In-Vitro Evaluation of Photofunctionalized Implant Surfaces in a High-Glucose Microenvironment Simulating Diabetics
Abstract
1. Introduction
2. Material and Methods
2.1. Photofunctionalization Unit
2.2. X-ray Photoelectron Spectroscopy (XPS) Based Implant Surface Analysis
2.3. Cell Viability and Attachment Assay
2.4. Mineralization Assay
2.5. Statistical Analysis
3. Results
3.1. XPS Implant Surface Analysis
3.2. XPS Core-Level Spectra of C, O, and Ti in the Study Groups
3.3. Morphology and Spreading Behavior of Osteoblasts Using Phase-Contrast Microscopy
3.4. Cell Viability Assay for the Dental Implant Surfaces
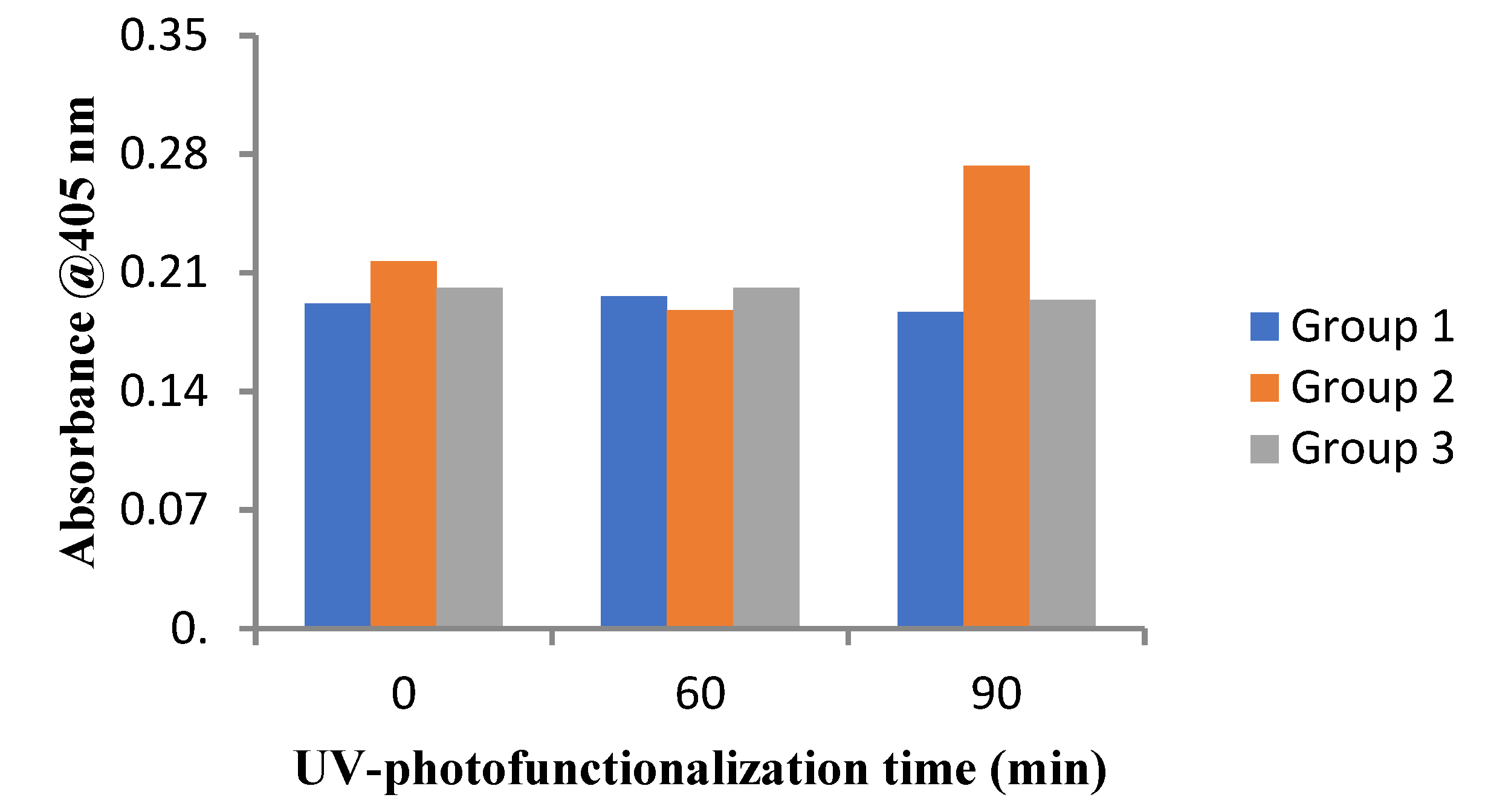
3.5. Alizarin Red S Assay for the Dental Implant Surfaces
4. Discussion
5. Conclusions
6. Clinical Relevance
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater 2017, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Sugita, Y.; Honda, Y.; Kato, D.D.S.I. Role of Photofunctionalization in Mitigating Impaired Osseointegration Associated with Type 2 Diabetes in Rats. Int. J. Oral Maxillofac. Implants 2014, 29, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Lin, J.H.; Ju, C.P.; Ong, H.C.; Chang, R.P. Structural characterization of pulsed laser-deposited hydroxyapatite film on titanium substrate. Biomaterials 1997, 18, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Mosca Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface, Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Dini, C.; Nagay, B.E.; Magno, M.B.; Maia, L.C.; Barão, V.A.R. Photofunctionalization as a suitable approach to improve the osseointegration of implants in animal models—A systematic review and meta-analysis. Clin. Oral Implants Res. 2020, 31, 785–802. [Google Scholar] [CrossRef]
