Titanium-Enriched Medium Promotes Environment-Induced Epigenetic Machinery Changes in Human Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
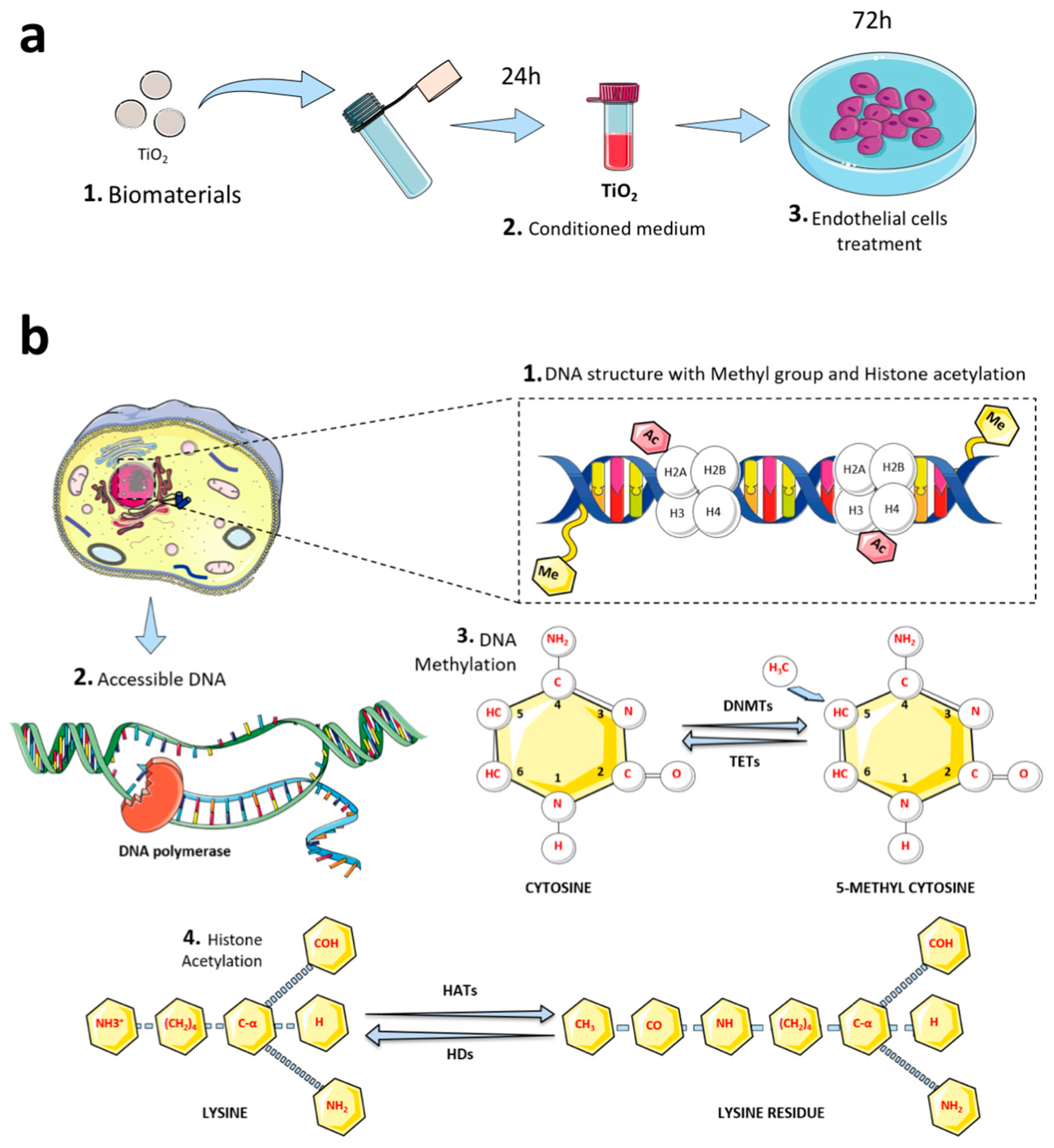
2.2. Titanium-Enriched Medium and Experimental Design
2.3. Cell Culture
2.4. Gene Expression
2.4.1. RNA Isolation and cDNA Synthesis
2.4.2. Gene Expression
2.5. Western Blot
2.6. Statistical Analysis
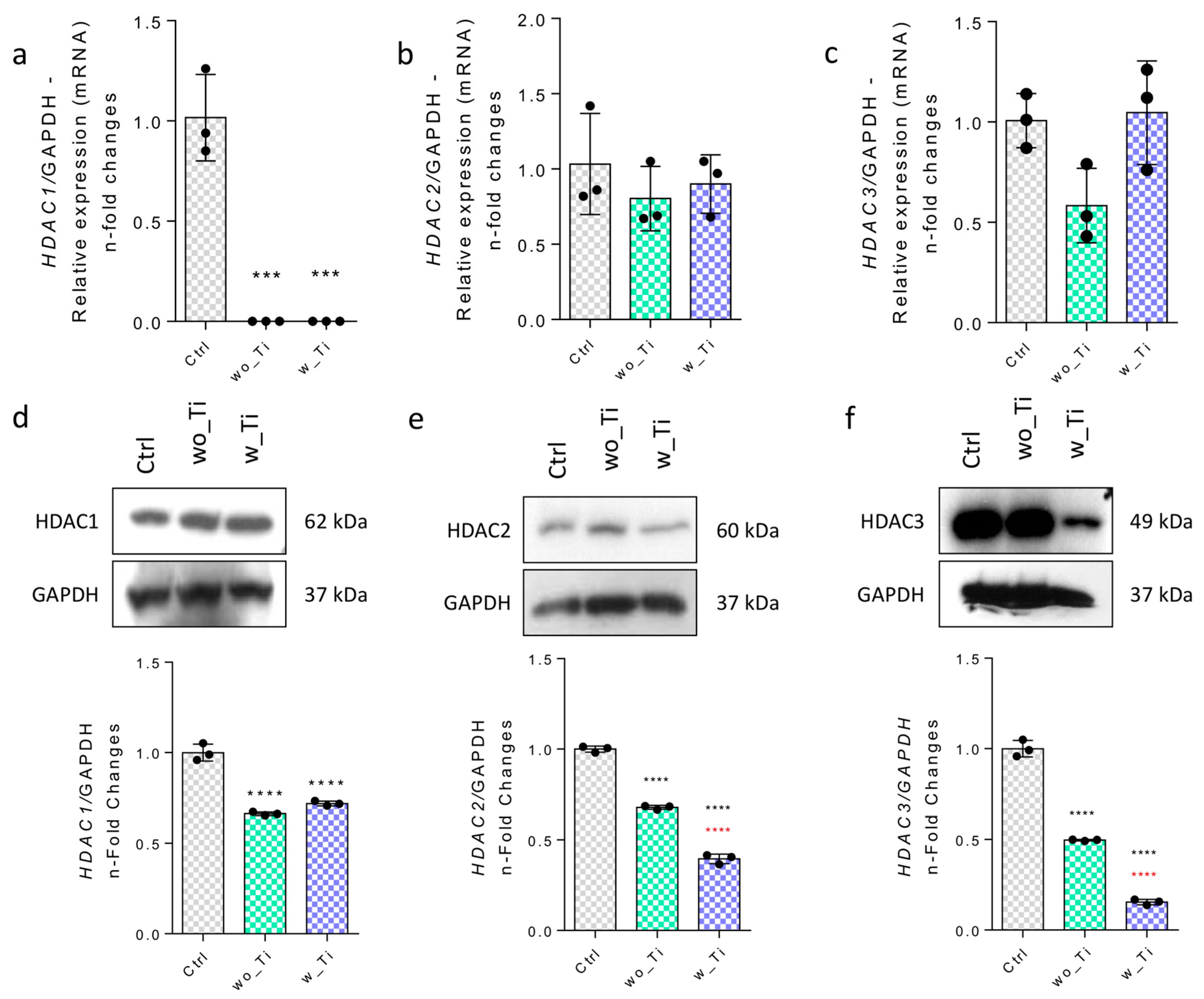
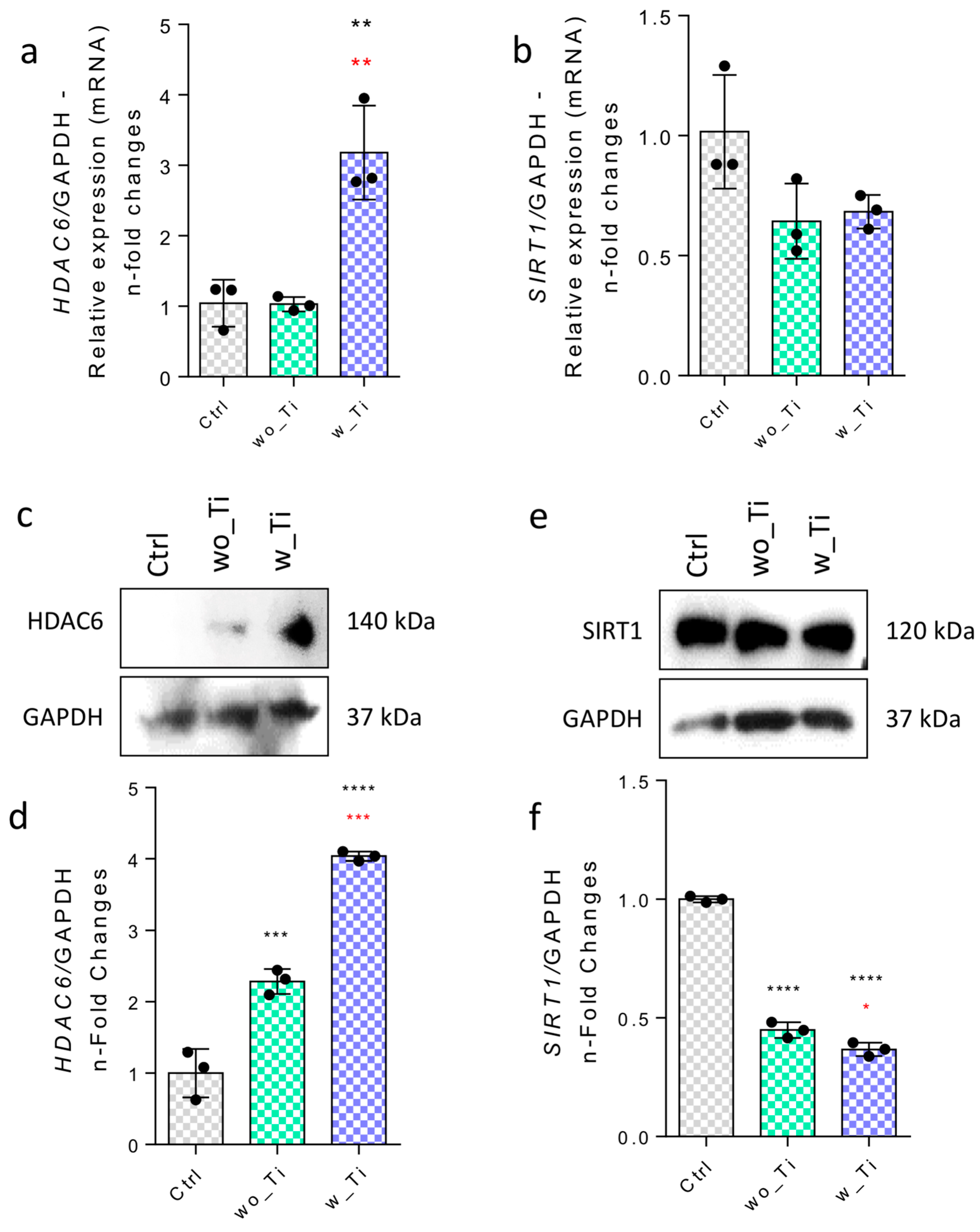
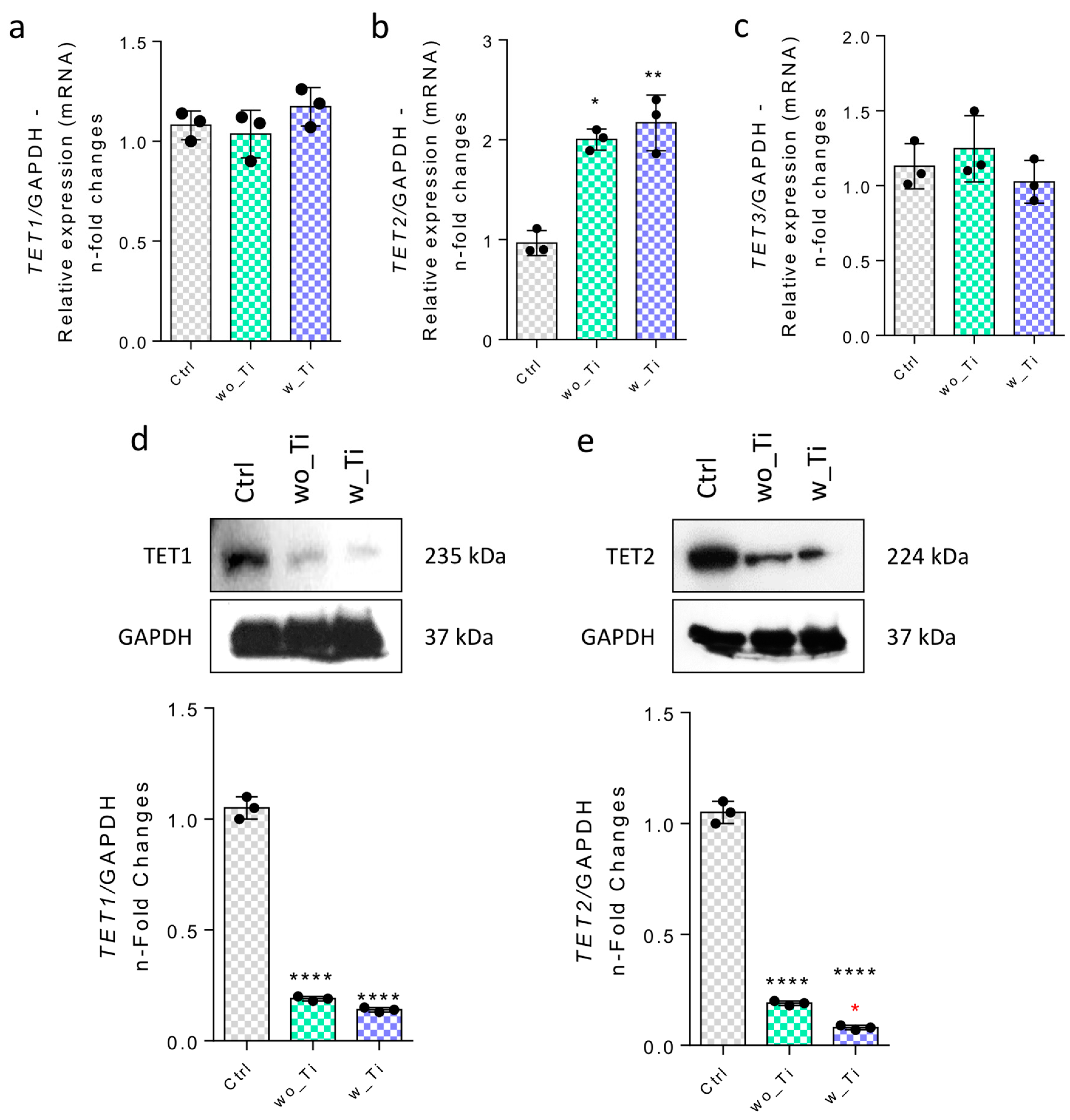
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busenlechner, D.; Furhauser, R.; Haas, R.; Watzek, G.; Mailath, G.; Pommer, B. Long-Term Implant Success at the Academy for Oral Implantology: 8-Year Follow-up and Risk Factor Analysis. J. Periodontal Implant. Sci. 2014, 44, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervaeke, S.; De Bruyn, H. Peri-implantitis. Rev. Belge. Med. Dent. 2008, 63, 161–170. [Google Scholar]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel Surfaces and Osseointegration in Implant Dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- Gemini-Piperni, S.; Takamori, E.R.; Sartoretto, S.C.; Paiva, K.B.S.; Granjeiro, J.M.; de Oliveira, R.C.; Zambuzzi, W.F. Cellular Behavior as a Dynamic Field for Exploring Bone Bioengineering: A Closer Look at Cell-Biomaterial Interface. Arch. Biochem. Biophys. 2014, 561, 88–98. [Google Scholar] [CrossRef]
- Zambuzzi, W.F.; Milani, R.; Teti, A. Expanding the Role of Src and Protein-Tyrosine Phosphatases Balance in Modulating Osteoblast Metabolism: Lessons from Mice. Biochimie 2010, 92, 327–332. [Google Scholar] [CrossRef]
- Zambuzzi, W.F.; Bonfante, E.A.; Jimbo, R.; Hayashi, M.; Andersson, M.; Alves, G.; Takamori, E.R.; Beltrao, P.J.; Coelho, P.G.; Granjeiro, J.M. Nanometer Scale Titanium Surface Texturing Are Detected by Signaling Pathways Involving Transient FAK and Src Activations. PLoS ONE 2014, 9, e95662. [Google Scholar] [CrossRef]
- Da Costa Fernandes, C.J.; Bezerra, F.J.B.; de Campos Souza, B.; Campos, M.A.; Zambuzzi, W.F. Titanium-Enriched Medium Drives Low Profile of ECM Remodeling as a Pre-Requisite to Pre-Osteoblast Viability and Proliferative Phenotype. J. Trace Elem. Med. Biol. 2018, 50, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On Osseointegration in Relation to