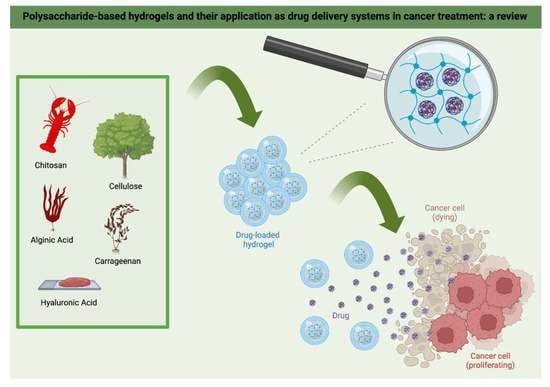
Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Classification
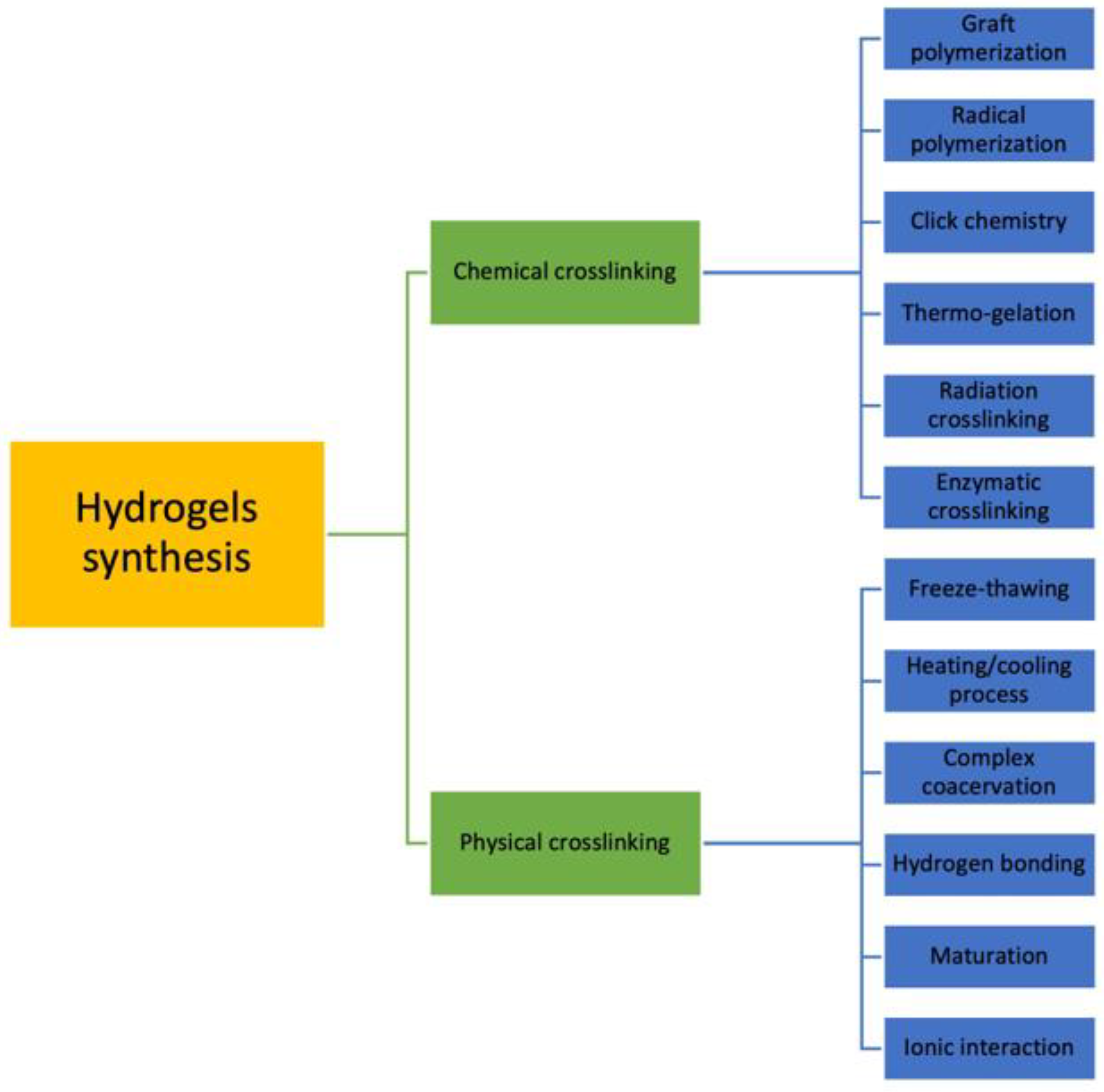
3.1. Crosslinking
3.2. Polymeric Composition
3.3. Source
3.4. Other Classifications of Hydrogels
4. Properties
4.1. Swelling Properties
4.2. Mechanical Properties
4.3. Biocompatibility and Biodegradability
5. Natural Hydrogels
- -
- Polysaccharides, such as chitosan (CHI), HA, and ALG;
- -
- Proteins, such as gelatin, collagen and silk, or peptides [28];
- -
- DNA.
