The Localized Ionic Microenvironment in Bone Modelling/Remodelling: A Potential Guide for the Design of Biomaterials for Bone Tissue Engineering
Abstract
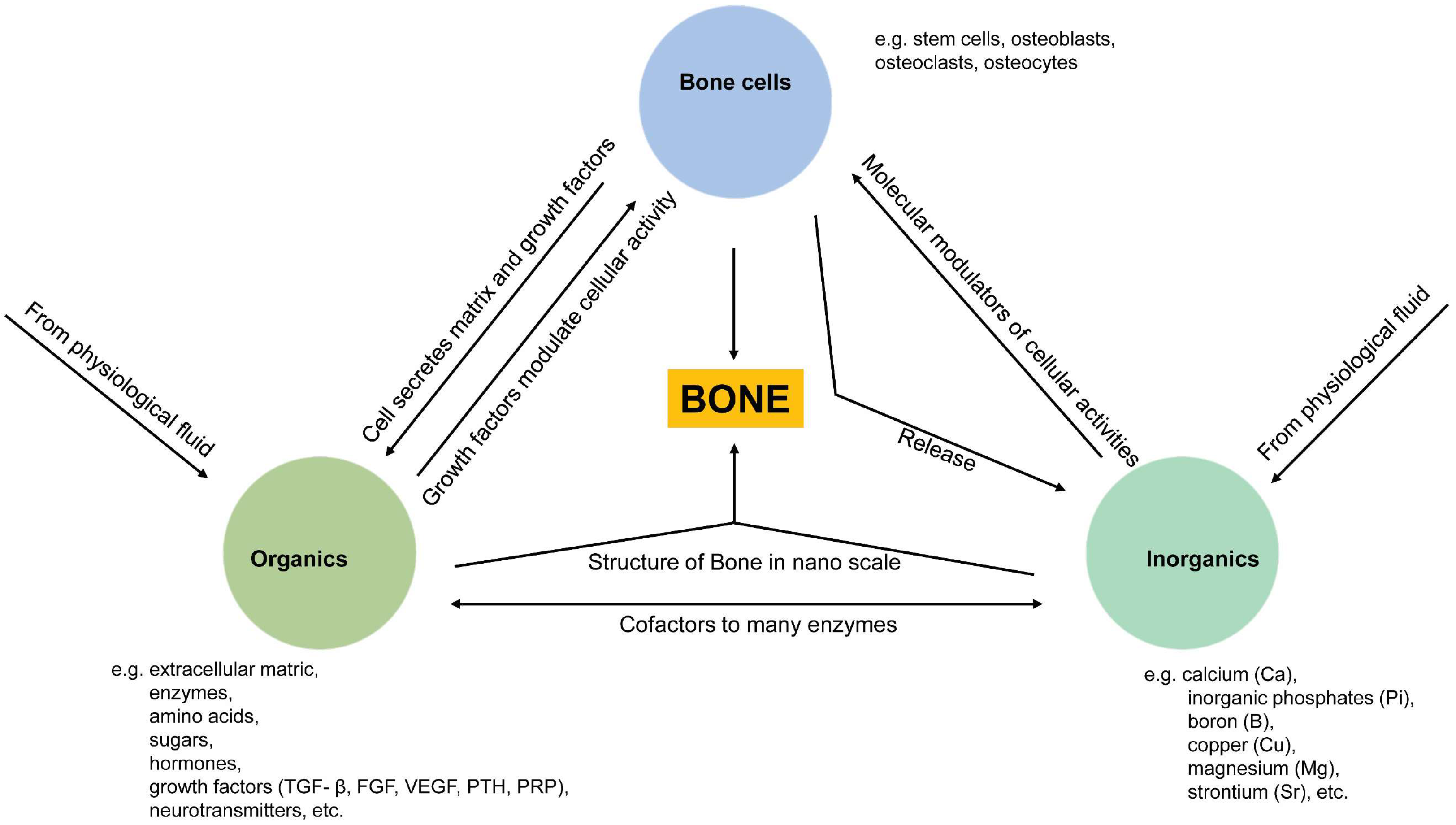
:1. Introduction
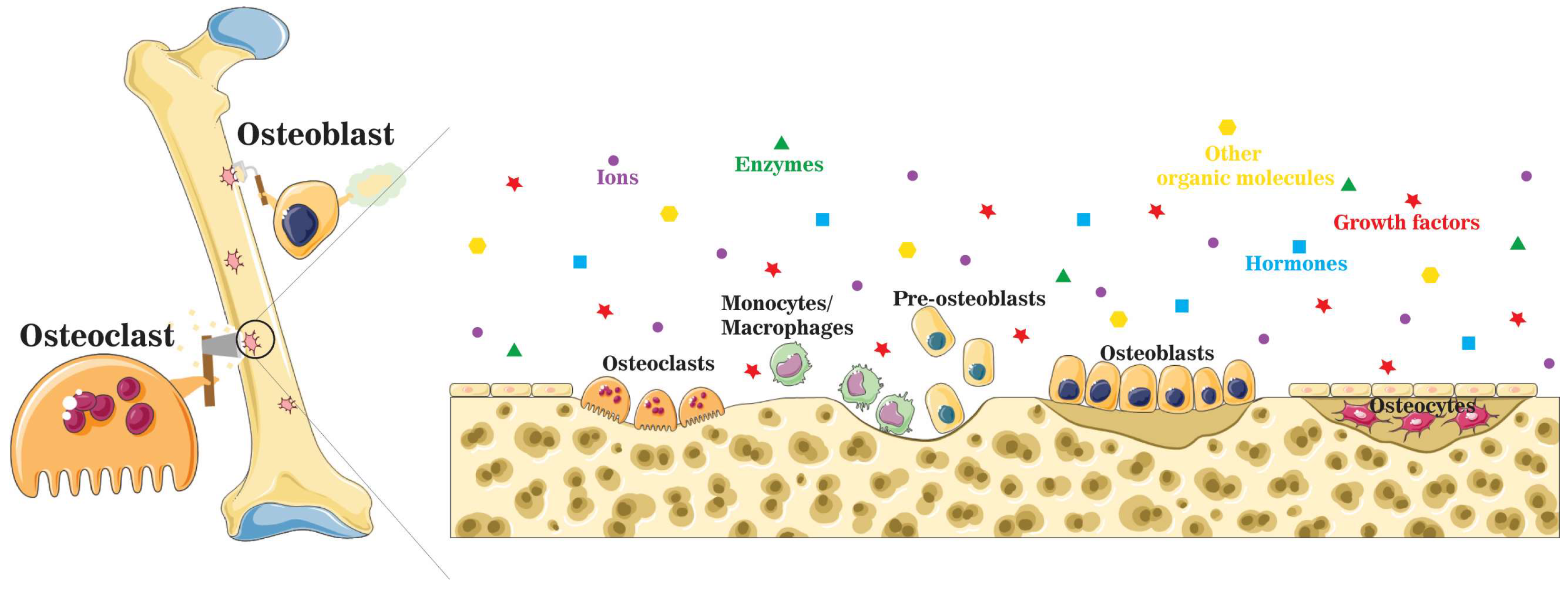
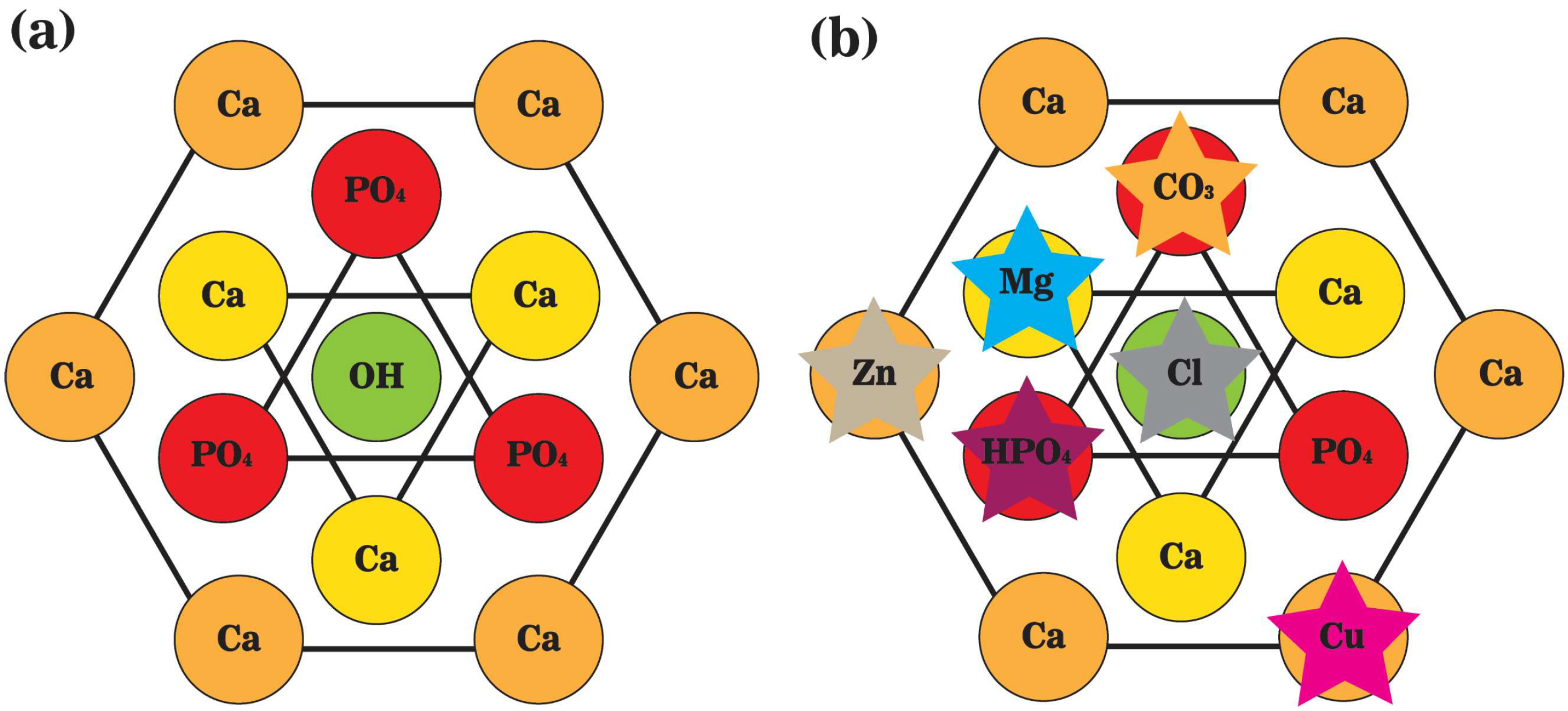
2. Bone Mineral Phase and Localized Ionic Microenvironment
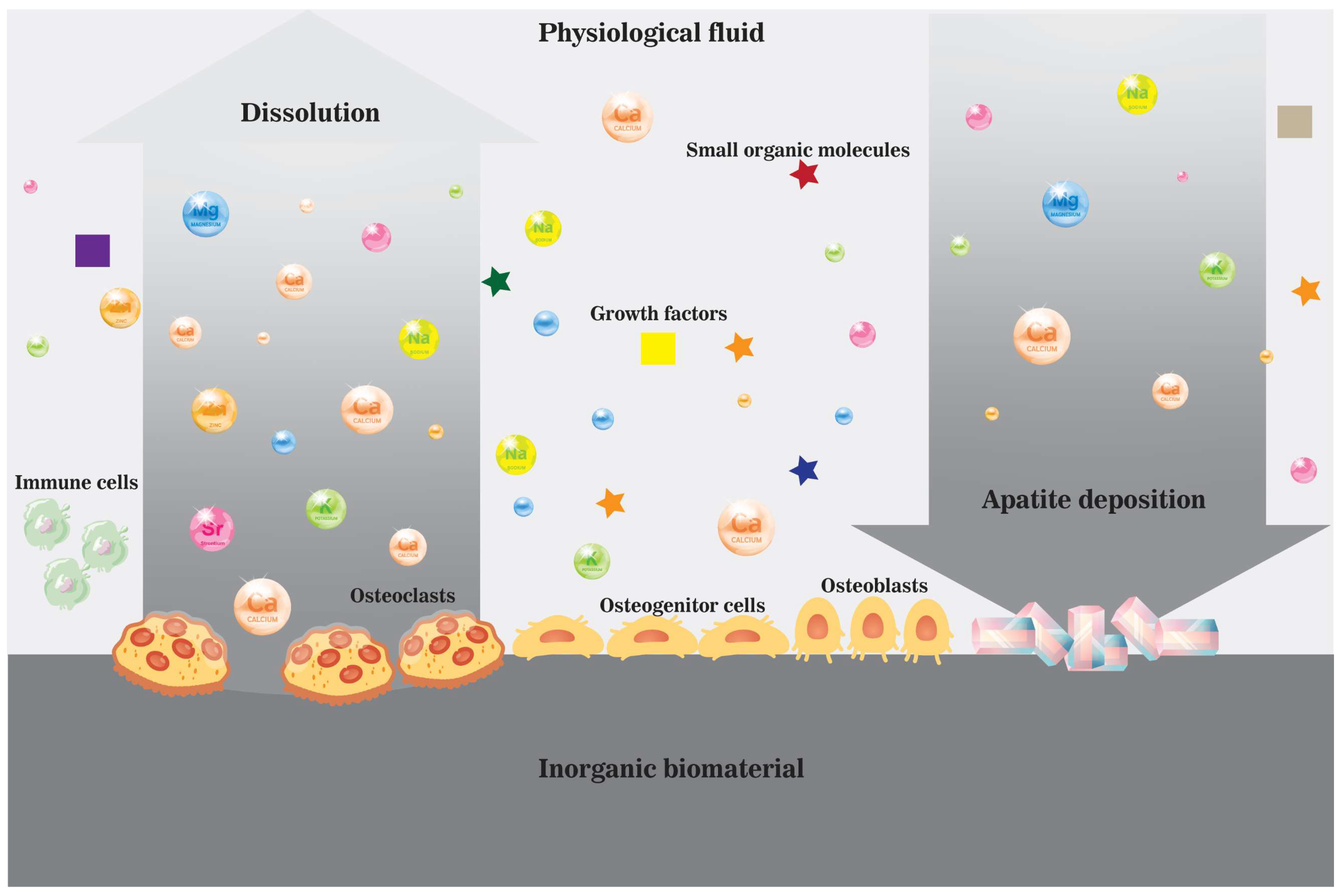
3. Active Osteoinductivity of Inorganic Biomaterials and Enriched Localized Microenvironment
4. Summary of Ions and Ionic Groups in the Maintenance of Bone Homeostasis
4.1. Extracellular Calcium-Ca2+
4.2. Inorganic Orthophosphate—Pi
4.3. Other Bioactive Inorganic Ions
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Itzstein, C.; Coxon, F.P.; Rogers, M.J. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2011, 2, 117–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Isab, A.A.; Ali, S.; Al-Arfaj, A.R. Perspectives in bioinorganic chemistry of some metal based therapeutic agents. Polyhedron 2006, 25, 1633–1645. [Google Scholar] [CrossRef]
- Cohen, S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 2007, 11, 115–120. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [Green Version]
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cells Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef]
- Xie, J.; Baumann, M.J.; McCabe, L.R. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J. Biomed. Mater. Res. Part A 2004, 71, 108–117. [Google Scholar] [CrossRef]
- Hu, C.; Zilm, M.; Wei, M. Fabrication of intrafibrillar and extrafibrillar mineralized collagen/apatite scaffolds with a hierarchical structure. J. Biomed. Mater. Res. Part A 2016, 104, 1153–1161. [Google Scholar] [CrossRef]
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef]
- Ripamonti, U. Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials 2006, 27, 807–822. [Google Scholar] [CrossRef]
- Legeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef]
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef]
- Solheim, E. Growth factors in bone. Int. Orthop. 1998, 22, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Ash, C.; Stone, R. A question of dose-Introduction. Science 2003, 300, 925. [Google Scholar] [CrossRef] [Green Version]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants: A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Orvig, C. Boon and bane of metal ions in medicine. Science 2003, 300, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Morgan, H.; Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P.; Anderson, P. Preparation and characterisation of monoclinic hydroxyapatite and its precipitated carbonate apatite intermediate. Biomaterials 2000, 21, 617–627. [Google Scholar] [CrossRef]
- Buddhachat, K.; Klinhom, S.; Siengdee, P.; Brown, J.L.; Nomsiri, R.; Kaewmong, P.; Thitaram, C.; Mahakkanukrauh, P.; Nganvongpanit, K. Elemental Analysis of Bone, Teeth, Horn and Antler in Different Animal Species Using Non-Invasive Handheld X-Ray Fluorescence. PLoS ONE 2016, 11, e0155458. [Google Scholar] [CrossRef]
- Castro, W.; Hoogewerff, J.; Latkoczy, C.; Almirall, J.R. Application of laser ablation (LA-ICP-SF-MS) for the elemental analysis of bone and teeth samples for discrimination purposes. Forensic Sci. Int. 2010, 195, 17–27. [Google Scholar] [CrossRef]
- Medvecký, Ľ.; Štulajterová, R.; Parilák, Ľ.; Trpčevská, J.; Ďurišin, J.; Barinov, S.M. Influence of manganese on stability and particle growth of hydroxyapatite in simulated body fluid. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 221–229. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, C.; Wei, J.; Wen, Z.; Zhang, S.; Lin, L. Calcium phosphate/chitosan composite coating: Effect of different concentrations of Mg2+ in the m-SBF on its bioactivity. Appl. Surf. Sci. 2013, 280, 256–262. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Omelon, S.J.; Grynpas, M.D. Relationships between Polyphosphate Chemistry, Biochemistry and Apatite Biomineralization. Chem. Rev. 2008, 108, 4694–4715. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Albee, F.H. Studies in bone growth–triple calcium phosphate as stimulus to osteogenesis. Ann. Surg. 1920, 71, 32. [Google Scholar] [CrossRef]
- Roberts, S.C.; Brilliant, J.D. Tricalcium phosphate as an adjunct to apical closure in pulpless permanent teeth. J. Endod. 1975, 1, 263–269. [Google Scholar] [CrossRef]
- Nery, E.; Lynch, K.; Hirthe, W.; Mueller, K. Bioceramic implants in surgically produced infrabony defects. J. Periodontol. 1975, 46, 328–347. [Google Scholar] [CrossRef]