Implant Surfaces. Clin. Implant Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Alayan, J.; Vaquette, C.; Saifzadeh, S.; Hutmacher, D.; Ivanovski, S. Comparison of Early Osseointegration of SLA((R)) and SLActive((R)) Implants in Maxillary Sinus Augmentation: A Pilot Study. Clin. Oral Implants Res. 2017, 28, 1325–1333. [Google Scholar] [CrossRef]
- Donos, N.; Hamlet, S.; Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Bosshardt, D.D.; Ivanovski, S. Gene Expression Profile of Osseointegration of a Hydrophilic Compared with a Hydrophobic Microrough Implant Surface. Clin. Oral Implants Res. 2011, 22, 365–372. [Google Scholar] [CrossRef]
- Gomes, O.P.; Feltran, G.S.; Ferreira, M.R.; Albano, C.S.; Zambuzzi, W.F.; Lisboa-Filho, P.N. A Novel BSA Immobilizing Manner on Modified Titanium Surface Ameliorates Osteoblast Performance. Colloids Surf. B Biointerfaces 2020, 190, 110888. [Google Scholar] [CrossRef]
- Albano, C.S.; Moreira Gomes, A.; da Silva Feltran, G.; da Costa Fernandes, C.J.; Trino, L.D.; Zambuzzi, W.F.; Lisboa-Filho, P.N. Biofunctionalization of Titanium Surfaces with Alendronate and Albumin Modulates Osteoblast Performance. Heliyon 2020, 6, e04455. [Google Scholar] [CrossRef]
- Albano, C.S.; Gomes, A.M.; da Silva Feltran, G.; da Costa Fernandes, C.J.; Trino, L.D.; Zambuzzi, W.F.; Lisboa-Filho, P.N. Bisphosphonate-Based Surface Biofunctionalization Improves Titanium Biocompatibility. J. Mater. Sci. Mater. Med. 2020, 31, 109. [Google Scholar] [CrossRef]
- Ferreira, M.R.; Milani, R.; Rangel, E.C.; Peppelenbosch, M.; Zambuzzi, W. OsteoBLAST: Computational Routine of Global Molecular Analysis Applied to Biomaterials Development. Front. Bioeng. Biotechnol. 2020, 8, 565901. [Google Scholar] [CrossRef] [PubMed]
- Da S. Feltran, G.; Bezerra, F.; da Costa Fernandes, C.J.; Ferreira, M.R.; Zambuzzi, W.F. Differential Inflammatory Landscape Stimulus during Titanium Surfaces-obtained Osteogenic Phenotype. J. Biomed. Mater. Res. A 2019, 107, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, M.; Fuhler, G.M.; Peppel, J.; Zambuzzi, W.F.; Leeuwen, J.P.; Eerden, B.C.J.; Peppelenbosch, M.P. Human Mesenchymal Stromal Cells in Adhesion to Cell-derived Extracellular Matrix and Titanium: Comparative Kinome Profile Analysis. J. Cell. Physiol. 2019, 234, 2984–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambuzzi, W.F.; Coelho, P.G.; Alves, G.G.; Granjeiro, J.M. Intracellular signal transduction as a factor in the development of “smart” biomaterials for bone tissue engineering. Biotechnol Bioeng. 2011, 108, 1246–1250. [Google Scholar] [CrossRef]