5.1. Synthesis
5.1.1. Ionic Interaction
5.1.2. Freeze-Drying
5.1.3. Heating/Cooling Process
5.1.4. Complex Coacervation
5.1.5. Hydrogen Bonding
5.2. Polysaccharides
5.2.1. Chitosan
5.2.2. Alginate
5.2.3. Hyaluronic Acid
5.2.4. Cellulose
5.2.5. Carrageenan
6. Drug Delivery in Cancer Therapy
6.1. Breast Cancer
6.2. Melanoma
6.3. Colorectal Cancer
6.4. Renal Cell Carcinoma
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Mohite, P.B.; Adhav, S. A hydrogels: Methods of preparation and applications. Int. J. Adv. Pharm. 2017, 6, 79–85. [Google Scholar]
- Jabbari, E.; Leijten, J.; Xu, Q.; Khademhosseini, A. The matrix reloaded: The evolution of regenerative hydrogels. Mater. Today 2016, 19, 190–196. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460–468. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47. [Google Scholar] [CrossRef]
- Singh, S.K.; Dhyani, A.; Juyal, D. Hydrogel: Preparation, characterization and applications. Pharma Innov. 2017, 6, 25. [Google Scholar]
- Novakovic, K.; Matcham, S.; Scott, A. Intelligent hydrogels as drug delivery systems. In Hydrogels; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–28. [Google Scholar]
- Nayak, A.K.; Das, B. Introduction to polymeric gels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–27. [Google Scholar]
- Mishra, S.; Rani, P.; Sen, G.; Dey, K.P. Preparation, properties and application of hydrogels: A review. In Hydrogels; Springer: Berlin/Heidelberg, Germany, 2018; pp. 145–173. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of hydrogels based on their source: A review and application in stem cell regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Kaith, B.S.; Singh, A.; Sharma, A.K.; Sud, D. Hydrogels: Synthesis, classification, properties and potential applications—A brief review. J. Polym. Environ. 2021, 29, 3827–3841. [Google Scholar] [CrossRef]
- Rana, P.; Ganarajan, G.; Kothiyal, P. Review on preparation and properties hydrogel formulation. World J. Pharm. Pharm. Sci. 2015, 4, 1069–1080. [Google Scholar]
- Gupta, N.V.; Shivakumar, H. Investigation of swelling behavior and mechanical properties of a pH-sensitive superporous hydrogel composite. Iran. J. Pharm. Res. 2012, 11, 481. [Google Scholar]
- Yahia, L.; Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 2. [Google Scholar] [CrossRef]
- Beckett, L.E.; Lewis, J.T.; Tonge, T.K.; Korley, L.T. Enhancement of the mechanical properties of hydrogels with continuous fibrous reinforcement. ACS Biomater. Sci. Eng. 2020, 6, 5453–5473. [Google Scholar] [CrossRef]
- Xue, B.; Tang, D.; Wu, X.; Xu, Z.; Gu, J.; Han, Y.; Zhu, Z.; Qin, M.; Zou, X.; Wang, W. Engineering hydrogels with homogeneous mechanical properties for controlling stem cell lineage specification. Proc. Natl. Acad. Sci. USA 2021, 118, e2110961118. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef]
- Kamath, K.R.; Park, K. Biodegradable hydrogels in drug delivery. Adv. Drug Deliv. Rev. 1993, 11, 59–84. [Google Scholar] [CrossRef]
- Saroia, J.; Yanen, W.; Wei, Q.; Zhang, K.; Lu, T.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Marshall, L.R.; Yoon, J.; Kulesha, A.; Edirisinghe, D.I.; Chandrasekaran, S.; Rathee, P.; Prabhakar, R.; Makhlynets, O.V. Peptide hydrogel with self-healing and redox-responsive properties. Nano Converg. 2022, 9, e3474. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Segura, T.; Chung, P.H.; Shea, L.D. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials 2005, 26, 1575–1584. [Google Scholar] [CrossRef]