- Köster, K.; Karbe, E.; Kramer, H.; Heide, H.; König, R. Experimental bone replacement with resorbable calcium phosphate ceramic (author’s transl). Langenbecks Arch. Fur. Chir. 1976, 341, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Denissen, H.W.; Groot, K.d. Immediate dental root implants from synthetic dense calcium hydroxylapatite. J. Prosthet. Dent. 1979, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.; Kirschner, M.; Wangemann, T.; Falk, C.; Mempel, W.; Hammer, C. Infections and immunological hazards of allogeneic bone transplantation. Arch. Orthop. Trauma Surg. 1995, 114, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Elsinger, E.C.; Leal, L. Coralline hydroxyapatite bone graft substitutes. J. Foot Ankle Surg. 1996, 35, 396–399. [Google Scholar] [CrossRef]
- Damien, E.; Revell, P.A. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J. Appl. Biomater. Biomech. JABB 2004, 2, 65. [Google Scholar]
- Chou, J.; Austin, C.; Doble, P.; Ben-Nissan, B.; Milthorpe, B. Trace elemental imaging of coralline hydroxyapatite by laser-ablation inductively coupled plasma–mass spectroscopy. J. Tissue Eng. Regen. Med. 2014, 8, 515–520. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium Enhances Osteogenic Differentiation of Mesenchymal Stem Cells and In vivo Bone Formation by Activating Wnt/Catenin Signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Ying, X.; Cheng, S.; Wang, W.; Lin, Z.; Chen, Q.; Zhang, W.; Kou, D.; Shen, Y.; Cheng, X.; Rompis, F.A.; et al. Effect of Boron on Osteogenic Differentiation of Human Bone Marrow Stromal Cells. Biol. Trace Elem. Res. 2011, 144, 306–315. [Google Scholar] [CrossRef]
- Meleti, Z.; Shapiro, I.M.; Adams, C.S. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 2000, 27, 359–366. [Google Scholar] [CrossRef]
- Mladenović, Ž.; Johansson, A.; Willman, B.; Shahabi, K.; Björn, E.; Ransjö, M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 2014, 10, 406–418. [Google Scholar] [CrossRef]
- Yamaguchi, M. Nutritional factors and bone homeostasis: Synergistic effect with zinc and genistein in osteogenesis. Mol. Cell. Biochem. 2012, 366, 201–221. [Google Scholar] [CrossRef]
- Verron, E.; Loubat, A.; Carle, G.F.; Vignes-Colombeix, C.; Strazic, I.; Guicheux, J.; Rochet, N.; Bouler, J.M.; Scimeca, J.-C. Molecular effects of gallium on osteoclastic differentiation of mouse and human monocytes. Biochem. Pharmacol. 2012, 83, 671–679. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Kasuya, S.; Kato-Kogoe, N.; Omori, M.; Yamamoto, K.; Taguchi, S.; Fujita, H.; Imagawa, N.; Sunano, A.; Inoue, K.; Ito, Y.; et al. New Bone Formation Process Using Bio-Oss and Collagen Membrane for Rat Calvarial Bone Defect: Histological Observation. Implant Dent. 2018, 27, 158–164. [Google Scholar] [CrossRef]
- Duda, M.; Pajak, J. The issue of bioresorption of the Bio-Oss xenogeneic bone substitute in bone defects. Ann. Univ. Mariae Curie Sklodowska Med. 2004, 59, 269–277. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2019, 24, 41–56. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Strates, B.S. Bone Morphogenetic Protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef] [PubMed]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urist, M.R.; Lietze, A.; Dawson, E. Beta-tricalcium phosphate delivery system for bone morphogenetic protein. Clin. Orthop. Relat. Res. 1984, 187, 277–280. [Google Scholar] [CrossRef]
- Wang, E.A.; Rosen, V.; Alessandro, J.S.; Bauduy, M.; Cordes, P.; Harada, T.; Israel, D.I.; Hewick, R.M.; Kerns, K.M.; LaPan, P. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 1990, 87, 2220–2224. [Google Scholar] [CrossRef] [Green Version]
- Wozney, J.; Rosen, V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin. Orthop. Relat. Res. 1998, 346, 26–37. [Google Scholar] [CrossRef]
- Boix, T.; Gómez-Morales, J.; Torrent-Burgués, J.; Monfort, A.; Puigdomènech, P.; Rodríguez-Clemente, R. Adsorption of recombinant human bone morphogenetic protein rhBMP-2m onto hydroxyapatite. J. Inorg. Biochem. 2005, 99, 1043–1050. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Kofron, M.D.; Laurencin, C.T. Bone tissue engineering by gene delivery. Adv. Drug Deliv. Rev. 2006, 58, 555–576. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Yano, S.; Tfelt-Hansen, J.; Rooney, P.; Kanuparthi, D.; Bandyopadhyay, S.; Ren, X.; Terwilliger, E.; Brown, E.M. Mitogenic Action of Calcium-Sensing Receptor on Rat Calvarial Osteoblasts. Endocrinology 2004, 145, 3451–3462. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, A.; Habibovic, P. Calcium Phosphates and Angiogenesis: Implications and Advances for Bone Regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-formation ability–predictor of “bioactivity”? Acta Biomater. 2010, 6, 4181–4188. [Google Scholar] [CrossRef]