- Li, J.; Qin, W.; Zhang, K.; Wu, F.; Yang, P.; He, Z.; Zhao, A.; Huang, N. Controlling Mesenchymal Stem Cells Differentiate into Contractile Smooth Muscle Cells on a TiO2 Micro/Nano Interface: Towards Benign Pericytes Environment for Endothelialization. Colloids Surf. B Biointerfaces 2016, 145, 410–419. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kammerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron Scale-Structured Hydrophilic Titanium Surfaces Promote Early Osteogenic Gene Response for Cell Adhesion and Cell Differentiation. Clin. Implant Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef]
- Gemini-Piperni, S.; Milani, R.; Bertazzo, S.; Peppelenbosch, M.; Takamori, E.R.; Granjeiro, J.M.; Ferreira, C.V.; Teti, A.; Zambuzzi, W. Kinome Profiling of Osteoblasts on Hydroxyapatite Opens New Avenues on Biomaterial Cell Signaling. Biotechnol. Bioeng. 2014, 111, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Tack, L.; Schickle, K.; Böke, F.; Fischer, H. Immobilization of specific proteins to titanium surface using self-assembled monolayer technique. Dent Mater. 2015, 31, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Abuna, R.P.F.; Oliveira, F.S.; Lopes, H.B.; Freitas, G.P.; Fernandes, R.R.; Rosa, A.L.; Beloti, M.M. The Wnt/β-catenin signaling pathway is regulated by titanium with nanotopography to induce osteoblast differentiation. Colloids Surf. B Biointerfaces 2019, 184, 110513. [Google Scholar] [CrossRef]
- Rossi, M.C.; Bezerra, F.J.B.; Silva, R.A.; Crulhas, B.P.; Fernandes, C.J.C.; Nascimento, A.S.; Pedrosa, V.A.; Padilha, P.; Zambuzzi, W.F. Titanium-Released from Dental Implant Enhances Pre-Osteoblast Adhesion by ROS Modulating Crucial Intracellular Pathways. J. Biomed. Mater. Res. A 2017, 105, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Silva, J.C.; Udangawa, R.N.; Cabral, J.M.S.; Ferreira, F.C.; da Silva, C.L.; Linhardt, R.J.; Vashishth, D. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 479–490. [Google Scholar] [CrossRef]