- Funato, A.; Yamada, D.D.S.M.; Ogawa, T. Success Rate, Healing Time, and Implant Stability of Photofunctionalized Dental Implants. Int. J. Oral Maxillofac. Implants 2013, 28, 1261–1271. [Google Scholar] [CrossRef]
- Funato, A.; Ogawa, T. Photofunctionalized Dental Implants: A Case Series in Compromised Bone. Int. J. Oral Maxillofac. Implants 2013, 28, 1589–1601. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Jimbo, R.; Shibata, Y.; Watanabe, I.; Wennerberg, A.; Sawase, T. Accelerated bone formation on photo-induced hydrophilic titanium implants: An experimental study in the dog mandible. Clin. Oral Implants Res. 2013, 24, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Okubo, T.; Saruta, J.; Hirota, M.; Kitajima, H.; Yaagisawa, N.; Ogawa, T. Osteoblast attachment compromised by high and low temperature of titanium and its restoration by UV photofunctionalization. Materials 2021, 14, 5493. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.; DiPietro, L. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Kanazawa, I.; Yamaguchi, T.; Yamamoto, M.; Yamauchi, M.; Kurioka, S.; Yano, S.; Sugimoto, T. Serum Osteocalcin Level Is Associated with Glucose Metabolism and Atherosclerosis Parameters in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2009, 94, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, J.C.; Mckenna, M.J.; Buderer, N.F.; Rao, D.S.; Whitehouse, F.W.; Parfitt, A.M. Bone Loss and Bone Turnover in Diabetes. Diabetes 1995, 44, 775–782. [Google Scholar] [CrossRef]
- Pedrazzoni, M.; Ciotti, G.; Pioli, G.; Girasole, G.; Davoli, L.; Palummeri, E.; Passeri, M. Osteocalcin levels in diabetic subjects. Calcif. Tissue Int. 1989, 45, 331–336. [Google Scholar] [CrossRef]