- Kim, E.H.; Lim, S.; Kim, T.E.; Jeon, I.O.; Choi, Y.S. Preparation of in situ injectable chitosan/gelatin hydrogel using an acid-tolerant tyrosinase. Biotechnol. Bioprocess Eng. 2018, 23, 500–506. [Google Scholar] [CrossRef]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels based drug delivery synthesis, characterization and administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Books on Demand: Norderstedt, Germany, 2011; p. 117150. [Google Scholar]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels—A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Patitucci, F.; Dattilo, M.; Malivindi, R.; Amone, F.; Morelli, C.; Nigro, A.; Sisci, D.; Puoci, F. Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect. J. Funct. Biomater. 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose Cryogels as Promising Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef]
- Butts, J.D.; Rehm, S.R. The effect of heating on the functional activity of iota carrageenan. J. Pharmacol. Methods 1985, 13, 53–57. [Google Scholar] [CrossRef]
- Lalevée, G.; David, L.; Montembault, A.; Blanchard, K.; Meadows, J.; Malaise, S.; Crépet, A.; Grillo, I.; Morfin, I.; Delair, T. Highly stretchable hydrogels from complex coacervation of natural polyelectrolytes. Soft Matter 2017, 13, 6594–6605. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, W. Dipole–dipole and H-bonding interactions significantly enhance the multifaceted mechanical properties of thermoresponsive shape memory hydrogels. Adv. Funct. Mater. 2015, 25, 471–480. [Google Scholar] [CrossRef]
- Takigami, M.; Amada, H.; Nagasawa, N.; Yagi, T.; Kasahara, T.; Takigami, S.; Tamada, M. Preparation and properties of CMC gel. Trans. Mater. Res. Soc. Jpn. 2007, 32, 713–716. [Google Scholar] [CrossRef]
- Gul, K.; Gan, R.-Y.; Sun, C.-X.; Jiao, G.; Wu, D.-T.; Li, H.-B.; Kenaan, A.; Corke, H.; Fang, Y.-P. Recent advances in the structure, synthesis, and applications of natural polymeric hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 3817–3832. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Omrani, M.; Naimi-Jamal, M.R.; Far, B.F. The design of multi-responsive nanohydrogel networks of chitosan for controlled drug delivery. Carbohydr. Polym. 2022, 298, 120143. [Google Scholar] [CrossRef]
- Bright, L.M.E.; Griffin, L.; Mondal, A.; Hopkins, S.; Ozkan, E.; Handa, H. Biomimetic gasotransmitter-releasing alginate beads for biocompatible antimicrobial therapy. J. Colloid Interface Sci. 2022, 628, 911–921. [Google Scholar] [CrossRef]
- Massana Roquero, D.; Bollella, P.; Katz, E.; Melman, A. Controlling porosity of calcium alginate hydrogels by interpenetrating polyvinyl alcohol–diboronate polymer network. ACS Appl. Polym. Mater. 2021, 3, 1499–1507. [Google Scholar] [CrossRef]
- Ren, M.; Li, N.; Jiang, X.; Liu, X.; Zou, A. Efficient oral delivery of water-soluble CT contrast agent using an W1/O/W2 alginate hydrogel matrix. Colloids Surf. B Biointerfaces 2022, 220, 112862. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian-Nodoushan, F.; Nikukar, H.; Soleimani, M.; JalaliJahromi, A.; Hosseinzadeh, S.; Khojasteh, A. A smart magnetic hydrogel containing exosome promotes osteogenic commitment of human adipose-derived mesenchymal stem cells. Iran. J. Basic Med. Sci. 2022, 25, 1123–1131. [Google Scholar] [PubMed]
- Dubashynskaya, N.V.; Petrova, V.A.; Romanov, D.P.; Skorik, Y.A. pH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers–Sodium Alginate Polyelectrolyte Complex. Materials 2022, 15, 5860. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Luo, H.; Wang, Z.; Chen, D.; Feng, Q.; Cao, X. Injectable and tissue adhesive EGCG-laden hyaluronic acid hydrogel depot for treating oxidative stress and inflammation. Carbohydr. Polym. 2023, 299, 120180. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Meng, M.; Wang, Q.; Wang, Y.; Lei, Y.; Zhang, Y.; Weng, L.; Chen, X. A dual-responsive hyaluronic acid nanocomposite hydrogel drug delivery system for overcoming multiple drug resistance. Chin. Chem. Lett. 2023, 34, 107583. [Google Scholar] [CrossRef]
- Nabipour, H.; Mansoorianfar, M.; Hu, Y. Carboxymethyl cellulose-coated HKUST-1 for baclofen drug delivery in vitro. Chem. Pap. 2022, 76, 6557–6566. [Google Scholar] [CrossRef]