- Bezerra, F.; Ferreira, M.R.; Fontes, G.N.; da Costa Fernandes, C.J.; Andia, D.C.; Cruz, N.C.; da Silva, R.A.; Zambuzzi, W.F. Nano Hydroxyapatite-Blasted Titanium Surface Affects Pre-Osteoblast Morphology by Modulating Critical Intracellular Pathways. Biotechnol. Bioeng. 2017, 114, 1888–1898. [Google Scholar] [CrossRef]
- Cortijo, S.; Wardenaar, R.; Colomé-Tatché, M.; Gilly, A.; Etcheverry, M.; Labadie, K.; Caillieux, E.; Hospital, F.; Aury, J.-M.; Wincker, P.; et al. Mapping the Epigenetic Basis of Complex Traits. Science 2014, 343, 1145–1148. [Google Scholar] [CrossRef]
- Larsson, L.; Pilipchuk, S.P.; Giannobile, W.V.; Castilho, R.M. When Epigenetics Meets Bioengineering-A Material Characteristics and Surface Topography Perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2065–2071. [Google Scholar] [CrossRef]
- Li, Y.; Chu, J.S.; Kurpinski, K.; Li, X.; Bautista, D.M.; Yang, L.; Sung, K.-L.P.; Li, S. Biophysical Regulation of Histone Acetylation in Mesenchymal Stem Cells. Biophys. J. 2011, 100, 1902–1909. [Google Scholar] [CrossRef] [Green Version]
- Lyu, M.; Zheng, Y.; Jia, L.; Zheng, Y.; Liu, Y.; Lin, Y.; Di, P. Genome-Wide DNA-Methylation Profiles in Human Bone Marrow Mesenchymal Stem Cells on Titanium Surfaces. Eur. J. Oral. Sci. 2019, 127, 196–209. [Google Scholar] [CrossRef]
- Goto, T.; Yoshinari, M.; Kobayashi, S.; Tanaka, T. The initial attachment and subsequent behavior of osteoblastic cells and oral epithelial cells on titanium. Biomed. Mater. Eng. 2004, 14, 537–544. [Google Scholar] [PubMed]
- Pinto, T.S.; Martins, B.R.; Ferreira, M.R.; Bezerra, F.; Zambuzzi, W.F. Nanohydroxyapatite-Blasted Bioactive Surface Drives Shear-Stressed Endothelial Cell Growth and Angiogenesis. Biomed. Res. Int. 2022, 2022, 1433221. [Google Scholar] [CrossRef]
- Fernandes, C.J.C.; Bezerra, F.; Ferreira, M.R.; Andrade, A.F.C.; Pinto, T.S.; Zambuzzi, W.F. Nano Hydroxyapatite-Blasted Titanium Surface Creates a Biointerface Able to Govern Src-Dependent Osteoblast Metabolism as Prerequisite to ECM Remodeling. Colloids Surf. B Biointerfaces 2018, 163, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markel, D.C.; Dietz, P.; Provenzano, G.; Bou-akl, T.; Ren, W.