- Shu, A.; Yin, M.T.; Stein, E.; Cremers, S.; Dworakowski, E.; Ives, R.; Rubin, M.R. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos. Int. 2012, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Chronic hyperglycemia as a risk factor in implant therapy. Periodontology 2000 2019, 81, 57–63. [Google Scholar] [CrossRef]
- Moraschini, V.; Barboza, E.S.P.; Peixoto, G.A. The impact of diabetes on dental implant failure: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 1237–1245. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, Y.; Liu, Z.; Tian, Z.; Zhu, S. Association between diabetes and dental implant complications: A systematic review and meta-analysis. Acta Odontol. Scand. 2021, 79, 9–18. [Google Scholar] [CrossRef]
- Al Ansari, Y.; Shahwan, H.; Chrcanovic, B.R. Diabetes Mellitus and Dental Implants: A Systematic Review and Meta-Analysis. Materials 2022, 15, 3227. [Google Scholar] [CrossRef] [PubMed]
- Pacicca, D.M.; Brown, T.; Watkins, D.; Kover, K.; Yan, Y.; Prideaux, M.; Bonewald, L. Elevated glucose acts directly on osteocytes to increase sclerostin expression in diabetes. Sci. Rep. 2019, 9, 17353. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet Photofunctionalization of Titanium Implants. Int. J. Oral Maxillofac. Implants 2014, 29, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper cobalt sulphide nanosheets realizing a promising electrocatalytic oxygen evolution reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Zhou, L.; Guo, Z.; Rong, M.; Liu, X.; Lai, C.; Ding, X. The Effects of Different Wavelength UV Photofunctionalization on Micro-Arc Oxidized Titanium. PLoS ONE 2013, 8, e68086. