Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review
Abstract
1. Introduction
- autologous or allographic human bone, i.e., taken from another donor (4%)
- xenographic: animal bone, coral (54%)
- alloplastic: bioglass, hydroxyapatite (HA), tricalcium β-phosphate (TCP), polyglycolic, and polylactic acid derivatives (33%)
- other (9%)
2. Materials and Methods
2.1. Search Processing
2.2. Inclusion Criteria
3. Results
4. The Autologous Tooth Graft
5. Devices for Tooth Processing
5.1. Step 1: Tooth Cleaning
5.2. Step 2: Tooth Grinding
5.3. Step 3: Treatment by Device
5.3.1. Bon Maker®
5.3.2. Tooth Transformer®
6. Tooth Transformer® Technique
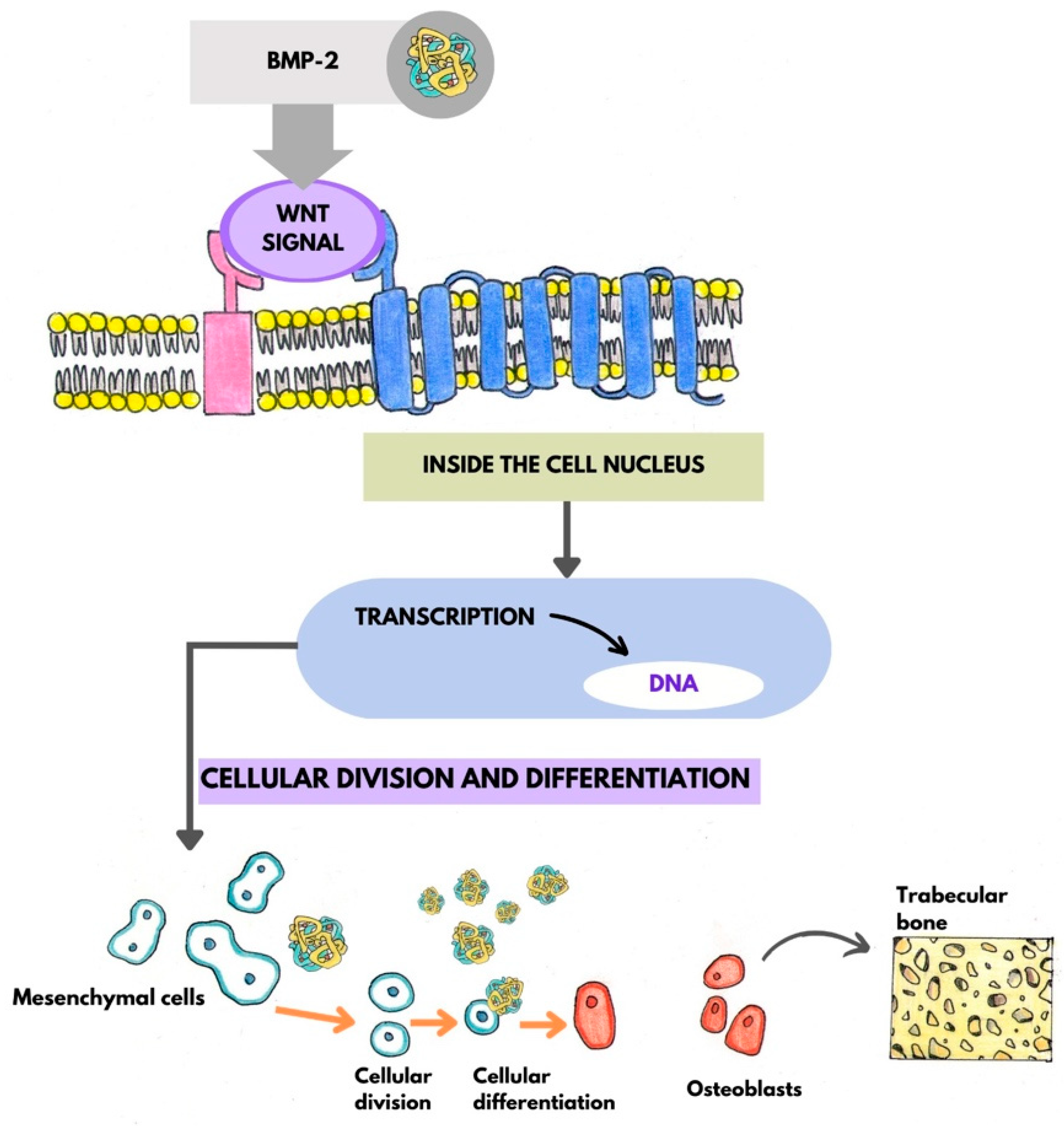
7. Microbiota and Bone Metabolism
- Gut microbiota homeostasis increases calbindin-D9k expression resulting in increased calcium reabsorption [139];
- The intestinal microbiota regulates the synthesis of serotonin and therefore bone metabolism [140];
- The homeostasis of the intestinal microbiota promotes the proliferation of enterocytes, strengthening the absorption of minerals [141];
- The intestinal microbiota promotes the production of the hormone IGF-1, which induces the differentiation of osteoblasts [146];
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| AR | Androgen Receptor |
| BMPs | Bone Morphogenetic Proteins |
| BSP | Bone Sialoprotein |
| DBM | Demineralized Bone Matrix |
| DDM | Demineralized Dentin Matrix |
| DFDBA | Demineralized Freeze-Dried Bone Allograft |
| DMP1 | Dentin Matrix Protein 1 |
| DSPP | Dentin Sialophosphoprotein |
| ER | Estrogen Receptor |
| FDA | Food and Drug Administration |
| FGFs | Fibroblast Growth Factors |
| GBR | Guided Bone Regeneration |
| GF | Germ-Free |
| HA | Hydroxyapatite |
| IGFII | Insulin Growth Factor II |
| LPS | Lipopolysaccharide |
| MMP | Matrix Metalloproteinases |
| MSC | Mesenchymal Stem Cell |
| NCP | Non-Collagen Proteins |
| NF-kB | Nuclear Factor kappa B |
| NFATc1 | Nuclear Factor of Activated T Cells 1 |
| OPG | Osteoprotegerin |
| OPN | Osteopontin |
| PDGF | Platelet-Derived Growth Factor |
| PRGF | Platelet Rich in Growth Factor |
| rhBMP-2 | Recombinant Human BMP-2 |
| RSV | Resveratrol |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SCFA | Short-Chain Fatty Acids |
| SEM | Scanning Electron Microscope |
| TCP | Tricalcium Beta-Phosphate |
| TGF | Transforming Growth Factor |
| TRAF6 | TNF Receptor Associated Factor 6 |
| TT® | Tooth Transformer® |
| VEGF | Vascular Endothelial Growth Factor |
References
- Quintessence Publishing Co., Inc. Guided Bone Regeneration in Implant Dentistry. Available online: http://www.quintpub.com/display_detail.php3?psku=B2494#.Y7sD6uzMKdY (accessed on 8 January 2023).