-P. Attachment and Growth of Fibroblasts and Tenocytes within a Porous Titanium Scaffold: A Bioreactor Approach. Arthroplast. Today 2022, 14, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, A.; Milani, R.; da Silva, R.A.; da Costa Fernandes, C.J.; Granjeiro, J.M.; Ferreira, C.V.; Peppelenbosch, M.P.; Zambuzzi, W.F. Phosphoproteome Analysis Reveals a Critical Role for Hedgehog Signalling in Osteoblast Morphological Transitions. Bone 2017, 103, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.R.; da Costa Fernandes, C.J.; da Silva, R.A.; Constantino, V.R.L.; Koh, I.H.J.; Zambuzzi, W.F. Mg-Al and Zn-Al Layered Double Hydroxides Promote Dynamic Expression of Marker Genes in Osteogenic Differentiation by Modulating Mitogen-Activated Protein Kinases. Adv. Healthc. Mater. 2018, 7, 1700693. [Google Scholar] [CrossRef]
- Da Costa Fernandes, C.J.; de Almeida, G.S.; Pinto, T.S.; Teixeira, S.A.; Bezerra, F.J.; Zambuzzi, W.F. Metabolic Effects of CoCr-Enriched Medium on Shear-Stressed Endothelial Cell and Osteoblasts: A Possible Mechanism Involving a Hypoxic Condition on Bone Healing. Mater. Sci. Eng. C 2021, 128, 112353. [Google Scholar] [CrossRef]
- Abuna, R.P.F.; Oliveira, F.S.; Adolpho, L.F.; Fernandes, R.R.; Rosa, A.L.; Beloti, M.M. Frizzled 6 disruption suppresses osteoblast differentiation induced by nanotopography through the canonical Wnt signaling pathway. J. Cell Physiol. 2020, 235, 8293–8303. [Google Scholar] [CrossRef]
- Martins, B.R.; Pinto, T.S.; da Costa Fernandes, C.J.; Bezerra, F.; Zambuzzi, W.F. PI3K/AKT Signaling Drives Titanium-Induced Angiogenic Stimulus. J. Mater. Sci. Mater. Med. 2021, 32, 18. [Google Scholar] [CrossRef]
- Moura, C.G.; Souza, M.; Kohal, R.J.; Dechichi, P.; Zanetta-Barbosa, D.; Jimbo, R.; Teixeira, C.C.; Teixeira, H.S.; Tovar, N.; Coelho, P.G. Evaluation of osteogenic cell culture and osteogenic/peripheral blood mononuclear human cell co-culture on modified titanium surfaces. Biomed. Mater. 2013, 8, 035002. [Google Scholar] [CrossRef] [Green Version]
- Robert, M.-F.; Morin, S.; Beaulieu, N.; Gauthier, F.; Chute, I.C.; Barsalou, A.; MacLeod, A.R. DNMT1 Is Required to Maintain CpG Methylation and Aberrant Gene Silencing in Human Cancer Cells. Nat. Genet. 