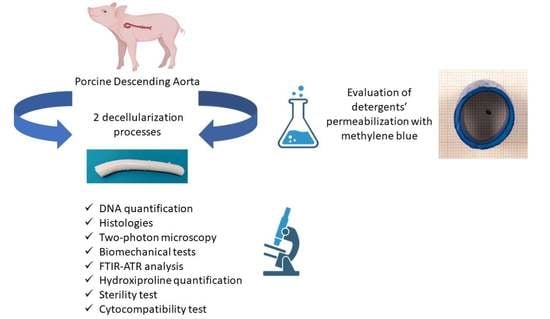
Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Porcine Descending Aorta Procurement
2.2. Descending Aorta Decellularization
2.2.1. Decellularization Procedure 1 (D1)
2.2.2. Decellularization Procedure 2 (D2)
2.2.3. Decellularization Procedure 3 (D3)
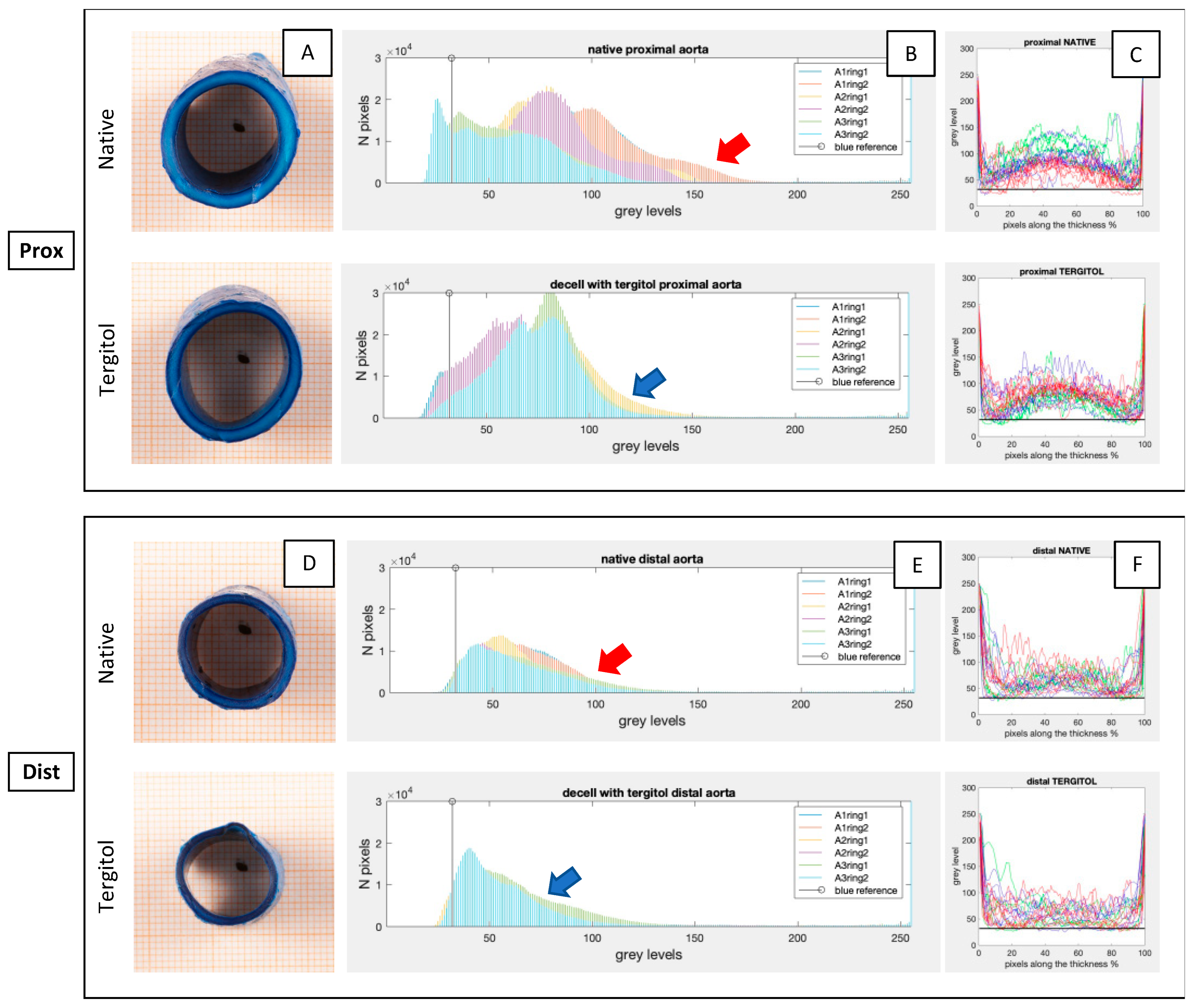
2.3. Comparison of Permeabilization Capacity of Different Detergents
2.4. DNA Quantification
2.5. Histological Stains