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Palla, G.; Fischer, D.S.; Regev, A.; Theis, F.J. Spatial components of molecular tissue biology. Nat. Biotechnol. 2022, 40, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Hazballa, D.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; Minetti, E.; di Venere, D.; Limongelli, L.; Bordea, I.R.; Scarano, A.; et al. The Effectiveness of Autologous Demineralized Tooth Graft for the Bone Ridge Preservation: A Systematic Review of the Literature. J. Biol. Regul. Homeost. Agents 2021, 35, 283–294. [Google Scholar] [CrossRef]
- Minetti, E.; Carasco, A.; Carasco, M.; Giacometti, E.; Ho, K.L.H.; Palermo, A.; Savadori, P.; Taschieri, S. Il Dente Come Materiale Da Innesto, 1st ed.; EDRA: Wood Buffalo, AB, Canada, 2020; Volume UNICO, ISBN 978-88-214-5353-3. [Google Scholar]
- Migliorini, F.; Cuozzo, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Autologous Bone Grafting in Trauma and Orthopaedic Surgery: An Evidence-Based Narrative Review. J. Clin. Med. 2021, 10, 4347. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Kadow-Romacker, A.; Pruss, A.; Haas, N.P.; Schmidmaier, G. Quantification of growth factors in allogenic bone grafts extracted with three different methods. Cell Tissue Bank. 2006, 8, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Alkaabi, S.; Alsabri, G.; NatsirKalla, D.; Alavi, S.; Mueller, W.; Forouzanfar, T.; Helder, M. A systematic review on regenerative alveolar graft materials in clinical trials: Risk of bias and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2021, 75, 356–365. [Google Scholar] [CrossRef]
- von Arx, T.; Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Lateral ridge augmentation and implant placement: An experimental study evaluating implant osseointegration in different augmentation materials in the canine mandible. Int. J. Oral Maxillofac. Implant. 2001, 16, 343–354. [Google Scholar]
- Piattelli, A.; Degidi, M.; di Stefano, D.A.; Rubini, C.; Fioroni, M.; Strocchi, R. Microvessel Density in Alveolar Ridge Regeneration with Autologous and Alloplastic Bone. Implant. Dent. 2002, 11, 370–375. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Sieverts, M.; Obata, Y.; Rosenberg, J.L.; Woolley, W.; Parkinson, D.Y.; Barnard, H.S.; Pelt, D.M.; Acevedo, C. Unraveling the effect of collagen damage on bone fracture using in situ synchrotron microtomography with deep learning. Commun. Mater. 2022, 3, 1–13. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-derived bone graft material. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.T.; Ritchie, H. The nature and functional significance of dentin extracellular matrix proteins. Int. J. Dev. Biol. 1995, 39, 169–179. [Google Scholar]
- Chen, J.; Shapiro, H.S.; Sodek, J. Developmental expression of bone sialoprotein mRNA in rat mineralized connective tissues. J. Bone Miner. Res. 2009, 7, 987–997. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Q.; Chen, J. Applications of transgenics in studies of bone sialoprotein. J. Cell. Physiol. 2009, 220, 30–34. [Google Scholar] [CrossRef]
- Schmidt-Schultz, T.H.; Schultz, M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: A storehouse for the study of human evolution in health and disease. Biol. Chem. 2005, 386, 19. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Andreacchio, D.; Koster, G.; Cochran, D.L.; Dean, D.D.; Boyan, B.D.; Schwartz, Z. Tissue response and osteoinduction of human bone grafts in vivo. Arch. Orthop. Trauma Surg. 2001, 121, 583–590. [Google Scholar] [CrossRef]
- Włodarski, K.H.; Szczęsny, G.; Kuzaka, B.; Wlodarski, P. Long-term preservation of Bone Morphogenetic Activity in stored demineralized murine incisors. Pol. Orthop. Traumatol. 2013, 78, 97–100. [Google Scholar]
- Dijke, P.T.; Miyazono, K.; Heldin, C.-H. Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr. Opin. Cell Biol. 1996, 8, 139–145. [Google Scholar] [CrossRef]
- Ripamonti, U.; Ramoshebi, L.N.; Matsaba, T.; Tasker, J.; Crooks, J.; Teare, J. Bone Induction by BMPs/OPs and Related Family Members in Primates. J. Bone Jt. Surg. 2001, 83, S116–S127. [Google Scholar] [CrossRef]
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Canalis, E.; Pash, J.; Varghese, S. Skeletal Growth Factors. Crit. Rev. Eukaryot. Gene Expr. 1993, 3, 155–166. [Google Scholar] [PubMed]
- Tabatabaei, F.S.; Torshabi, M. Effects of Non-Collagenous Proteins, TGF-β1, and PDGF-BB on Viability and Proliferation of Dental Pulp Stem Cells. J. Oral Maxillofac. Res. 2016, 7, e4. [Google Scholar] [CrossRef]
- Luu, H.H.; Song, W.-X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.-L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.-C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone Morphogenetic Proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, G.; Gong, Y.; Li, B.; Li, W.; Liu, D.; Yang, X. BMP-2 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells by Enhancing Mitochondrial Activity. J. Musculoskelet. Neuronal. Interact. 2022, 22, 123–131. [Google Scholar] [PubMed]