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Sawase, T.; Hai, K.; Yoshida, K.; Baba, K.; Hatada, R.; Atsuta, M. Spectroscopic studies of three osseointegrated implants. J. Dent. 1998, 26, 119–124. [Google Scholar] [CrossRef]
- Roy, M.; Pompella, A.; Kubacki, J.; Szade, J.; Roy, R.A.; Hedzelek, W. Photofunctionalization of Titanium: An Alternative Explanation of Its Chemical-Physical Mechanism. PLoS ONE 2016, 11, e0157481. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakamoto, K.; Martra, G.; Coluccia, S.; Anpo, M. Mechanism of Photoinduced Superhydrophilicity on the TiO2 Photocatalyst Surface. J. Phys. Chem. B 2005, 109, 15422–15428. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Lee, M.C.-I.; Ogawa, T. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T. Photofunctionalization of TiO2 for Optimal Bone-titanium Integration: A Novel Phenomenon of Super Osseointegration. In Environmentally Benign Photocatalysts. Nanostructure Science and Technology; Anpo, M., Kamat, P., Eds.; Springer: New York, NY, USA, 2010; pp. 699–713. ISBN 978-0-387-48441-9. [Google Scholar] [CrossRef]
- Ogawa, T.; Iwasa, F.; Tsukimura, N.; Att, W.; Kodali-Kanuru, R.; Kubo, K.; Hasnain, H. TiO2 micro-nano-hybrid surface to alleviate biological aging of UV-photofunctionalized titanium. Int. J. Nanomed. 2011, 6, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.-A.; Ogawa, T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.A.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminosn, M.; Gil, F.J. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Sammons, R.L.; Lumbikanonda, N.; Gross, M.; Cantzler, P. Comparison of osteoblast spreading on microstructured dental implant surfaces and cell behaviour in an explant model of osseointegration. Clin. Oral Implants Res. 2005, 16, 657–666. [Google Scholar] [CrossRef]