- Dermience, M.; Lognay, G.; Mathieu, F.; Goyens, P. Effects of thirty elements on bone metabolism. J. Trace Elem. Med. Biol. 2015, 32, 86–106. [Google Scholar] [CrossRef]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Weiss, P.; Guicheux, J.; Beck, L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell. Mol. Life Sci. 2011, 68, 205–218. [Google Scholar] [CrossRef]
- Liu, Y.K.; Lu, Q.Z.; Pei, R.; Ji, H.J.; Zhou, G.S.; Zhao, X.L.; Tang, R.K.; Zhang, M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: Implication for bone tissue engineering. Biomed. Mater. 2009, 4, 025004. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, S.L.; Slatopolsky, E. Sustained activation of the extracellular signal-regulated kinase pathway is required for extracellular calcium stimulation of human osteoblast proliferation. J. Biol. Chem. 2001, 276, 21351. [Google Scholar] [CrossRef] [Green Version]
- Fromigué, O.; Haÿ, E.; Barbara, A.; Petrel, C.; Traiffort, E.; Ruat, M.; Marie, P.J. Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J. Cell. Mol. Med. 2009, 13, 2189–2199. [Google Scholar] [CrossRef]
- Mentaverri, R.; Yano, S.; Chattopadhyay, N.; Petit, L.; Kifor, O.; Kamel, S.; Terwilliger, E.F.; Brazier, M.; Brown, E.M. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J. 2006, 20, 2562–2564. [Google Scholar] [CrossRef]
- Zaidi, M.; Adebanjo, O.A.; Moonga, B.S.; Sun, L.; Huang, C.L.H. Emerging Insights into the Role of Calcium Ions in Osteoclast Regulation. J. Bone Miner. Res. 1999, 14, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Bassett, D.C.; Doillon, C.J.; Gerard, C.; McKee, M.D.; Barralet, J.E. Collagen biomineralization in vivo by sustained release of inorganic phosphate ions. Adv. Mater. 2010, 22, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, Y.; Candeliere, G.A.; Maeda, N.; Aubin, J.E. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol. Cell. Biol. 2007, 27, 4465–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, C.S.; Mansfield, K.; Perlot, R.L.; Shapiro, I.M. Matrix regulation of skeletal cell apoptosis. Role of calcium and phosphate ions. J. Biol. Chem. 2001, 276, 20316–20322. [Google Scholar] [CrossRef] [Green Version]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Kanzawa, M.; Chihara, K. Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell. Physiol. 2003, 196, 180–189. [Google Scholar] [CrossRef]
- Mozar, A.; Haren, N.; Chasseraud, M.; Louvet, L.; Mazière, C.; Wattel, A.; Mentaverri, R.; Morlière, P.; Kamel, S.; Brazier, M. High extracellular inorganic phosphate concentration inhibits RANK–RANKL signaling in osteoclast-like cells. J. Cell. Physiol. 2008, 215, 47–54. [Google Scholar] [CrossRef]
- Brown, R.B.; Razzaque, M.S. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. Bonekey Rep. 2015, 4, 705. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, F.H. Dietary fat composition modifies the effect of boron on bone characteristics and plasma lipids in rats. Biofactors 2004, 20, 161–171. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Steimetz, T.; Nielsen, F.H.; Guglielmotti, M.B. Histomorphometric study of alveolar bone healing in rats fed a boron-deficient diet. Anat. Rec. 2008, 291, 441–447. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Stoecker, B.J. Boron and fish oil have different beneficial effects on strength and trabecular microarchitecture of bone. J. Trace Elem. Med. Biol. 2009, 23, 195–203. [Google Scholar] [CrossRef]
- Uysal, T.; Ustdal, A.; Sonmez, M.F.; Ozturk, F. Stimulation of Bone Formation by Dietary Boron in an Orthopedically Expanded Suture in Rabbits. Angle Orthod. 2009, 79, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Movahedi Najafabadi, B.-A.-H.; Abnosi, M.H. Boron Induces Early Matrix Mineralization via Calcium Deposition and Elevation of Alkaline Phosphatase Activity in Differentiated Rat Bone Marrow Mesenchymal Stem Cells. Cell J. 2016, 18, 62–73. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.S.; Hakki, E.E. Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J. Trace Elem. Med. Biol. 2010, 24, 243–250. [Google Scholar] [CrossRef]
- Roughead, Z.K.; Lukaski, H.C. Inadequate Copper Intake Reduces Serum Insulin-Like Growth Factor-I and Bone Strength in Growing Rats Fed Graded Amounts of Copper and Zinc. J. Nutr. 2003, 133, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 1998, 67, 996S–1002S. [Google Scholar] [CrossRef] [Green Version]
- Uauy, R.; Maass, A.; Araya, M. Estimating risk from copper excess in human populations. Am. J. Clin. Nutr. 2008, 88, 867S–871S. [Google Scholar] [CrossRef] [Green Version]
- Barralet, J.; Gbureck, U.; Habibovic, P.; Vorndran, E.; Gerard, C.; Doillon, C.J. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. Part A 2009, 15, 1601–1609. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Ahmed, I.; Pratten, J.; Nazhat, S.N.; Knowles, J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254. [Google Scholar] [CrossRef]