- Mehwish, N.; Xu, M.; Zaeem, M.; Lee, B.H. Mussel-Inspired Surface Functionalization of Porous Albumin Cryogels Supporting Synergistic Antibacterial/Antioxidant Activity and Bone-Like Apatite Formation. Gels 2022, 8, 679. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Murata, M.; Kusano, K.; Zakaria, S.M.; Noor, A.H.M.; Khuda, F.; Hossain, I.; Sultana, S.; Saito, T. Radiological Evaluation of Human Dentin Autografts in Bangladesh. J. Hard Tissue Biol. 2014, 23, 363–370. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; García, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef]
- Sotiropoulou, P.; Fountos, G.; Martini, N.; Koukou, V.; Michail, C.; Kandarakis, I.; Nikiforidis, G. Bone calcium/phosphorus ratio determination using dual energy X-ray method. Phys. Med. 2015, 31, 307–313. [Google Scholar] [CrossRef]
- Dozza, B.; Lesci, I.G.; Duchi, S.; della Bella, E.; Martini, L.; Salamanna, F.; Falconi, M.; Cinotti, S.; Fini, M.; Lucarelli, E.; et al. When size matters: Differences in demineralized bone matrix particles affect collagen structure, mesenchymal stem cell behavior, and osteogenic potential. J. Biomed. Mater. Res. Part A 2017, 105, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.-I.; Takashi, I.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone Regeneration Using Dentin Matrix Depends on the Degree of Demineralization and Particle Size. PLoS ONE 2016, 11, e0147235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Yun, P.-Y.; Yeo, I.-S.; Jin, S.-C.; Oh, J.-S.; Kim, H.-J.; Yu, S.-K.; Lee, S.-Y.; Kim, J.-S.; et al. Autogenous teeth used for bone grafting: A comparison with traditional grafting materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Ferrante, F.; Schmitz, J.H.; Ho, H.K.L.; Hann, S.N.D.; Giacometti, E.; Gambardella, U.; Contessi, M.; Celko, M.; et al. Autologous Tooth Graft after Endodontical Treated Used for Socket Preservation: A Multicenter Clinical Study. Appl. Sci. 2019, 9, 5396. [Google Scholar] [CrossRef]
- Umebayashi, M.; Ohba, S.; Kurogi, T.; Noda, S.; Asahina, I. Full Regeneration of Maxillary Alveolar Bone Using Autogenous Partially Demineralized Dentin Matrix and Particulate Cancellous Bone and Marrow for Implant-Supported Full Arch Rehabilitation. J. Oral Implant. 2020, 46, 122–127. [Google Scholar] [CrossRef]
- Supplement, D.; Minetti, E.; Palermo, A.; Savadori, P.; Barlattani, A.; Franco, R.; Michele, M.; Gianfreda, F.; Bollero, P. Autologous tooth graft: A histological comparison between dentin mixed with xenograft and dentin alone grafts in socket preservation. J. Biol. Regul. Homeost. Agents 2019, 33, 189–197. [Google Scholar]
- Liu, X.; Li, Q.; Wang, F.; Wang, Z. Maxillary sinus floor augmentation and dental implant placement using dentin matrix protein-1 gene-modified bone marrow stromal cells mixed with deproteinized boving bone: A comparative study in beagles. Arch. Oral Biol. 2016, 64, 102–108. [Google Scholar] [CrossRef]
- Andrade, C.; Camino, J.; Nally, M.; Quirynen, M.; Martínez, B.; Pinto, N. Combining autologous particulate dentin, L-PRF, and fibrinogen to create a matrix for predictable ridge preservation: A pilot clinical study. Clin. Oral Investig. 2019, 24, 1151–1160. [Google Scholar] [CrossRef]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18, 2–4. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Cazzolla, A.P.; di Cosola, M.; Greco Lucchina, A.; Santacroce, L.; Charitos, I.A.; Topi, S.; Malcangi, G.; Hazballa, D.; Scarano, A.; et al. The integumentary system and its microbiota between health and disease. J. Biol. Regul. Homeost. Agents 2021, 35, 303–321. [Google Scholar] [CrossRef]
- Pei, Z.; Bini, E.J.; Yang, L.; Zhou, M.; Francois, F.; Blaser, M.J. Bacterial biota in the human distal esophagus. Proc. Natl. Acad. Sci. USA 2004, 101, 4250–4255. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; de Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; de Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; Roa, R.I.L.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; E Kulling, S. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Nargeh, H.; Aliabadi, F.; Ajami, M.; Pazoki-Toroudi, H. Role of Polyphenols on Gut Microbiota and the Ubiquitin-Proteasome System in Neurodegenerative Diseases. J. Agric. Food Chem. 2021, 69, 6119–6144. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Li, Y.-J.; Lu, P.-P.; Dai, G.-C.; Chen, X.-X.; Rui, Y.-F. The modulatory effect and implication of gut microbiota on osteoporosis: From the perspective of “brain–gut–bone” axis. Food Funct. 2021, 12, 5703–5718. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Pal, S.; Paterson, C.W.; Li, J.-Y.; Tyagi, A.M.; Adams, J.; Coopersmith, C.M.; Weitzmann, M.N.; Pacifici, R. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Asil, Y.; Glüer, C.-C.; Schrezenmeir, J. Prebiotics, Probiotics, and Synbiotics Affect Mineral Absorption, Bone Mineral Content, and Bone Structure. J. Nutr. 2007, 137, S838–S846. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Larsbrink, J.; Rogers, T.E.; Hemsworth, G.R.; McKee, L.S.; Tauzin, A.S.; Spadiut, O.; Klinter, S.; Pudlo, N.A.; Urs, K.; Koropatkin, N.M.; et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 2014, 506, 498–502. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Denef, V.J.; Costello, E.K.; Thomas, B.C.; Poroyko, V.; Relman, D.A.; Banfield, J.F. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc. Natl. Acad. Sci. USA 2010, 108, 1128–1133. [Google Scholar] [CrossRef]