2003, 33, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Otrock, Z.; Mahfouz, R.; Makarem, J.; Shamseddine, A. Understanding the Biology of Angiogenesis: Review of the Most Important Molecular Mechanisms. Blood Cells Mol. Dis. 2007, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Schoelermann, J.; Mustafa, K.; Cimpan, M.R. TiO2 nanoparticles disrupt cell adhesion and the architecture of cytoskeletal networks of human osteoblast-like cells in a size dependent manner. J. Biomed. Mater Res. A 2018, 106, 2582–2593. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [Green Version]
- Boyault, C.; Sadoul, K.; Pabion, M.; Khochbin, S. HDAC6, at the Crossroads between Cytoskeleton and Cell Signaling by Acetylation and Ubiquitination. Oncogene 2007, 26, 5468–5476. [Google Scholar] [CrossRef] [Green Version]
- Verdel, A.; Khochbin, S. Identification of a New Family of Higher Eukaryotic Histone Deacetylases. Coordinate Expression of Differentiation-Dependent Chromatin Modifiers. J. Biol. Chem. 1999, 274, 2440–2445. [Google Scholar] [CrossRef] [Green Version]
- Kaluza, D.; Kroll, J.; Gesierich, S.; Yao, T.-P.; Boon, R.A.; Hergenreider, E.; Tjwa, M.; Rossig, L.; Seto, E.; Augustin, H.G.; et al. Class IIb HDAC6 Regulates Endothelial Cell Migration and Angiogenesis by Deacetylation of Cortactin. EMBO J. 2011, 30, 4142–4156. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, H.; Suetake, I.; Shimahara, H.; Ura, K.; Tate, S.; Tajima, S. Distinct DNA Methylation Activity of Dnmt3a and Dnmt3b towards Naked and Nucleosomal DNA. J. Biochem. 2006, 139, 503–515. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for de Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA Methylation in the Mammalian Life Cycle: Building and Breaking Epigenetic Barriers. Philos. Trans. R Soc. Lond. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef] [Green Version]
- Logan, P.C.; Mitchell, M.D.; Lobie, P.E. DNA Methyltransferases and TETs in the Regulation of Differentiation and Invasiveness of Extra-Villous Trophoblasts. Front. Genet. 2013, 4, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imashiro, C.; Morikura, T.; Hayama, M.; Ezura, A.; Komotori, J.; Miyata, S.; Sakaguchi, K.; Shimizu, T. Metallic Vessel with Mesh Culture Surface Fabricated Using Three-Dimensional Printing Engineers Tissue Culture Environment. Biotechnol. Bioprocess Eng. 2023. [Google Scholar] [CrossRef]