- Choi, B.; Lee, Y.C.; Oh, K.C.; Lee, J.H. Effects of photofunctionalization on early osseointegration of titanium dental implants in the maxillary posterior region: A randomized double-blinded clinical trial. Int. J. Implant Dent. 2021, 7, 37. [Google Scholar] [CrossRef]
- Chang, L.C. Clinical Applications of Photofunctionalization on Dental Implant Surfaces: A Narrative Review. J. Clin. Med. 2022, 11, 5823. [Google Scholar] [CrossRef]
- Roy, M.; Corti, A.; Dorocka-Bobkowska, B.; Pompella, A. Positive Effects of UV-Photofunctionalization of Titanium Oxide Surfaces on the Survival and Differentiation of Osteogenic Precursor Cells—An In Vitro Study. J. Funct. Biomater. 2022, 13, 265. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res. A 2014, 102, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet Treatment Overcomes Time-Related Degrading Bioactivity of Titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef] [PubMed]

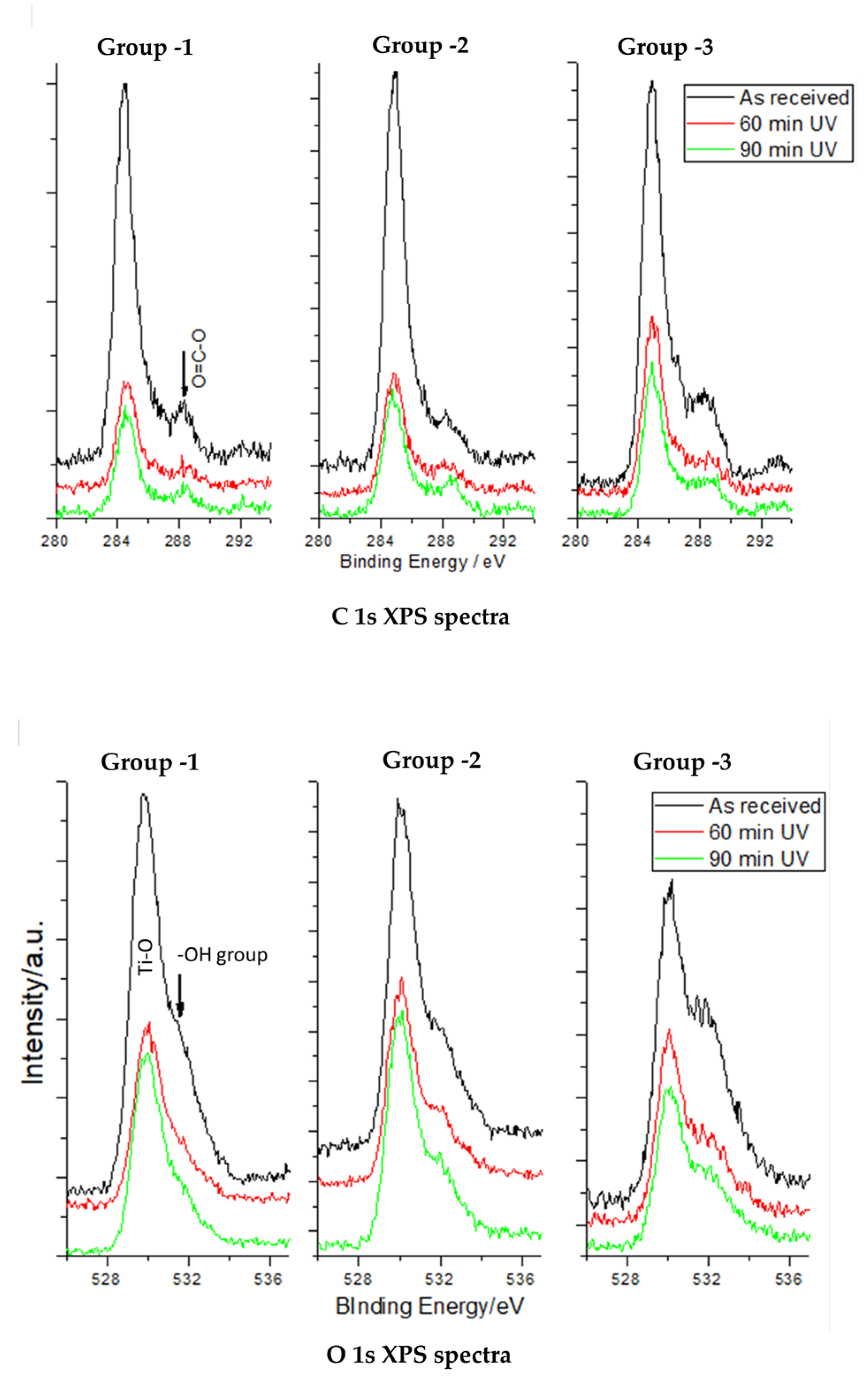
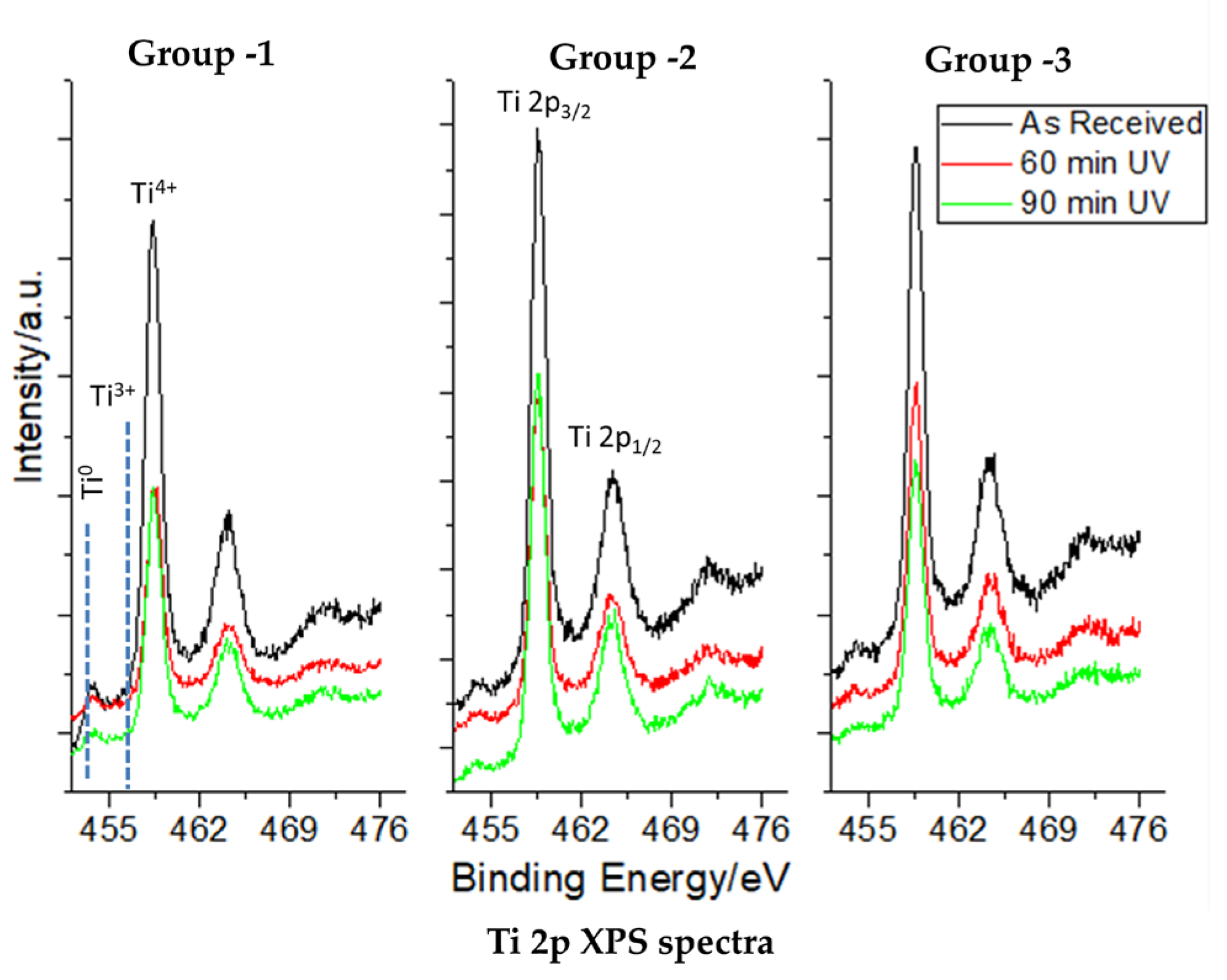


| Control (Mean ± SD) | 60 min (Mean ± SD) | 90 min (Mean ± SD) | |
|---|---|---|---|
| C 1s | |||
| Group 1 | 20.63 ± 0.058 | 10.10 ± 0.100 | 7.50 ± 0.033 |
| Group 2 | 20.77 ± 0.058 | 12.76 ± 0.100 | 9.45 ± 0.033 |
| Group 3 | 22.67 ± 0.580 | 18.50 ± 0.580 | 16.20 ± 0.010 |
| O 1s | |||
| Group 1 | 40.60 ± 0.100 | 43.87 ± 0.58 | 43.80 ± 0.100 |
| Group 2 | 40.33 ± 0.577 | 42.70 ± 0.100 | 44.60 ± 0.100 |
| Group 3 | 41.53 ± 0.153 | 41.76 ± 0.058 | 44.10 ± 0.100 |