- Finney, L.; Mandava, S.; Ursos, L.; Zhang, W.; Rodi, D.; Vogt, S.; Legnini, D.; Maser, J.; Ikpatt, F.; Olopade, O.I. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Verron, E.; Bouler, J.M.; Scimeca, J.C. Gallium as a potential candidate for treatment of osteoporosis. Drug Discov. Today 2012, 17, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Bockman, R. The effects of gallium nitrate on bone resorption. Semin. Oncol. 2003, 2003, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.J.; Chambers, T.J. Gallium inhibits bone resorption by a direct effect on osteoclasts. Bone Miner. 1990, 8, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone 2005, 37, 211–219. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, A.M.J. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022. [Google Scholar] [CrossRef] [Green Version]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Miyamoto, I.; Xue, Y.; Andersson, M.; Mustafa, K.; Wennerberg, A.; Jimbo, R. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014, 10, 5193–5201. [Google Scholar] [CrossRef]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High magnesium inhibits human osteoblast differentiation in vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C.; García-Pérez, J. Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin. Dial. 2009, 22, 37–44. [Google Scholar] [CrossRef]
- Katsumata, S.-i.; Katsumata-Tsuboi, R.; Uehara, M.; Suzuki, K. Severe Iron Deficiency Decreases Both Bone Formation and Bone Resorption in Rats. J. Nutr. 2008, 139, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [Green Version]
- Isomura, H.; Fujie, K.; Shibata, K.; Inoue, N.; Iizuka, T.; Takebe, G.; Takahashi, K.; Nishihira, J.; Izumi, H.; Sakamoto, W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology 2004, 197, 92–99. [Google Scholar] [CrossRef]
- He, Y.-F.; Ma, Y.; Gao, C.; Zhao, G.-y.; Zhang, L.-L.; Li, G.-F.; Pan, Y.-Z.; Li, K.; Xu, Y.-J. Iron Overload Inhibits Osteoblast Biological Activity Through Oxidative Stress. Biol. Trace Elem. Res. 2013, 152, 292–296. [Google Scholar] [CrossRef]
- Beattie, J.H.; Avenell, A. Trace element nutrition and bone metabolism. Nutr. Res. Rev. 1992, 5, 167–188. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Vieira, S.I.; Cerqueira, A.R.; Pina, S.; da Cruz Silva, O.A.B.; Abrantes, J.C.C.; Ferreira, J.M.F. Effects of Mn-doping on the structure and biological properties of β-tricalcium phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar]
- Miola, M.; Brovarone, C.V.; Maina, G.; Rossi, F.; Bergandi, L.; Ghigo, D.; Saracino, S.; Maggiora, M.; Canuto, R.A.; Muzio, G. In vitro study of manganese-doped bioactive glasses for bone regeneration. Mater. Sci. Eng. C 2014, 38, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Barrioni, B.R.; Norris, E.; Li, S.; Naruphontjirakul, P.; Jones, J.R.; de Magalhães Pereira, M. Osteogenic potential of sol–gel bioactive glasses containing manganese. J. Mater. Sci. Mater. Med. 2019, 30, 86. [Google Scholar] [CrossRef]
- Yu, L.; Tian, Y.; Qiao, Y.; Liu, X. Mn-containing titanium surface with favorable osteogenic and antimicrobial functions synthesized by PIII&D. Colloids Surf. B Biointerfaces 2017, 152, 376–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hreha, J.; Wey, A.; Cunningham, C.; Krell, E.S.; Brietbart, E.A.; Paglia, D.N.; Montemurro, N.J.; Nguyen, D.A.; Lee, Y.-J.; Komlos, D.; et al. Local manganese chloride treatment accelerates fracture healing in a rat model. J. Orthop. Res. 2015, 33, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Downey, C.M.; Horton, C.R.; Carlson, B.A.; Parsons, T.E.; Hatfield, D.L.; Hallgrímsson, B.; Jirik, F.R. Osteo-chondroprogenitor–specific deletion of the selenocysteine trna gene, Trsp, leads to chondronecrosis and abnormal skeletal development: A putative model for kashin-beck disease. PLoS Genet. 2009, 5, e1000616. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Reyes, R.; Egrise, D.; Nève, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef]
- Ren, F.L.; Guo, X.; Zhang, R.J.; Wang, S.J.; Zuo, H.; Zhang, Z.T.; Geng, D.; Yu, Y.; Su, M. Effects of selenium and iodine deficiency on bone, cartilage growth plate and chondrocyte differentiation in two generations of rats. Osteoarthr. Cartil. 2007, 15, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wolf, E.; Röser, K.; Delling, G.; Müller, P.K. Selenium deficiency and fulvic acid supplementation induces fibrosis of cartilage and disturbs subchondral ossification in knee joints of mice: An animal model study of Kashin-Beck disease. Virchows Arch. A 1993, 423, 483–491. [Google Scholar] [CrossRef]