- Albiero, M.; Fullin, A.; Villano, G.; Biasiolo, A.; Quarta, S.; Bernardotto, S.; Turato, C.; Ruvoletto, M.; Fadini, G.P.; Pontisso, P. Semisolid Wet Sol–Gel Silica/Hydroxypropyl Methyl Cellulose Formulation for Slow Release of Serpin B3 Promotes Wound Healing In Vivo. Pharmaceutics 2022, 14, 1944. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.R.; Roy, D.; Majee, S.B. Effect of Cellulosic Polymer on Physico Mechanical Properties of Superporous Hydrogel of an Antihypertensive Drug and Drug Release Kinetics from It. Int. J. Appl. Pharm. 2019, 11, 257–263. [Google Scholar] [CrossRef]
- Permana, A.D.; Elim, D.; Ananda, P.W.R.; Zaman, H.S.; Muslimin, W.; Tunggeng, M.G.R. Enhanced and sustained transdermal delivery of primaquine from polymeric thermoresponsive hydrogels in combination with Dermarollers®®®. Colloids Surf. B Biointerfaces 2022, 219, 112805. [Google Scholar] [CrossRef]
- Saramas, T.; Sakunpongpitiporn, P.; Rotjanasuworapong, K.; Morarad, R.; Niamlang, S.; Sirivat, A. Metformin delivery via iontophoresis based on κ-carrageenan cryogels. Int. J. Biol. Macromol. 2022, 223, 702–712. [Google Scholar] [CrossRef]
- Kochkina, N.; Nikitina, M.; Agafonov, M.; Delyagina, E.; Terekhova, I. iota-Carrageenan hydrogels for methotrexate delivery. J. Mol. Liq. 2022, 368, 120790. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Parisi, O.I.; Scrivano, L.; Sinicropi, M.S.; Puoci, F. Polymeric nanoparticle constructs as devices for antibacterial therapy. Curr. Opin. Pharmacol. 2017, 36, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Bhise, K.S.; Dhumal, R.S.; Paradkar, A.R.; Kadam, S.S. Effect of drying methods on swelling, erosion and drug release from chitosan–naproxen sodium complexes. AAPS PharmSciTech 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Jarrah, R.; El Sammak, S.; Onyedimma, C.; Ghaith, A.K.; Moinuddin, F.; Bhandarkar, A.R.; Siddiqui, A.; Madigan, N.; Bydon, M. The role of alginate hydrogels as a potential treatment modality for spinal cord injury: A comprehensive review of the literature. Neurospine 2022, 19, 272–280. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrød, O.; Wachtmeister, C.A.; Kristiansen, L.; Jensen, K.A. Fractionation of alginates by precipitation with calcium and magnesium ions. Acta Chem. Scand. 1965, 19, 1221–1226. [Google Scholar] [CrossRef]
- Roquero, D.M.; Othman, A.; Melman, A.; Katz, E. Iron (III)-cross-linked alginate hydrogels: A critical review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, X.; Chen, Y.; Guo, J.; Chen, Q.; Jiang, X. pH-induced self-assembly and capsules of sodium alginate. Biomacromolecules 2005, 6, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Colinet, I.; Dulong, V.; Mocanu, G.; Picton, L.; Le Cerf, D. New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2009, 73, 345–350. [Google Scholar] [CrossRef]
- Silva, E.A.; Mooney, D.J. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 2010, 31, 1235–1241. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef]
- Kaniewska, K.; Karbarz, M.; Katz, E. Nanocomposite hydrogel films and coatings–Features and applications. Appl. Mater. Today 2020, 20, 100776. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik, Y.A. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile. Biomolecules 2019, 9, 291. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586. [Google Scholar] [CrossRef]
- Golshani, R.; Lopez, L.; Estrella, V.; Kramer, M.; Iida, N.; Lokeshwar, V.B. Hyaluronic acid synthase-1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008, 68, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Biomaterials from Chemically-Modified Hyaluronan. Available online: https://www.glycoforum.gr.jp/article/05A4.html: (accessed on 5 December 2022).