2.6. Immunofluorescence
2.7. Two-Photon Microscopy
2.8. Mechanical Tests
2.9. FTIR Analysis
2.10. Hydroxyproline Quantification
2.11. Descending Aorta Sterilization
2.12. Sterility Assessment
2.13. Tests for In Vitro Cytotoxicity
2.13.1. Live/Dead Assay
2.13.2. WST Assay
2.13.3. Whole Mount Immunofluorescence
3. Results
3.1. Evaluation of Detergents’ Permeabilization
3.2. Evaluation of Decellularization Efficacy
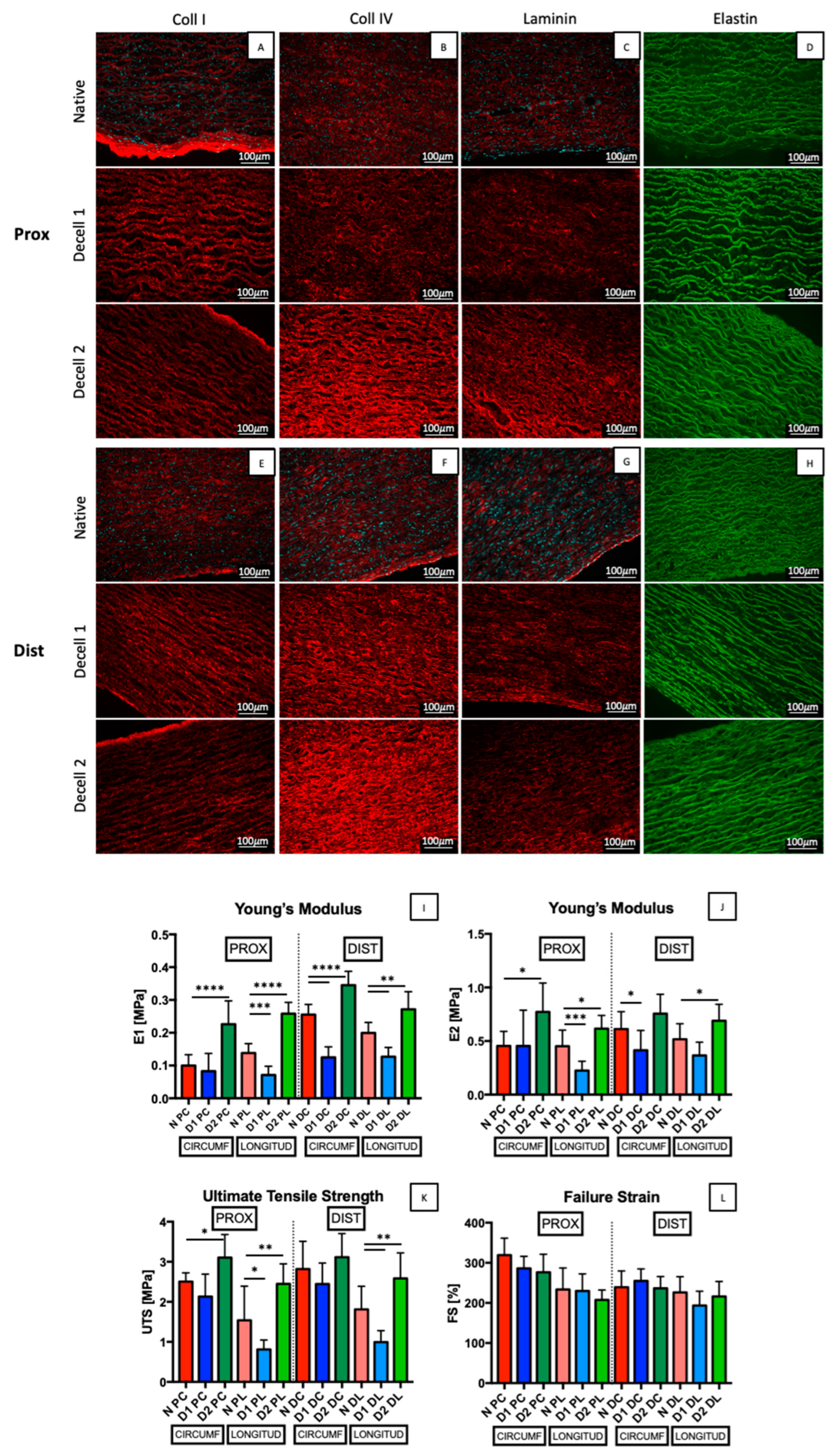