- Martiniaková, M.; Boboňová, I.; Omelka, R.; Grosskopf, B.; Stawarz, R.; Toman, R. Structural changes in femoral bone tissue of rats after subchronic peroral exposure to selenium. Acta Vet. Scand. 2013, 55, 8. [Google Scholar] [CrossRef] [Green Version]
- Turan, B.; Bayari, S.; Balcik, C.; Severcan, F.; Akkas, N. A biomechanical and spectroscopic study of bone from rats with selenium deficiency and toxicity. Biometals 2000, 13, 113–121. [Google Scholar] [CrossRef]
- Hoeg, A.; Gogakos, A.; Murphy, E.; Mueller, S.; Köhrle, J.; Reid, D.M.; Glüer, C.C.; Felsenberg, D.; Roux, C.; Eastell, R.; et al. Bone Turnover and Bone Mineral Density Are Independently Related to Selenium Status in Healthy Euthyroid Postmenopausal Women. J. Clin. Endocrinol. Metab. 2012, 97, 4061–4070. [Google Scholar] [CrossRef] [Green Version]
- McCann, J.C.; Ames, B.N. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: Why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011, 25, 1793–1814. [Google Scholar] [CrossRef] [Green Version]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic Products of Bioactive Glass Dissolution Increase Proliferation of Human Osteoblasts and Induce Insulin-like Growth Factor II mRNA Expression and Protein Synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef]
- Xynos, I.D.; Hukkanen, M.V.J.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass ®45S5 Stimulates Osteoblast Turnover and Enhances Bone Formation In vitro: Implications and Applications for Bone Tissue Engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [Green Version]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Xiao, Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomater. Sci. 2013, 1, 379–392. [Google Scholar] [CrossRef]
- Zhou, X.; Moussa, F.M.; Mankoci, S.; Ustriyana, P.; Zhang, N.; Abdelmagid, S.; Molenda, J.; Murphy, W.L.; Safadi, F.F.; Sahai, N. Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation. Acta Biomater. 2016, 39, 192–202. [Google Scholar] [CrossRef]
- Schröder, H.C.; Wang, X.H.; Wiens, M.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Müller, W.E.G. Silicate modulates the cross-talk between osteoblasts (SaOS-2) and osteoclasts (RAW 264.7 cells): Inhibition of osteoclast growth and differentiation. J. Cell. Biochem. 2012, 113, 3197–3206. [Google Scholar] [CrossRef]
- Henstock, J.R.; Ruktanonchai, U.R.; Canham, L.T.; Anderson, S.I. Porous silicon confers bioactivity to polycaprolactone composites in vitro. J. Mater. Sci. Mater. Med. 2014, 25, 1087–1097. [Google Scholar] [CrossRef]
- Shi, M.; Zhou, Y.; Shao, J.; Chen, Z.; Song, B.; Chang, J.; Wu, C.; Xiao, Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015, 21, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N.; Quinn, S.J.; Kifor, O.; Ye, C.; Brown, E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, G.; Luk, K.D.K.; Cheung, K.M.C.; Li, Z.; Lam, W.M.; Zhou, Z.; Lu, W.W. Strontium Promotes Osteogenic Differentiation of Mesenchymal Stem Cells Through the Ras/MAPK Signaling Pathway. Cell. Physiol. Biochem. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Choudhary, S.; Halbout, P.; Alander, C.; Raisz, L.; Pilbeam, C. Strontium Ranelate Promotes Osteoblastic Differentiation and Mineralization of Murine Bone Marrow Stromal Cells: Involvement of Prostaglandins. J. Bone Miner. Res. 2007, 22, 1002–1010. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: A physiological approach for optimizing bone formation and resorption. Bone 2006, 38, 10–14. [Google Scholar] [CrossRef]
- Brennan, T.C.; Rybchyn, M.S.; Green, W.; Atwa, S.; Conigrave, A.D.; Mason, R.S. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol. 2009, 157, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- Wornham, D.P.; Hajjawi, M.O.; Orriss, I.R.; Arnett, T.R. Strontium potently inhibits mineralisation in bone-forming primary rat osteoblast cultures and reduces numbers of osteoclasts in mouse marrow cultures. Osteoporos. Int. 2014, 25, 2477–2484. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Neale Weitzmann, M. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 2012, 359, 399–407. [Google Scholar] [CrossRef]
- Peng, S.; Liu, X.S.; Huang, S.; Li, Z.; Pan, H.; Zhen, W.; Luk, K.D.K.; Guo, X.E.; Lu, W.W. The cross-talk between osteoclasts and osteoblasts in response to strontium treatment: Involvement of osteoprotegerin. Bone 2011, 49, 1290–1298. [Google Scholar] [CrossRef]
- Baron, R.; Tsouderos, Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur. J. Pharmacol. 2002, 450, 11–17. [Google Scholar] [CrossRef]