- De Oliveira, C.P.M.; Viana, M.M.; Amaral, M.C.S. Coupling Photocatalytic Degradation Using a Green TiO2 Catalyst to Membrane Bioreactor for Petroleum Refinery Wastewater Reclamation. J. Water Process Eng. 2020, 34, 101093. [Google Scholar] [CrossRef]
- Imashiro, C.; Takeshita, H.; Morikura, T.; Miyata, S.; Takemura, K.; Komotori, J. Development of Accurate Temperature Regulation Culture System with Metallic Culture Vessel Demonstrates Different Thermal Cytotoxicity in Cancer and Normal Cells. Sci. Rep. 2021, 11, 21466. [Google Scholar] [CrossRef]
- Fingerle, M.; Köhler, O.; Rösch, C.; Kratz, F.; Scheibe, C.; Davoudi, N.; Müller-Renno, C.; Ziegler, C.; Huster, M.; Schlegel, C.; et al. Cleaning of Titanium Substrates after Application in a Bioreactor. Biointerphases 2015, 10, 019007. [Google Scholar] [CrossRef]
- Dearman, B.L.; Greenwood, J.E. Scale-up of a Composite Cultured Skin Using a Novel Bioreactor Device in a Porcine Wound Model. J. Burn. Care Res. 2021, 42, 1199–1209. [Google Scholar] [CrossRef]
- Olmer, R.; Lange, A.; Selzer, S.; Kasper, C.; Haverich, A.; Martin, U.; Zweigerdt, R. Suspension Culture of Human Pluripotent Stem Cells in Controlled, Stirred Bioreactors. Tissue Eng. Part C Methods 2012, 18, 772–784. [Google Scholar] [CrossRef] [Green Version]


| Gene (ID) | Primer | 5′–3′ Sequence | Reaction Conditions | Product Size |
|---|---|---|---|---|
| HDAC1 (3065) | Forward | CTG GCC ATC ATC TCC TTG AT | 95 °C—10 s; 58 °C—30 s; 72 °C—30 s | 216 pb |
| Reverse | ACC AGA GAC GTG GAA ACT GG | |||
| HDAC2 (3066) | Forward | TTC TCA GTG CAC CCA GTC AG | 95 °C—10 s; 59 °C—30 s; 72 °C—30 s | 170 pb |
| Reverse | CCA GTA TCC TTG GGG GAA AT | |||
| HDAC3 (8841) | Forward | ACG TGG GCA ACT TCC ACT AC | 95 °C—10 s; 58 °C—30 s; 72 °C—30 s | 219 pb |
| Reverse | GAC TCT TGG TGA AGC CTT GC | |||
| HDAC6 (10013) | Forward | AAG TAG GCA GAA CCC CCA GT | 95 °C—10 s; 59 °C—30 s; 72 °C—30 s | 416 pb |
| Reverse | GTG CTT CAG CCT CAA GGT TC | |||
| SIRT 1 (23411) | Forward | GCA GAT TAG TAG GCG GCT TG | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 152 pb |
| Reverse | TCT GGC ATG TCC CAC TAT CA | |||
| DNMT1 (1786) | Forward | AGG ACC CAG ACA GAG AAG CA | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 201 pb |
| Reverse | GTA CGG GAA TGC TGA GTG GT | |||
| DNMT3A (1788) | Forward | AGG AAG CCC ATC CGG GTG CTA | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 225 pb |
