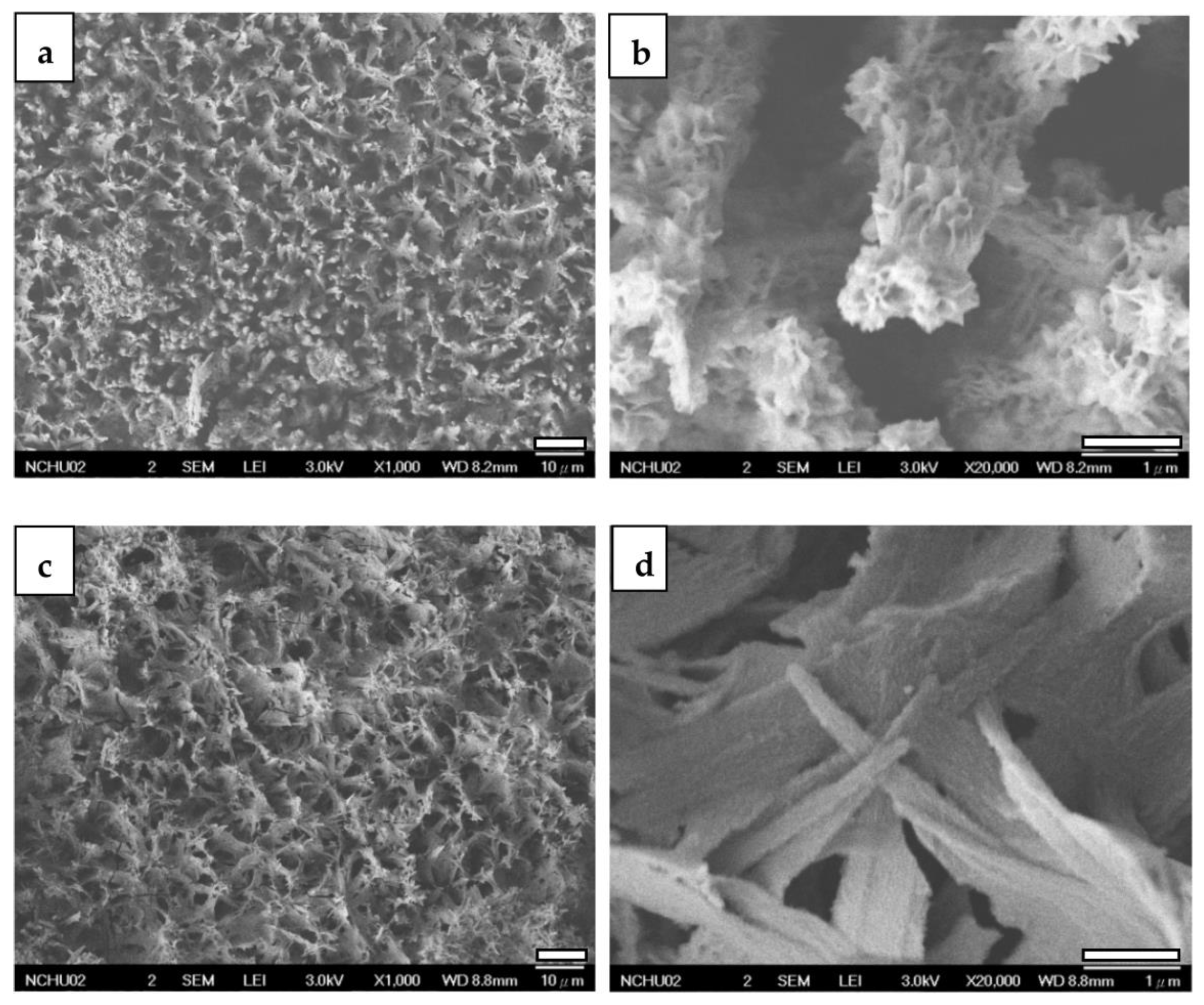
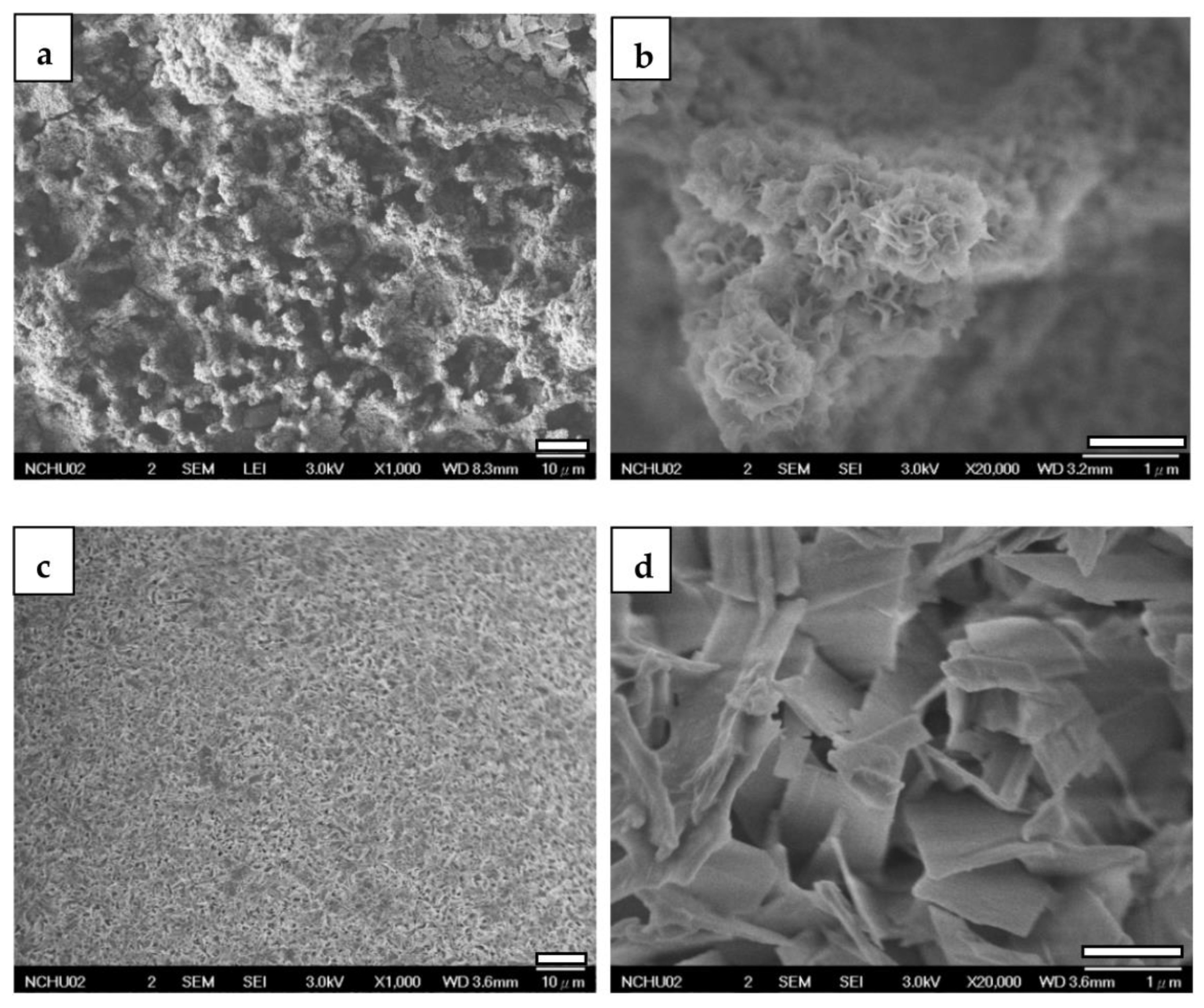



| Element | Mg | Al | Cu | Mn | Ni | Si | Fe |
|---|---|---|---|---|---|---|---|
| % | 99.93 | 0.018 | 0.0033 | 0.019 | 0.0003 | 0.018 | 0.004 |
| Solution | pH Levels | O2 Levels (ppm) | Conductivity (mS/cm) |
|---|---|---|---|
| DI water | 6.82 | 7.12 | 0.007 |
| Hep | 6.77 | 7.60 | 9.30 |
| Calcium phosphate (CaP) | 4.28 | 7.99 | 8.73 |
| CaP-Hep | 4.40 | 7.98 | 9.32 |
| CaP-Gel | 4.78 | 7.44 | 8.46 |
| CaP-Gel-Hep | 4.84 | 7.24 | 8.70 |
| Component | Plasma | MEM + FBS |
|---|---|---|
| Na+ (mmol/L) | 142 | 151 |
| K+ (mmol/L) | 5.0 | 5.33 |
| Ca2+ (mmol/L) | 2.5 | 1.80 |
| Mg2+ (mmol/L) | 1.5 | 0.81 |
| HCO3− (mmol/L) | 27.0 | 26.2 |
| Cl− (mmol/L) | 103.0 | 125 |
| HPO4− (mmol/L) | 1.0 | 1.01 |
| SO42− (mmol/L) | 0.5 | 0.81 |
| Glucose (mmol/L) | 3.6–5.2 | 5.56 |
| Protein (g/L) | 63–80 | 4.3 |
| Amino acid (g/L) | ND | 0.86 |
| Phenol red (mmol/L) | - | 0.03 |