- Hurtel-Lemaire, A.S.; Mentaverri, R.; Caudrillier, A.; Cournarie, F.; Wattel, A.; Kamel, S.; Terwilliger, E.F.; Brown, E.M.; Brazier, M. The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J. Biol. Chem. 2009, 284, 575. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials 2013, 34, 3184–3195. [Google Scholar] [CrossRef]
- Luo, X.; Barbieri, D.; Zhang, Y.; Yan, Y.; Bruijn, J.D.; Yuan, H. Strontium-Containing Apatite/Poly Lactide Composites Favoring Osteogenic Differentiation and in vivo Bone Formation. ACS Biomater. Sci. Eng. 2015, 1, 85–93. [Google Scholar] [CrossRef]
- Hill, T.; Meunier, N.; Andriollo-Sanchez, M.; Ciarapica, D.; Hininger-Favier, I.; Polito, A.; O’Connor, J.M.; Coudray, C.; Cashman, K.D. The relationship between the zinc nutritive status and biochemical markers of bone turnover in older European adults: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, S73–S78. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Migliaccio, S.; Corsi, A.; Marzia, M.; Bianco, P.; Teti, A.; Gambelli, L.; Cianfarani, S.; Paoletti, F.; Branca, F. Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J. Nutr. 2001, 131, 1142–1146. [Google Scholar] [CrossRef] [Green Version]
- Yusa, K.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In vitro prominent bone regeneration by release zinc ion from Zn-modified implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Hadley, K.B.; Newman, S.M.; Hunt, J.R. Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats. J. Nutr. Biochem. 2010, 21, 297–303. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of zinc in bone formation and bone resorption. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 1998, 11, 119–135. [Google Scholar] [CrossRef]
- Kwun, I.-S.; Cho, Y.-E.; Lomeda, R.-A.R.; Shin, H.-I.; Choi, J.-Y.; Kang, Y.-H.; Beattie, J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 2010, 46, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moonga, B.S.; Dempster, D.W. Zinc is a potent inhibitor of osteoclastic bone resorption in vitro. J. Bone Miner. Res. 1995, 10, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Weitzmann, M.N. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 2011, 355, 179. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. The calcium-sensing receptor in bone cells: A potential therapeutic target in osteoporosis. Bone 2010, 46, 571–576. [Google Scholar] [CrossRef]
- Melita, M.D.; Ashia, S.; Donald, T.W.; Carter, D.H.; Sarah, L.D.; Edward, F.N.; Daniela, R. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. USA 2004, 101, 5140. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.; González, A.; Planell, J.A.; Engel, E. Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2010, 393, 156–161. [Google Scholar] [CrossRef]
- Gustavsson, J.; Ginebra, M.; Planell, J.; Engel, E. Osteoblast-like cellular response to dynamic changes in the ionic extracellular environment produced by calcium-deficient hydroxyapatite. J. Mater. Sci. Mater. Med. 2012, 23, 2509–2520. [Google Scholar] [CrossRef]
- Yamauchi, M.; Yamaguchi, T.; Kaji, H.; Sugimoto, T.; Chihara, K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E608–E616. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.C.; Roberts, S.J.; Schrooten, J.; Luyten, F.P. Probing the Osteoinductive Effect of Calcium Phosphate by Using an In vitro Biomimetic Model. Tissue Eng. Part A 2010, 17, 1083–1097. [Google Scholar] [CrossRef]
- Nakade, O.; Takahashi, K.; Takuma, T.; Aoki, T.; Kaku, T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J. Bone Miner. Metab. 2001, 19, 13–19. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Godwin, S.L.; Soltoff, S.P. Calcium-sensing receptor-mediated activation of phospholipase C-γ1 is downstream of phospholipase C-β and protein kinase C in MC3T3-E1 osteoblasts. Bone 2002, 30, 559–566. [Google Scholar] [CrossRef]
- Choudhary, S.; Kumar, A.; Kale, R.K.; Raisz, L.G.; Pilbeam, C.C. Extracellular calcium induces COX-2 in osteoblasts via a PKA pathway. Biochem. Biophys. Res. Commun. 2004, 322, 395–402. [Google Scholar] [CrossRef]
- Honda, Y.; Fitzsimmons, R.J.; Baylink, D.J.; Mohan, S. Effects of extracellular calcium on insulin-like growth factor II in human bone cells. J. Bone Miner. Res. 1995, 10, 1660–1665. [Google Scholar] [CrossRef]
- Choudhary, S.; Wadhwa, S.; Raisz, L.G.; Alander, C.; Pilbeam, C.C. Extracellular Calcium Is a Potent Inducer of Cyclo-oxygenase-2 in Murine Osteoblasts Through an ERK Signaling Pathway. J. Bone Miner. Res. 2003, 18, 1813–1824. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsumoto, T.; Sasaki, J.-I.; Egusa, H.; Lee, K.Y.; Nakano, T.; Sohmura, T.; Nakahira, A. Effect of Calcium Ion Concentrations on Osteogenic Differentiation and Hematopoietic Stem Cell Niche-Related Protein Expression in Osteoblasts. Tissue Eng. Part A 2010, 16, 2467–2473. [Google Scholar] [CrossRef]