- Prestwich, G.D.; Kuo, J.-W. Chemically-modified HA for therapy and regenerative medicine. Curr. Pharm. Biotechnol. 2008, 9, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Choh, S.-Y.; Cross, D.; Wang, C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules 2011, 12, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shu, X.Z.; Gray, S.D.; Prestwich, G.D. Disulfide-crosslinked hyaluronan–gelatin sponge: Growth of fibrous tissue in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Qiu, L.; Ge, L.; Zhou, J.; Ji, Q.; Yang, Y.; Long, M.; Wang, D.; Teng, L.; Chen, J. Overcoming multidrug resistance by intracellular drug release and inhibiting p-glycoprotein efflux in breast cancer. Biomed. Pharmacother. 2021, 134, 111108. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-based hydrogels in tissue engineering applications. Cellul. Chem. Technol. 2019, 53, 907–923. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Di Giuseppe, E. Analogue Materials in Experimental Tectonics; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Cellulose-based hydrogels for pharmaceutical and biomedical applications. In Cellulose-Based Superabsorbent Hydrogels: Springer Nature; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–28. [Google Scholar]
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-derivatives-based hydrogels as vehicles for dermal and transdermal drug delivery. Emerg. Concepts Anal. Appl. Hydrogels 2016, 2, 64. [Google Scholar]
- Trombino, S.; Cassano, R.; Bloise, E.; Muzzalupo, R.; Leta, S.; Puoci, F.; Picci, N. Design and synthesis of cellulose derivatives with antioxidant activity. Macromol. Biosci. 2008, 8, 86–95. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, S. “Smart” materials based on cellulose: A review of the preparations, properties, and applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef]
- Marques-Marinho, F.D.; Vianna-Soares, C.D. Cellulose and its derivatives use in the pharmaceutical compounding practice. In Cellulose-Medical, Pharmaceutical and Electronic Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Murata, M.; Nakazoe, J.-I. Production and use of marine aIgae in Japan. Jpn. Agric. Res. Q. 2001, 35, 281–290. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis–A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Bui, V.T.; Nguyen, B.T.; Nicolai, T.; Renou, F. Mixed iota and kappa carrageenan gels in the presence of both calcium and potassium ions. Carbohydr. Polym. 2019, 223, 115107. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Jo, D.-G.; Park, J.H. Polysaccharide-based nanoparticles: A versatile platform for drug delivery and biomedical imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Wang, M.-J. Removal of heavy metal ions by poly (vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustain. Chem. Eng. 2016, 4, 2830–2837. [Google Scholar] [CrossRef]
- Ma, X.; Yang, C.; Zhang, R.; Yang, J.; Zu, Y.; Shou, X.; Zhao, Y. Doxorubicin loaded hydrogel microparticles from microfluidics for local injection therapy of tumors. Colloids Surf. B. Biointerfaces 2022, 220, 112894. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Gulfam, M.; Jo, S.-H.; Gal, Y.-S.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef] [PubMed]
- King, J.L.; Maturavongsadit, P.; Hingtgen, S.D.; Benhabbour, S.R. Injectable pH Thermo-Responsive Hydrogel Scaffold for Tumoricidal Neural Stem Cell Therapy for Glioblastoma Multiforme. Pharmaceutics 2022, 14, 2243. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater. Sci. Eng. C 2020, 112, 110853. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chang, R.; Shen, J.; Wang, Y.; Song, H.; Kang, X.; Zhao, Y.; Guo, S.; Qin, J. Self-healing pectin/cellulose hydrogel loaded with limonin as TMEM16A inhibitor for lung adenocarcinoma treatment. Int. J. Biol. Macromol. 2022, 219, 754–766. [Google Scholar] [CrossRef]
- Miao, Y.; Lu, J.; Yin, J.; Zhou, C.; Guo, Y.; Zhou, S. Yb3+-containing chitosan hydrogels induce B-16 melanoma cell anoikis via a Fak-dependent pathway. Nanotechnol. Rev. 2019, 8, 645–660. [Google Scholar] [CrossRef]