| Tests * | A * | B * | C * | D * | E * |
|---|---|---|---|---|---|
| Specimens | |||||
| Pure magnesium | −1.69 | 1.33 × 10−5 | −1.50 | 0.300 | 7.442 |
| ZrO2 coated | −1.87 | 7.10 × 10−6 | −1.38 | 0.160 | 3.973 |
| CaP coated | −1.60 | 4.17 × 10−6 | −1.41 | 0.094 | 2.333 |
| CaP/ZrO2 coated | −1.53 | 3.86 × 10−6 | −1.35 | 0.087 | 2.160 |
| CaP-Hep/CaP/ZrO2 coated | −1.69 | 1.04 × 10−5 | −1.37 | 0.235 | 5.820 |
| CaP-Gel-Hep/CaP-Hep/CaP/ZrO2 coated | −1.62 | 7.42 × 10−6 | −1.39 | 0.168 | 4.152 |
| Items | Medication Encapsulation (μg/cm2) | Weight Increase (mg/cm2) | Drug Content/Weight Gain (×100%) |
|---|---|---|---|
| Specimens | |||
| CaP/ZrO2 | 0.61 ± 0.03 | ||
| CaP-Hep | 243.56 ± 55.18 | 1.32 ± 0.53 | 18.45% |
| CaP-Gel-Hep | 379.05 ± 13.63 * | 2.95 ± 0.4 * | 12.84% |
| CaP-Hep/CaP/ZrO2 | 277.87 ± 16.14 * | 1.36 ± 0.24 * | 20.43% |