- Bergwitz, C.; Jüppner, H. Regulation of Phosphate Homeostasis by PTH, Vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Chihara, K. IGF-I mediates the stimulatory effect of high phosphate concentration on osteoblastic cell proliferation. J. Cell. Physiol. 2002, 190, 306–312. [Google Scholar] [CrossRef]
- Conrads, K.A.; Yi, M.; Simpson, K.A.; Lucas, D.A.; Camalier, C.E.; Yu, L.-R.; Veenstra, T.D.; Stephens, R.M.; Conrads, T.P.; Beck, G.R. A Combined Proteome and Microarray Investigation of Inorganic Phosphate-induced Pre-osteoblast Cells. Mol. Cell. Proteom. 2005, 4, 1284. [Google Scholar] [CrossRef] [Green Version]
- Beck, G.R.; Knecht, N. Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent. J. Biol. Chem. 2003, 278, 41921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-Dependent Regulation of MGP in Osteoblasts: Role of ERK1/2 and Fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Khoshniat, S.; Bourgine, A.; Julien, M.; Petit, M.; Pilet, P.; Rouillon, T.; Masson, M.; Gatius, M.; Weiss, P.; Guicheux, J.; et al. Phosphate-dependent stimulation of MGP and OPN expression in osteoblasts via the ERK1/2 pathway is modulated by calcium. Bone 2011, 48, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Bakiri, L.; Jimenez, M.; Schinke, T.; Amling, M.; Wagner, E.F. Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production. J. Cell Biol. 2010, 190, 1093–1106. [Google Scholar] [CrossRef] [Green Version]
- Yates, A.J.; Oreffo, R.O.C.; Mayor, K.; Mundy, G.R. Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts. J. Bone Miner. Res. 1991, 6, 473–478. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Q.; Lu, S. From selenoprotein to endochondral ossification: A novel mechanism with microRNAs potential in bone related diseases? Med. Hypotheses 2011, 77, 807–811. [Google Scholar] [CrossRef]
- Sripanyakorn, S.; Jugdaohsingh, R.; Thompson, R.P.H.; Powell, J.J. Dietary silicon and bone health. Nutr. Bull. 2005, 30, 222–230. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A requirement in bone formation independent of vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort. J. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Nieves, J.W. Skeletal effects of nutrients and nutraceuticals, beyond calcium and vitamin D. Osteoporos. Int. 2013, 24, 771–786. [Google Scholar] [CrossRef]
- Hott, M.; de Pollak, C.; Modrowski, D.; Marie, P.J. Short-term effects of organic silicon on trabecular bone in mature ovariectomized rats. Calcif. Tissue Int. 1993, 53, 174–179. [Google Scholar] [CrossRef]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef]
- Buehler, J.; Chappuis, P.; Saffar, J.L.; Tsouderos, Y.; Vignery, A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis). Bone 2001, 29, 176–179. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Kaufman, J.M.; Goemaere, S.; Devogelaer, J.P.; Benhamou, C.L.; Felsenberg, D.; Diaz-Curiel, M.; Brandi, M.L.; Badurski, J.; Wark, J.; et al. Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos. Int. 2012, 23, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Verberckmoes, S.C.; De Broe, M.E.; D’Haese, P.C. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003, 64, 534–543. [Google Scholar] [CrossRef] [Green Version]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clin. Toxicol. 2011, 3, 0495. [Google Scholar] [CrossRef] [Green Version]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Hallab, N.J.; Vermes, C.; Messina, C.; Roebuck, K.A.; Glant, T.T.; Jacobs, J.J. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J. Biomed. Mater. Res. 2002, 60, 420–433. [Google Scholar] [CrossRef]
- Anissian, L.; Stark, A.; Dahlstrand, H.; Granberg, B.; Good, V.; Bucht, E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop. Scand. 2002, 73, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queally, J.M.; Devitt, B.M.; Butler, J.S.; Malizia, A.P.; Murray, D.; Doran, P.P.; O’Byrne, J.M. Cobalt ions induce chemokine secretion in primary human osteoblasts. J. Orthop. Res. 2009, 27, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials 2010, 31, 3580–3589. [Google Scholar] [CrossRef] [Green Version]
- Birgani, Z.T.; Gharraee, N.; Malhotra, A.; Van Blitterswijk, C.A.; Habibovic, P. Combinatorial incorporation of fluoride and cobalt ions into calcium phosphates to stimulate osteogenesis and angiogenesis. Biomed. Mater. 2016, 11, 015020. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Milan, P.B.; Hamzehlou, S.; Barati, M.; Baino, F.; Hill, R.G.; Joghataei, M.T. Strontium-and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef]

| Ion | Related Disorders or Diseases | Effects on Cellular Activities | References | |