- Doneda, E.; Bianchi, S.E.; Pittol, V.; Kreutz, T.; Scholl, J.N.; Ibañez, I.L.; Bracalente, C.; Durán, H.; Figueiró, F.; Klamt, F. 3-O-Methylquercetin from Achyrocline satureioides—Cytotoxic activity against A375-derived human melanoma cell lines and its incorporation into cyclodextrins-hydrogels for topical administration. Drug Deliv. Transl. Res. 2021, 11, 2151–2168. [Google Scholar] [CrossRef]
- Omtvedt, L.A.; Kristiansen, K.A.; Strand, W.I.; Aachmann, F.L.; Strand, B.L.; Zaytseva-Zotova, D.S. Alginate hydrogels functionalized with β-cyclodextrin as a local paclitaxel delivery system. J. Biomed. Mater. Res. Part A 2021, 109, 2625–2639. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Jafari, H.; Atlasi, Z.; Mahdavinia, G.R.; Hadifar, S.; Sabzi, M. Magnetic κ-carrageenan/chitosan/montmorillonite nanocomposite hydrogels with controlled sunitinib release. Mater. Sci. Eng. C 2021, 124, 112042. [Google Scholar] [CrossRef] [PubMed]
- Corrie, P.; Hategan, M.; Fife, K.; Parkinson, C. Management of melanoma. Br. Med. Bull. 2014, 111, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Teo, R.D.; Termini, J.; Gray, H.B. Lanthanides: Applications in cancer diagnosis and therapy: Miniperspective. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.K.; Mohiuddin, A.; Ming, L.C. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, Z.; Li, M.; Qu, Q.; Ma, X.; Yu, S.H.; Zhao, Y. A preloaded amorphous calcium carbonate/doxorubicin@ silica nanoreactor for ph-responsive delivery of an anticancer drug. Angew. Chem. Int. Ed. 2015, 54, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.; Kessler, E.R.; Bernard, B.; Flaig, T.W.; Lam, E.T. Renal cell carcinoma: A review of biology and pathophysiology. F1000Research 2018, 7, 307. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17009. [Google Scholar] [CrossRef]
- Parisi, O.I.; Morelli, C.; Scrivano, L.; Sinicropi, M.S.; Cesario, M.G.; Candamano, S.; Puoci, F.; Sisci, D. Controlled release of sunitinib in targeted cancer therapy: Smart magnetically responsive hydrogels as restricted access materials. RSC Adv. 2015, 5, 65308–65315. [Google Scholar] [CrossRef]

| Criteria | Classification | Features |
|---|---|---|
| Crosslinking | Chemical | Polymers are covalently crosslinked by permanent junctions. It can be carried out by the addition of crosslinker molecules, polymer–polymer conjugation, or photoinitiators. |
| Physical | Polymers are hold together by chain entanglements and/or hydrogen bonds or hydrophobic or ionic interactions. | |
| Polymeric composition | Homopolymer | Hydrogel derived from a single species of monomer. |
| Copolymer | Hydrogel consists of two or more different monomers with at least one hydrophilic component. | |
| Semi-interpenetrating network | Hydrogel consists of one crosslinked monomer and another non-crosslinked component. | |
| Interpenetrating network | Hydrogel are made of two independent crosslinked polymeric chains contained in a network form. | |
| Source | Natural | Polysaccharides and proteins are examples of polymers for natural hydrogels. They are biocompatible and biodegradable. |
| Synthetic | Synthetic hydrogels have higher strength and can be designed to have specific mechanical and chemical properties. | |
| Hybrid | Hydrogels consists of a combination of synthetic and natural polymers. | |
| Physical structure | Amorphous | They contain randomly arranged macromolecular chains. |
| Crystalline | They possess dense regions of ordered macromolecular chains. | |
| Semicrystalline | A mixture of amorphous and crystalline phases. | |
| Network electrical charge | Nonionic | They do not present any charged groups and have ultra-durable and permanent connections. |
| Ionic | They can be positive or negative and have different behaviors according to the pH. | |
| Amphoteric | Hydrogels contain both acidic and basic groups. | |
| Zwitterionic | They present an equal amount of positive and negative charge. |
| Hydrogel Source | Additional Components | Synthesis Method | Loaded Drug | Reference |
|---|---|---|---|---|
| Chitosan | --- | Formaldehyde crosslinking | DOX/5-FU | [47] |