| Ti 2p | |||
| Group 1 | 37.55 ± 0.306 | 43.30 ± 0.100 | 46.07 ± 0.058 |
| Group 2 | 39.20 ± 0.100 | 41.80 ± 0.100 | 42.33 ± 0.058 |
| Group 3 | 31.40 ± 0.100 | 34.65 ± 0.026 | 36.67 ± 0.577 |
| Baseline Mean (%) ± SD | 90 min Mean (%) ± SD | |
|---|---|---|
| Group 1 | 100 ± 3 a | 125 ± 3.75 b |
| Group 2 | 100 ± 3 a | 110.04 ± 3.30 a |
| Group 3 | 100 ± 3 a | 125 ± 3.75 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheur, S.; Kheur, M.; Madiwal, V.; Sandhu, R.; Lakha, T.; Rajwade, J.; Eyüboğlu, T.F.; Özcan, M. In-Vitro Evaluation of Photofunctionalized Implant Surfaces in a High-Glucose Microenvironment Simulating Diabetics. J. Funct. Biomater. 2023, 14, 130. https://doi.org/10.3390/jfb14030130
Kheur S, Kheur M, Madiwal V, Sandhu R, Lakha T, Rajwade J, Eyüboğlu TF, Özcan M. In-Vitro Evaluation of Photofunctionalized Implant Surfaces in a High-Glucose Microenvironment Simulating Diabetics. Journal of Functional Biomaterials. 2023; 14(3):130. https://doi.org/10.3390/jfb14030130
Chicago/Turabian StyleKheur, Supriya, Mohit Kheur, Vaibhav Madiwal, Ramandeep Sandhu, Tabrez Lakha, Jyutika Rajwade, Tan Fırat Eyüboğlu, and Mutlu Özcan. 2023. "In-Vitro Evaluation of Photofunctionalized Implant Surfaces in a High-Glucose Microenvironment Simulating Diabetics" Journal of Functional Biomaterials 14, no. 3: 130. https://doi.org/10.3390/jfb14030130
APA StyleKheur, S., Kheur, M., Madiwal, V., Sandhu, R., Lakha, T., Rajwade, J., Eyüboğlu, T. F., & Özcan, M. (2023). In-Vitro Evaluation of Photofunctionalized Implant Surfaces in a High-Glucose Microenvironment Simulating Diabetics. Journal of Functional Biomaterials, 14(3), 130. https://doi.org/10.3390/jfb14030130