| Reverse | AGC GGT CCA CTT GGA TGC CC | |||
| DNMT3B (1789) | Forward | TCG ACT TGG TGG TTA TTG TCT G | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 129 pb |
| Reverse | TCG AGC TAC AAG ACT GCT TGG | |||
| TET1 (80312) | Forward | GCC CCT CTT CAT TAC CAA GTC | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 211 pb |
| Reverse | CGC CAG TTG CTT ATC AAA ATC | |||
| TET2 (54790) | Forward | GGT GCC TCT GGA GTG ACT GT | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 245 pb |
| Reverse | GGA AAA TGC AAG CCC TAT GA | |||
| TET3 (200424) | Forward | GGT CAG GCT GGT TTA CAA CG | 95 °C—15 s; 60 °C—30 s; 72 °C—30 s | 198 pb |
| Reverse | GGC ATA GAC CCA CAC ACA TCT | |||
| GAPDH (2597) | Forward | AAG GTG AAG GTC GGA GTC AA | 95 °C—10 s; 58 °C—30 s; 72 °C—30 s | 345 pb |
| Reverse | AAT GAA GGG GTC ATT GAT GG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.J.d.C.; da Silva, R.A.F.; Wood, P.F.; Ferreira, M.R.; de Almeida, G.S.; de Moraes, J.F.; Bezerra, F.J.; Zambuzzi, W.F. Titanium-Enriched Medium Promotes Environment-Induced Epigenetic Machinery Changes in Human Endothelial Cells. J. Funct. Biomater. 2023, 14, 131. https://doi.org/10.3390/jfb14030131
Fernandes CJdC, da Silva RAF, Wood PF, Ferreira MR, de Almeida GS, de Moraes JF, Bezerra FJ, Zambuzzi WF. Titanium-Enriched Medium Promotes Environment-Induced Epigenetic Machinery Changes in Human Endothelial Cells. Journal of Functional Biomaterials. 2023; 14(3):131. https://doi.org/10.3390/jfb14030131
Chicago/Turabian StyleFernandes, Célio Júnior da C., Rodrigo A. Foganholi da Silva, Patrícia F. Wood, Marcel Rodrigues Ferreira, Gerson S. de Almeida, Julia Ferreira de Moraes, Fábio J. Bezerra, and Willian F. Zambuzzi. 2023. "Titanium-Enriched Medium Promotes Environment-Induced Epigenetic Machinery Changes in Human Endothelial Cells" Journal of Functional Biomaterials 14, no. 3: 131. https://doi.org/10.3390/jfb14030131
APA StyleFernandes, C. J. d. C., da Silva, R. A. F., Wood, P. F., Ferreira, M. R., de Almeida, G. S., de Moraes, J. F., Bezerra, F. J., & Zambuzzi, W. F. (2023). Titanium-Enriched Medium Promotes Environment-Induced Epigenetic Machinery Changes in Human Endothelial Cells. Journal of Functional Biomaterials, 14(3), 131. https://doi.org/10.3390/jfb14030131