| Sodium alginate | --- | Ionic crosslinking | S-nitrosoglutathione | [48] |
| Sodium alginate | Polyvinyl alcohol/ benzeneboronic acid | Ionic crosslinking | Proteins | [49] |
| Sodium alginate | --- | Ionic crosslinking | Iohexol | [50] |
| Sodium alginate | Polyvinyl pyrrolidone | Ionic crosslinking | Exosomes | [51] |
| Sodium alginate | Chitin nanowhiskers | Ionic crosslinking | Metronidazole | [52] |
| Hyaluronic acid | Gelatin | UV radiation | Epigallocatechin- 3-gallate | [53] |
| Hyaluronic acid | Core–shell SiO2 nanoparticles | UV radiation | Doxorubicin/ glucose oxidase | [54] |
| CMC | HKUST-1 | Ionic crosslinking | Baclofen | [55] |
| HPMC | SiO2/Glycerol | Chemical crosslinking | Serpin B3 | [56] |
| HPMC | Carbopol 971p | Chemical crosslinking | Atenolol | [57] |
| HPMC | Pluronic F127 (PF127) and F68 (PF68) | Temperature-dependent gelation | Primaquine | [58] |
| κ-carrageenan | --- | Freeze-drying process | Metformin | [59] |
| ι-carrageenan | β-cyclodextrins | Maturation | Methotrexate | [60] |
| Cancer Type | Hydrogel Origin | Loaded Drug | In Vitro/In Vivo Outcomes | |
|---|---|---|---|---|
| Breast cancer | Hyaluronic acid | Doxorubicin | HA scaffold had great antitumor activity when combined with near-infrared light, showing synergistic antitumor and photothermal effect. | [117] |
| Breast cancer | CMC | Doxorubicin | The system showed tumor inhibition effect with a strong apoptotic signal and no significant changes in bodyweight. | [118] |
| Glioblastoma | Cellulose/chitosan | TRAIL | Hydrogel scaffolds maintained cell viability and released TRAIL at concentrations that exhibited in vitro efficient tumor cell killing. | [119] |
| Colorectal cancer | CMC/alginate | Methotrexate/aspirin | The system showed concentration-dependent cytotoxicity with a colon cancer cell viability decrease of up to 10%. | [120] |
| Colorectal cancer | Chitosan/chondroitin sulfate | Curcumin | Hydrogel scaffold did not present significant level of cytotoxicity and allowed efficient drug release and absorption preferentially by cancer cells. | [121] |
| Lung cancer | Acylhydrazide-functionalized CMC | Limonin | Limonin-loaded hydrogels exhibited enhanced tumor suppression efficiency through a sustained release process with no difference in tissue morphology. | [122] |
| Melanoma | Chitosan | Ytterbium (Yb3+) | Chitosan hydrogel induced in vitro melanoma cells’ anoikis and inhibited tumor growth in animal experiment. | [123] |
| Melanoma | HPMC/Cyclodextrins | 3-O-Methylquercetin (3OMQ) | The formulation achieved complete 3OMQ release using a Franz cell model, reaching the whole skin layer. | [124] |
| Prostate cancer | Alginate/ cyclodextrins | Paclitaxel | The combined ALG–CD complex prevented Paclitaxel crystallization and allowed its diffusion out of the network, decreasing the metabolic activity of prostate cancer cells in a dose-dependent manner. | [125] |
| Hepatocellular carcinoma | N-carboxyethyl chitosan | Doxorubicin | The pH-responsive system showed good degradability properties in tumor acidic microenvironment with enhanced drug efficiency to kill tumor cells and less side effects for normal tissue. | [126] |
| Renal cell carcinoma | Κ-carrageenan/ chitosan | Sunitinib | In vitro release studies showed pH-dependent release of the drug, with an increase at acidic pH similar to damaged cancerous tissues. | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. https://doi.org/10.3390/jfb14020055
Dattilo M, Patitucci F, Prete S, Parisi OI, Puoci F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. Journal of Functional Biomaterials. 2023; 14(2):55. https://doi.org/10.3390/jfb14020055
Chicago/Turabian StyleDattilo, Marco, Francesco Patitucci, Sabrina Prete, Ortensia Ilaria Parisi, and Francesco Puoci. 2023. "Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review" Journal of Functional Biomaterials 14, no. 2: 55. https://doi.org/10.3390/jfb14020055
APA StyleDattilo, M., Patitucci, F., Prete, S., Parisi, O. I., & Puoci, F. (2023). Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. Journal of Functional Biomaterials, 14(2), 55. https://doi.org/10.3390/jfb14020055