- Signorini, L.; Ballini, A.; Arrigoni, R.; de Leonardis, F.; Saini, R.; Cantore, S.; de Vito, D.; Coscia, M.F.; Dipalma, G.; Santacroce, L.; et al. Evaluation of a Nutraceutical Product with Probiotics, Vitamin D, Plus Banaba Leaf Extracts (Lagerstroemia speciosa) in Glycemic Control. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 1356–1365. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Greenbaum, J.; Shen, H.; Deng, H.-W. Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective. J. Clin. Endocrinol. Metab. 2017, 102, 3635–3646. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, Z.; Sun, J.; Huang, S.; Chen, Y.; Li, C.; Sun, X.; Xia, B.; Tian, L.; Guo, C.; et al. Gut Microbiome Reveals Specific Dysbiosis in Primary Osteoporosis. Front. Cell. Infect. Microbiol. 2020, 10, 160. [Google Scholar] [CrossRef]
- Cairoli, E.; Zhukouskaya, V.V.; Eller-Vainicher, C.; Chiodini, I. Perspectives on osteoporosis therapies. J. Endocrinol. Investig. 2015, 38, 303–311. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, J.; Murata, M.; Nakanishi, Y.; Ochi, M.; Hirose, Y.; Akazawa, T.; Yodogawa, S.; Hino, J.; Ito, K.; Kitajo, H.; et al. Simultaneous Implantation of Dental Implants and Autogenous Human Dentin. Key Eng. Mater. 2011, 493–494, 426–429. [Google Scholar] [CrossRef]
- Jun, S.-H.; Ahn, J.-S.; Lee, J.-I.; Ahn, K.-J.; Yun, P.-Y.; Kim, Y.-K. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J. Adv. Prosthodont. 2014, 6, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Lee, I.-K.; Kang, J.-Y.; Lee, E.-Y. Various autogenous fresh demineralized tooth forms for alveolar socket preservation in anterior tooth extraction sites: A series of 4 cases. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 27. [Google Scholar] [CrossRef]
- Bono, N.; Tarsini, P.; Candiani, G. Demineralized Dentin and Enamel Matrices as Suitable Substrates for Bone Regeneration. J. Appl. Biomater. Funct. Mater. 2017, 15, 236–243. [Google Scholar] [CrossRef]
- Li, P.; Zhu, H.; Huang, D. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant. Dent. Relat. Res. 2018, 20, 923–928. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Nevins, M.; Schupbach, P. New Bone Formation Using an Extracted Tooth as a Biomaterial: A Case Report with Histologic Evidence. Int. J. Periodontics Restor. Dent. 2019, 39, 157–163. [Google Scholar] [CrossRef]
- Kadkhodazadeh, M.; Fathiazar, A.; Yadegari, Z.; Amid, R. Comparison of osteopromoting ability of human tooth powder with the demineralized freeze-dried bone allograft, a bovine xenograft, and a synthetic graft: An in vitro study. J. Adv. Periodontol. Implant. Dent. 2020, 12, 19–23. [Google Scholar] [CrossRef]
- Minetti, E.; Taschieri, S.; Corbella, S. Autologous Deciduous Tooth-Derived Material for Alveolar Ridge Preservation: A Clinical and Histological Case Report. Case Rep. Dent. 2020, 2020, 2936878. [Google Scholar] [CrossRef]
- Sánchez-Labrador, L.; Martín-Ares, M.; Ortega-Aranegui, R.; López-Quiles, J.; Martínez-González, J.M. Autogenous Dentin Graft in Bone Defects after Lower Third Molar Extraction: A Split-Mouth Clinical Trial. Materials 2020, 13, 3090. [Google Scholar] [CrossRef]
- Korsch, M.; Peichl, M. Retrospective Study: Lateral Ridge Augmentation Using Autogenous Dentin: Tooth-Shell Technique vs. Bone-Shell Technique. Int. J. Environ. Res. Public Health 2021, 18, 3174. [Google Scholar] [CrossRef]
- Cervera-Maillo, J.M.; Morales-Schwarz, D.; Morales-Melendez, H.; Mahesh, L.; Calvo-Guirado, J.L. Autologous Tooth Dentin Graft: A Retrospective Study in Humans. Medicina 2021, 58, 56. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Corbella, S.; Taschieri, S.; Canullo, L. Tooth as graft material: Histologic study. Clin. Implant. Dent. Relat. Res. 2022, 24, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Ishikawa, M.; Shakya, M.; Hosono, H.; Maehara, O.; Ohkawara, T.; Ohnishi, S.; Akazawa, T.; Murata, M. Autograft of Demineralized Dentin Matrix Prepared Immediately after Extraction for Horizontal Bone Augmentation of the Anterior Atrophic Maxilla: A First Case of Non-Vital Tooth-Derived Dentin. J. Hard Tissue Biol. 2022, 31, 47–54. [Google Scholar] [CrossRef]
- Pohl, S.; Prasad, H.; Prasad, S.; Kotsakis, G. Human Histologic Analysis of Implant Osseointegration in a Healed Site Grafted with Nondemineralized Autologous Tooth-Derived Graft Material. Int. J. Periodontics Restor. Dent. 2022, 42, e199–e207. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Gianfreda, F.; Palermo, A.; Bollero, P. Autogenous Dentin Particulate Graft for Alveolar Ridge Augmentation with and without Use of Collagen Membrane: Preliminary Histological Analysis on Humans. Materials 2022, 15, 4319. [Google Scholar] [CrossRef]
- Bhaskar, S.N.; Orban, B.J. Orban’s Oral Histology and Embryology, 9th ed.; Mosby: St. Louis, MO, USA, 1980; ISBN 978-0-8016-4609-6. [Google Scholar]
- Tanoue, R.; Ohta, K.; Miyazono, Y.; Iwanaga, J.; Koba, A.; Natori, T.; Iwamoto, O.; Nakamura, K.-I.; Kusukawa, J. Three-dimensional ultrastructural analysis of the interface between an implanted demineralised dentin matrix and the surrounding newly formed bone. Sci. Rep. 2018, 8, 2858. [Google Scholar] [CrossRef] [PubMed]