| CaP-Gel-Hep/CaP-Hep/CaP/ZrO2 | 484.19 ± 19.26 * | 3.97 ± 0.22 * | 12.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-L.; Lin, C.-R.; Yen, C.-C.; Yen, S.-K. Heparin-Loaded Composite Coatings on Porous Stent from Pure Magnesium for Biomedical Applications. J. Funct. Biomater. 2023, 14, 519. https://doi.org/10.3390/jfb14100519
Lai Y-L, Lin C-R, Yen C-C, Yen S-K. Heparin-Loaded Composite Coatings on Porous Stent from Pure Magnesium for Biomedical Applications. Journal of Functional Biomaterials. 2023; 14(10):519. https://doi.org/10.3390/jfb14100519
Chicago/Turabian StyleLai, Yu-Liang, Cheng-Rui Lin, Chao-Chun Yen, and Shiow-Kang Yen. 2023. "Heparin-Loaded Composite Coatings on Porous Stent from Pure Magnesium for Biomedical Applications" Journal of Functional Biomaterials 14, no. 10: 519. https://doi.org/10.3390/jfb14100519
APA StyleLai, Y.-L., Lin, C.-R., Yen, C.-C., & Yen, S.-K. (2023). Heparin-Loaded Composite Coatings on Porous Stent from Pure Magnesium for Biomedical Applications. Journal of Functional Biomaterials, 14(10), 519. https://doi.org/10.3390/jfb14100519