|---|---|---|---|---|
| + | − | |||
| Ca | Deficiency: rickets, osteomalacia, and osteoporosis; Overload: poor bone health, kidney stone formation, and abnormal heart and brain function | MSC mineralization, osteoblast cell proliferation, survival and differentiation, osteoclast cell apoptosis | Osteoblast cell apoptosis, bone resorption | [9,74,76,77,78,79,80] |
| Pi | Deficiency: impaired bone mineralization, dysfunction in blood, muscle, central nervous system, cardio and respiratory system; Overload: kidney disease, cardiovascular disease, cancer, and skeletal disorder | Osteoblast and osteoclast cell apoptosis (high Pi level), osteoblastic differentiation and mineralization, bone resorption (low Pi level) | Bone resorption (at high Pi levels) | [50,75,81,82,83,84,85,86] |
| B | Deficiency: reduced osteogenesis, inhibited bone formation, decreased bone volume, and reduced mechanical strength | MSC and osteoblast osteogenic differentiation and mineralization | * | [49,87,88,89,90,91,92] |
| Cu | Deficiency: abnormal bone formation with impaired quality and strength, severe neurological issues, or liver diseases | angiogenesis, innate antibacterial property, extracellular matrix formation | * | [4,93,94,95,96,97,98,99] |
| Ga | * | Bone formation and mineralization | Osteoclast differentiation, bone resorption | [53,100,101,102] |
| Mg | Deficiency: impaired bone growth, disrupted mineral metabolism, and osteoporosis | MSC osteogenic differentiation and mineralization | Osteoblast differentiation (high Mg level) | [103,104,105,106,107,108,109,110,111,112] |
| Fe | Deficiency: overall loss in bone mass and density, impaired biomechanical strength Overload: metabolic bone diseases such as osteoporosis, altered bone microarchitecture, and reduced biomechanical strength | Bone resorption (high Fe level) | Osteoblast cell maturation and differentiation (high Fe level) | [113,114,115,116] |
| Mn | Deficiency: abnormal bone growth, such as stunted bone growth and osteoporosis; Overload: impaired bone development and neurotoxicity | Osteoblast proliferation, adhesion, and spreading, osteoblastic differentiation, collagen deposition, angiogenesis, and bone healing | * | [21,117,118,119,120,121,122,123] |
| Se | Deficiency: impaired bone and cartilage metabolism, osteopenia, osteoporosis, and Kashin-Beck disease (together with iodine); Overload: decreased mineral content, altered bone structure, and reduced biomechanical strength | ** | * | [74,124,125,126,127,128,129,130,131] |
| Si | Deficiency: abnormal bone growth | Osteoblast cell growth, proliferation, and differentiation | Osteoclast formation, recruitment, and bone resorption, as well as osteoblast-induced osteoclastogenesis | [132,133,134,135,136,137,138,139,140] |
| Sr | * | Pre-osteoblast cell replication and collagen synthesis, osteoblast cell proliferation, survival, differentiation, mineralization, osteoclast cell apoptosis | Osteoclast cell survival, differentiation, osteoblast-induced osteoclastogenesis, and bone resorption | [48,78,141,142,143,144,145,146,147,148,149,150,151,152,153] |
| Zn | Deficiency: abnormal immune response, impaired wound healing, overall bone mass, and health, and bone turnover rate | MSC viability, osteoblastic differentiation, and mineralization, osteoblast cell proliferation, differentiation, and mineralization | Osteoclastogenesis and bone resorption | [154,155,156,157,158,159,160,161,162,163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Y.; Du, Z.; Xiao, L.; Gao, W.; Crawford, R.; Xiao, Y. The Localized Ionic Microenvironment in Bone Modelling/Remodelling: A Potential Guide for the Design of Biomaterials for Bone Tissue Engineering. J. Funct. Biomater. 2023, 14, 56. https://doi.org/10.3390/jfb14020056
Mu Y, Du Z, Xiao L, Gao W, Crawford R, Xiao Y. The Localized Ionic Microenvironment in Bone Modelling/Remodelling: A Potential Guide for the Design of Biomaterials for Bone Tissue Engineering. Journal of Functional Biomaterials. 2023; 14(2):56. https://doi.org/10.3390/jfb14020056
Chicago/Turabian StyleMu, Yuqing, Zhibin Du, Lan Xiao, Wendong Gao, Ross Crawford, and Yin Xiao. 2023. "The Localized Ionic Microenvironment in Bone Modelling/Remodelling: A Potential Guide for the Design of Biomaterials for Bone Tissue Engineering" Journal of Functional Biomaterials 14, no. 2: 56. https://doi.org/10.3390/jfb14020056
APA StyleMu, Y., Du, Z., Xiao, L., Gao, W., Crawford, R., & Xiao, Y. (2023). The Localized Ionic Microenvironment in Bone Modelling/Remodelling: A Potential Guide for the Design of Biomaterials for Bone Tissue Engineering. Journal of Functional Biomaterials, 14(2), 56. https://doi.org/10.3390/jfb14020056