- Alvares, K. The role of acidic phosphoproteins in biomineralization. Connect. Tissue Res. 2014, 55, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Mah, Y.-J.; Kim, D.-H.; Kim, E.-S.; Park, E.-J. Demineralized deciduous tooth as a source of bone graft material: Its biological and physicochemical characteristics. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- van Orten, A.; Goetz, W.; Bilhan, H. Tooth-Derived Granules in Combination with Platelet-Rich Fibrin (“Sticky Tooth”) in Socket Preservation: A Histological Evaluation. Dent. J. 2022, 10, 29. [Google Scholar] [CrossRef]
- Pohl, S.; Binderman, I.; Tomac, J. Maintenance of Alveolar Ridge Dimensions Utilizing an Extracted Tooth Dentin Particulate Autograft and Platelet-Rich fibrin: A Retrospective Radiographic Cone-Beam Computed Tomography Study. Materials 2020, 13, 1083. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Miron, R.J. Platelet rich fibrin: A second-generation platelet concentrate. In Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications; Miron, R.J., Choukroun, J., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2017; pp. 1–14. ISBN 978-1-119-40679-2. [Google Scholar]
- Fujioka-Kobayashi, M.; Miron, R.J. Biological components of platelet rich fibrin: Growth factor release and cellular activity. In Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications; Miron, R.J., Choukroun, J., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2017; pp. 15–31. ISBN 978-1-119-40679-2. [Google Scholar]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbé, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the Fibrin Architecture and Leukocyte Content Influence the Growth Factor Release of Platelet Concentrates? An Evidence-based Answer Comparing a Pure Platelet-Rich Plasma (P-PRP) Gel and a Leukocyte- and Platelet-Rich Fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.-B. Three-Dimensional Architecture and Cell Composition of a Choukroun’s Platelet-Rich Fibrin Clot and Membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Simonpieri, A.; del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef]
- del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Everts, P.A. In Search of a Consensus Terminology in the Field of Platelet Concentrates for Surgical Use: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Fibrin Gel Polymerization and Leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar] [CrossRef]
- Kanazirski, N.; Kanazirska, P. Auto-tooth bone graft material for reconstruction of bone defects in the oral region: Case reports. Folia Medica 2022, 64, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W. Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, R.A.; Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Bischof, J.C.; He, X. Thermal Stability of Proteins. Ann. N. Y. Acad. Sci. 2005, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Freeman, C.; Blackwood, K. Processed bovine dentine as a bone substitute. Br. J. Oral Maxillofac. Surg. 2008, 46, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Berardini, M.; Trisi, P. A New Tooth Processing Apparatus Allowing to Obtain Dentin Grafts for Bone Augmentation: The Tooth Transformer. Open Dent. J. 2019, 13, 6–14. [Google Scholar] [CrossRef]
- Carano, R.A.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Contessi, M.; Gambardella, U.; Schmitz, J.H.; Giacometti, E.; Celko, M.; Trisi, P. Autologous tooth graft for maxillary sinus augmentation: A multicenter clinical study. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 45. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Nava, M.M.; Draghi, L.; Giordano, C.; Pietrabissa, R. The Effect of Scaffold Pore Size in Cartilage Tissue Engineering. J. Appl. Biomater. Funct. Mater. 2016, 14, e223–e229. [Google Scholar] [CrossRef]
- Passos, A.D.; Tziafas, D.; Mouza, A.A.; Paras, S.V. Computational Modelling for Efficient Transdentinal Drug Delivery. Fluids 2017, 3, 4. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.-N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Tiedemann, K.; le Nihouannen, D.; Fong, J.E.; Hussein, O.; Barralet, J.E.; Komarova, S.V. Regulation of Osteoclast Growth and Fusion by mTOR/raptor and mTOR/rictor/Akt. Front. Cell Dev. Biol. 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- ScienceDirect Topics. Osteoblast—An Overview. Available online: https://www.sciencedirect.com/topics/materials-science/osteoblast (accessed on 25 December 2022).
- Pohl, V.; Pohl, S.; Sulzbacher, I.; Fuerhauser, R.; Mailath-Pokorny, G.; Haas, R. Alveolar Ridge Augmentation Using Dystopic Autogenous Tooth: 2-Year Results of an Open Prospective Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.-S.; Tulio, A.-V.M.; Kang, C.-D.; Fabian, O.-A.; Kim, G.E.A.; Kim, H.-G. Socket preservation using demineralized tooth graft: A case series report with histological analysis. Int. J. Growth Factors Stem Cells Dent. 2020, 3, 27. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Stankoska, K.; Swain, M.V. Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans. Micron 2012, 43, 229–236. [Google Scholar] [CrossRef]
- Arends, J.; Stokroos, I.; Jongebloed, W.; Ruben, J. The Diameter of Dentinal Tubules in Human Coronal Dentine after Demineralization and Air Drying. Caries Res. 1995, 29, 118–121. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Next-Generation Biomaterials for Bone & Periodontal Regeneration. Available online: http://www.quintpub.com/display_detail.php3?psku=B7963 (accessed on 30 October 2020).
- Bono, N.; Tarsini, P.; Candiani, G. BMP-2 and type I collagen preservation in human deciduous teeth after demineralization. J. Appl. Biomater. Funct. Mater. 2018, 17, 2280800018784230. [Google Scholar] [CrossRef]
- Lim, J.Y.; Taylor, A.F.; Li, Z.; Vogler, E.A.; Donahue, H.J. Integrin Expression and Osteopontin Regulation in Human Fetal Osteoblastic Cells Mediated by Substratum Surface Characteristics. Tissue Eng. 2005, 11, 19–29. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Kim, K.-W.; Um, I.-W.; Murata, M.; Ito, K. Analysis of Organic Components and Osteoinductivity in Autogenous Tooth Bone Graft Material. J. Korean Assoc. Maxillofac. Plast. Reconstr. Surg. 2013, 35, 353–359. [Google Scholar] [CrossRef]
- Hedenbjörk-Lager, A.; Hamberg, K.; Pääkkönen, V.; Tjäderhane, L.; Ericson, D. Collagen degradation and preservation of MMP-8 activity in human dentine matrix after demineralization. Arch. Oral Biol. 2016, 68, 66–72. [Google Scholar] [CrossRef]
- Bertassoni, L.E. Dentin on the nanoscale: Hierarchical organization, mechanical behavior and bioinspired engineering. Dent. Mater. 2017, 33, 637–649. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; E Bertassoni, L. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2017, 10, 024101. [Google Scholar] [CrossRef]
- Toyosawa, S.; Shintani, S.; Fujiwara, T.; Ooshima, T.; Sato, A.; Ijuhin, N.; Komori, T. Dentin Matrix Protein 1 Is Predominantly Expressed in Chicken and Rat Osteocytes but Not in Osteoblasts. J. Bone Miner. Res. 2001, 16, 2017–2026. [Google Scholar] [CrossRef]
- Ling, Y.; Rios, H.F.; Myers, E.R.; Lu, Y.; Feng, J.Q.; Boskey, A.L. DMP1 depletion decreases bone mineralization in vivo: An FTIR imaging analysis. J. Bone Miner. Res. 2005, 20, 2169–2177. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, L.; Yu, S.; Zhang, S.; Xie, Y.; McKee, M.D.; Li, Y.C.; Kong, J.; Eick, J.D.; Dallas, S.L.; et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev. Biol. 2007, 303, 191–201. [Google Scholar] [CrossRef]
- Beniash, E.; Deshpande, A.S.; Fang, P.A.; Lieb, N.S.; Zhang, X.; Sfeir, C.S. Possible role of DMP1 in dentin mineralization. J. Struct. Biol. 2011, 174, 100–106. [Google Scholar] [CrossRef]
- Almushayt, A.; Narayanan, K.; E Zaki, A.; George, A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2005, 13, 611–620. [Google Scholar] [CrossRef]
- Narayanan, K.; Srinivas, R.; Ramachandran, A.; Hao, J.; Quinn, B.; George, A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. USA 2001, 98, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Li, R.H.; Mikos, A.G.; Barry, M.A. An optimized method for the chemiluminescent detection of alkaline phosphatase levels during osteodifferentiation by bone morphogenetic protein 2. J. Cell. Biochem. 2000, 80, 532–537. [Google Scholar] [CrossRef]
- Inchingolo, F.; DiPalma, G.; Cirulli, N.; Cantore, S.; Saini, R.S.; Altini, V.; Santacroce, L.; Ballini, A.; Saini, R. Microbiological results of improvement in periodontal condition by administration of oral probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1323–1328. [Google Scholar] [PubMed]
- Isacco, C.G.; Ballini, A.; de Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 777–784. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.R.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Isacco, C.G.; Ballini, A.; Paduanelli, G.; Inchingolo, A.D.; Nguyen, K.C.D.; Inchingolo, A.M.; Pham, V.H.; Aityan, S.K.; Schiffman, M.; Tran, T.C.; et al. Bone decay and beyond: How can we approach it better. J. Biol. Regul. Homeost. Agents 2019, 33, 143–154. [Google Scholar]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’Toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Bäckhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- Ballini, A.; DiPalma, G.; Isacco, C.G.; Boccellino, M.; Di Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Bongers, A.; Heuvel, E.G.H.M.V.D. Prebiotics and the Bioavailability of Minerals and Trace Elements. Food Rev. Int. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 1–24. [Google Scholar] [CrossRef]
- Fukushima, A.; Aizaki, Y.; Sakuma, K. Short-chain fatty acids increase the level of calbindin-D9k messenger RNA in Caco-2 cells. J. Nutr. Sci. Vitaminol. 2012, 58, 287–291. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2017, 102, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, E.-J.; Lee, J.-C.; Kim, W.-K.; Kim, H.-S. Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-κB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Jarchum, I.; Pamer, E.G. Regulation of innate and adaptive immunity by the commensal microbiota. Curr. Opin. Immunol. 2011, 23, 353–360. [Google Scholar] [CrossRef]
- MacPherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Hajishengallis, G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014, 49, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral probiotics influence oral and respiratory tract infections in pediatric population: A randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. OSTEOIMMUNOLOGY: Interplay Between the Immune System and Bone Metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Nocini, P.F.; Albanese, M.; Zotti, F.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. Beneficial Effects of Concentrated Growth Factors and Resveratrol on Human Osteoblasts In Vitro Treated with Bisphosphonates. BioMed Res. Int. 2018, 2018, 4597321. [Google Scholar] [CrossRef]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Isacco, C.G.; Ballini, A.; de Vito, D.; Inchingolo, A.M.; Cantore, S.; Paduanelli, G.; Nguyen, K.C.D.; Inchingolo, A.D.; Dipalma, G.; Inchingolo, F. Probiotics in health and immunity: A first step toward understanding the importance of microbiota system in translational medicine. In Prebiotics and Probiotics; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; de Vito, D.; Abbinante, A.; Altini, V.; DiPalma, G.; Inchingolo, F.; Saini, R. Clinical results of improvement in periodontal condition by administration of oral probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334. [Google Scholar] [PubMed]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; de Nitto, E.; Topi, S. The Human Respiratory System and its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Santacroce, L.; Cantore, S.; Ballini, A.; del Prete, R.; Topi, S.; Saini, R.; Dipalma, G.; Arrigoni, R. Probiotics and EpiCor® in human health. J. Biol. Regul. Homeost. Agents 2019, 33, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef]
- Inchingolo, A.; Inchingolo, A.; Bordea, I.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.; Isacco, C.; et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 2021, 9, 793. [Google Scholar] [CrossRef]




| Authors (Year) | Type of the Study | Aim of the Study | Materials | Results |
|---|---|---|---|---|
| Tazaki et al. (2012) [65] | Clinical report | The use of autologous dentin grafts for the treatment of bone defects. | The extracted tooth was crushed by a newly developed automatic mill. The crushed granules were demineralised in 2% HNO3. The granules were washed in distilled cold water and freeze-dried (size: 0.5–2.0 mm). | The human dentin can be used as an autogenous biomaterial for local bone engineering. |
| Kabir et al. (2014) [31] | Clinical report | These studies imply that dentin may actually replace bone as a viable biomaterial. | Case 1: a 29-year-old male patient. A #38 was used to create tooth-derived granules, which were then demineralized in 2% HNO3 for 30 min and thoroughly cleaned. DDM was transplanted into the bone gap. Case 2: A 20-year-old woman who had an impacted third molar (#48). The DDM autograft and extraction of the affected tooth were completed concurrently. | 90%–95% of patient-own recycled dentin matrix had remodeled into bone, resulting in excellent bone defect repair. |
| Jun et al. (2014) [66] | A prospective clinical study | Evaluation of DDM and Bio-Oss as sinus bone grafts in patients with residual bone height less than 5.0 mm in the maxillary posterior area. | 43 patients, 21 in the control (Bio-Oss) and 22 in the test group (DDM 0.5–1.0 mm). After four months the sites were analyzed with microcomputed tomography analysis and histomorphometric analysis. | In the two groups, there was no difference in bone density and height, instead, there was a significant difference in osteoid thickness. |
| Kim et al. (2015) [67] | Case reports | To evaluate the clinical use of the chairside-prepared demineralised tooth immediately after extraction for alveolus preservation. | The use of the extracted tooth as graft material radiographic and histological evaluation of the graft site. | The use of the dental block is effective in maintaining height and thickness of the bone in the preservation of the alveolus. |
| Bono et al. (2017) [68] | In vitro study | To examinate the effects of demineralization on the physical–chemical and biological behavior of D and E. | Human dentin and enamel were minced into particles (Ø < 1 mm), demineralized, and sterilized. Thorough physical–chemical and biochemical characterizations of native and demineralized materials were performed by SEM and EDS analysis and ELISA kits to determine mineral, collagen type I, and BMP-2 contents. In addition, MG63 and SAOS-2 cells were seeded on tooth-derived materials and Bio-Oss®, and a comparison of cell responses in terms of adhesion and proliferation was carried out. | The demineralization process determined an increase in BMP-2 bioavailability, favouring the development of more effective, osteoinductive tooth-derived materials for bone regeneration and replacement. |
| Li et al. (2018) [69] | A prospective clinical study | Evaluation of DDM and Bio-Oss as bone substitutes in GBR for immediate placement of implants in periodontal post-extraction sites. | For 40 patients, DDM (size 0.5–1.0 mm) and Bio-Oss were used as bone grafts in the test group and control group, respectively. The implant stability, evaluated by Osstell Mentor, and marginal bone resorption, evaluated with x-ray examinations, were measured at T0, at 6 and 18 months after surgery. | The values of implant stability and marginal bone resorption were superimposable in the two groups. |
| Minetti et al. (2019) [38] | Multicenter Clinical Study | Studied the use of extracted tooth as autologous tooth graft after endodontic root canal therapies used for socket preservation and evaluated the implant insertion in regenerated bone with six-month follow up. | A total of 98 patients (29 men and 69 women) with an average age 53.7 years. Autologous tooth as a graft after the TT® device procedure. | The success rate of the tooth graft procedure was 99.1% (one site was infected and lost the regeneration and the implant). In all cases, after all implants were inserted, complete osseointegration after proper healing period was achieved. After the healing period, hard and soft tissues were stable. The healing of soft tissues after grafting procedures was free of complications. The implant success rate was 98.94% (one implant failed). |
| Cardaropoli et al. (2019) [70] | Case report | To evaluate the regenerative potential of particles obtained from a crushed extracted tooth. | After tooth removal, the clean root was ground, and the dentin and cementum granules were grafted into a fresh extraction socket for a ridge preservation procedure. | Tissue healing was evaluated by histologic and radiologic analysis. The volume of the ridge was preserved. Histologically, a dentin–bone complex was reported. New bone formation was evident, with an intimate contact between bone and both dentin/cementum. |
| Kadkhodazadeh et al. (2020) [71] | In vitro study | To evaluate the osteopromoting ability of human tooth powder and compare it with a bovine xenograft, a synthetic material, and a demineralized freeze-dried bone allograft (DFDBA). | A total of 30 teeth were collected. The samples were ground to a powder with particles <500 µm. Osteoblast-like cells of MG-63 were cultured with the tooth powder, Cerabone, DFDBA, and Osteon II. Cell proliferation was assessed by the MTT assay at 24 and 72 h intervals. | Tooth powder was able to increase osteogenic cell proliferation in comparison with the bovine xenograft, the synthetic graft, and the DFDBA. However, its osteopromoting ability was less than the osteogenic materials. |
| Minetti et al. (2020) [37] | Multicenter pilot study | Evaluation of post-extraction site preservation with DDM from vital or endodontically treated teeth and a collagen membrane. | A total of 28 patients and 32 extractions. After 4 months, 32 biopsies were performed, and histological and histomorphometric analyses were evaluated. | There was no significant difference in the bone regeneration of sites where DDM was obtained from vital or endodontically treated teeth. |
| Minetti et al. (2020) [72] | Case reports | Present a case of alveolar socket preservation by using tooth graft material and one implant-supported rehabilitation | One 26-year-old women, nonsmoker, ASA-1 Autologous Deciduous Tooth-Derived Material. | Good results in terms of clinical and radiographic outcomes showing the absence of bone resorption process and the stability of soft tissues after two years. |
| Sánchez- Labrador et al. (2020) [73] | A split-mouth clinical trial | Evaluation of post-extraction site preservation of the included third molar with and without DDM. | A total of 15 patients, 30 lower third molars were extracted, 15 sites post-extraction were grafted with DDM (300–1200 μm) and 15 contralateral sites were left to heal without graft. At three and six months, the sites were evaluated with x-ray and probing of periodontal tissue. | Bone regeneration and reduced periodontal defect on the distal side of the lower second molar were found in the sites grafted with DDM. |
| Korsch et al. (2021) [74] | Retrospective study | Using autogenous dentin for lateral ridge augmentation. | For the tooth-shell method (TST): 28 patients (15 females, 13 males) with 34 areas and 38 implants, autogenous dentin slices were taken from teeth and utilized to restore lateral ridge deficiencies. The control was the bone-shell technique (BST), which was performed on the autogenous bone on 31 patients (16 females and 15 males) with 32 areas and 41 implants. In both situations, implants were put in at the same time. A follow-up three months following implantation. | Between the two groups, there were no appreciable variations in the overall number of problems. One implant with TST and one with BST both showed horizontal hard tissue loss of 1 mm and 0.5 mm, respectively. |
| Cervera-Maillo et al. (2021) [75] | Prospective clinical trial | To evaluate the efficacy of extracted teeth processed into bacteria-free particulate dentin in a Smart dentin grinder and then grafted immediately into alveolus post extraction or into bone deficiencies. | Ten healthy, partially edentulous patients with few teeth in the mandible were recruited in the study. After their own teeth were grinded, particulate teeth were placed in empty sockets and bone defects after teeth extractions. Furthermore, after 3,6, 12, and 24 months, core samples using a 3 mm trephine were obtained. | Particulate dentin grafts should be considered as an alternative material for sockets’ preservation, split technique, and sinus lifting. Clinically and histologically, the performance of the dentin graft is at least comparable to extensively used xenogeneic or allogenic biomaterials. |
| Minetti et al. (2022) [76] | Histological specimen study | The aim of the study was to explore the histomorphometric outcomes of tooth derivative materials used as bone substitute material in socket preservation procedure. | The use of a demineralised tooth as grafting material was evaluated for the preservation of the alveolus. | No significant difference was noted between the maxillary and mandibular sites, including defect type and section position. |
| Okubo et al. (2022) [77] | Case report | To evaluate the effectiveness of DDM for GBR in a patient with severe bone defect on the anterior upper jaw. | Ridge augmentation with DDM in a patient with atrophic maxilla and implant placement. After one year from the surgery a biopsy was executed. | Histological findings revealed the direct formation of new bone on DDM residue. Radiographs showed in the region of upper lateral incisor an increase in horizontal breadth of 3,16 mm after implant placement. The upper level’s breadth grew from 3.38 mm to 5.92 mm. |
| Pohl et al. (2022) [78] | Case report | Assess concerns about the biological response of these ATDGs in preparation for implant placement and subsequent osseointegration. | After 12 weeks of extraction socket healing, an implant with an acid-etched surface was placed using osseodensification osteotomy preparation and was retrieved after 16 weeks of integration. Histologic analysis revealed ≥ 64% of direct bone-to-implant contact at multiple regions of interest along the implant surface. Residual dentin particles have been searched in contact with the implant. | Residual dentin particles were scarce and were never found in contact with the implant. The autologous tooth-derived grafts did not interfere with implant osseointegration. |
| Minetti et al. (2022) [79] | Clinical trial | Evaluation of alveolar ridge augmentation with and without collagen membrane associated with DDM. | Six patients with defects requiring bone augmentation and DDM (406 µ m and 815 µ m with peaks up to 1110 µ m). In Group 1, DDM was associated with a resorbable membrane, and in group 2 only DDM was used. At four months, a bone tissue biopsy was performed. | Histological analysis showed more bone volume and vital bone in the sites where membrane had been used in association with DDB. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. https://doi.org/10.3390/jfb14030132
Inchingolo AM, Patano A, Di Pede C, Inchingolo AD, Palmieri G, de Ruvo E, Campanelli M, Buongiorno S, Carpentiere V, Piras F, et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. Journal of Functional Biomaterials. 2023; 14(3):132. https://doi.org/10.3390/jfb14030132
Chicago/Turabian StyleInchingolo, Angelo Michele, Assunta Patano, Chiara Di Pede, Alessio Danilo Inchingolo, Giulia Palmieri, Elisabetta de Ruvo, Merigrazia Campanelli, Silvio Buongiorno, Vincenzo Carpentiere, Fabio Piras, and et al. 2023. "Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review" Journal of Functional Biomaterials 14, no. 3: 132. https://doi.org/10.3390/jfb14030132
APA StyleInchingolo, A. M., Patano, A., Di Pede, C., Inchingolo, A. D., Palmieri, G., de Ruvo, E., Campanelli, M., Buongiorno, S., Carpentiere, V., Piras, F., Settanni, V., Viapiano, F., Hazballa, D., Rapone, B., Mancini, A., Di Venere, D., Inchingolo, F., Fatone, M. C., Palermo, A., ... Malcangi, G. (2023). Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. Journal of Functional Biomaterials, 14(3), 132. https://doi.org/10.3390/jfb14030132





