3.3. Structural and Biomechanical Characterization
3.4. Collagen Evaluation with Two-Photon Microscopy
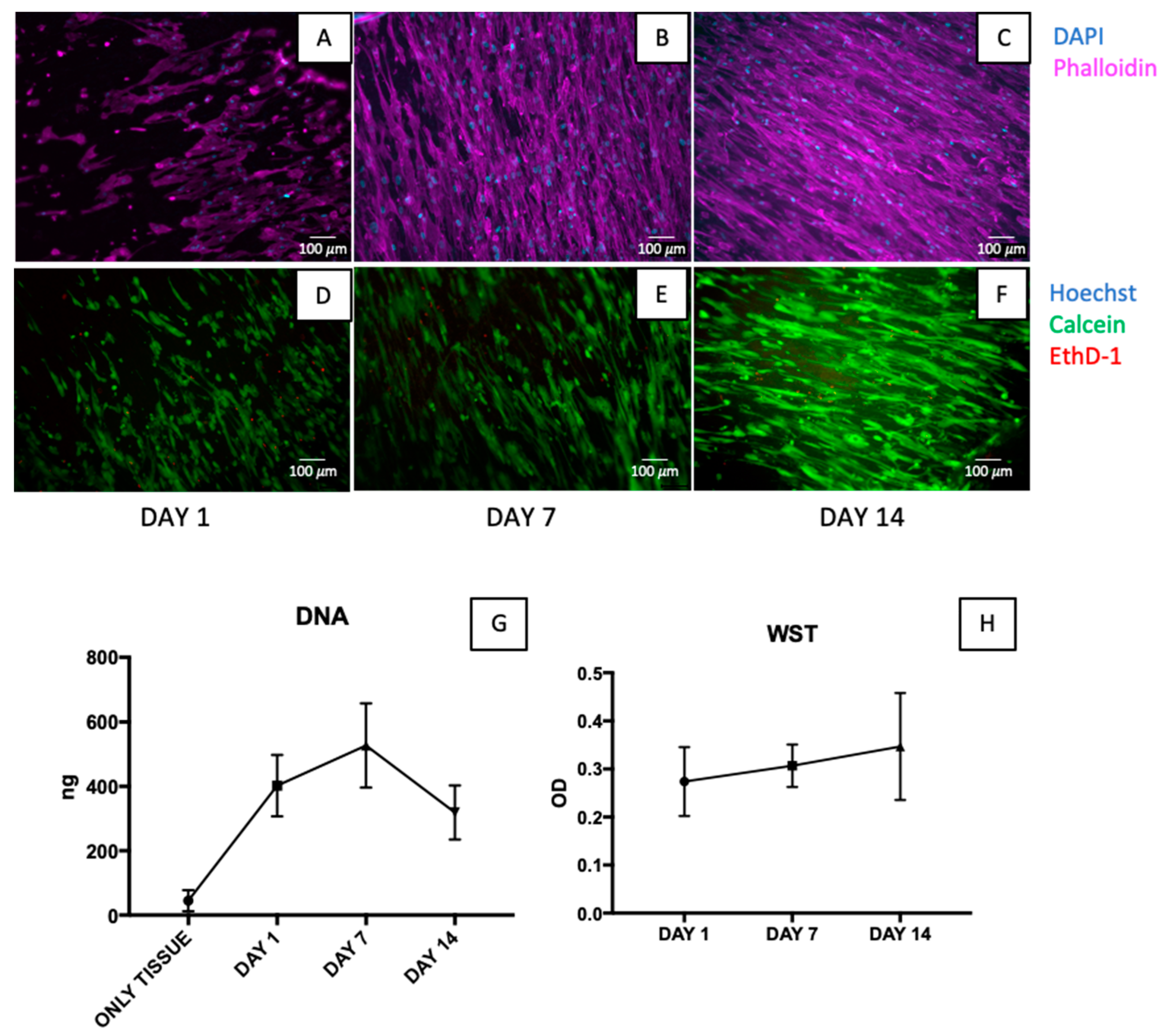
3.5. Sterility Assessment and Cytocompatibility Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering approaches for bladder regeneration. Int. J. Mol. Sci. 2018, 19, 19. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, S.T.; Djaladat, H.; Ahmadi, H.; Miranda, G.; Cai, J.; Schuckman, A.K.; Daneshmand, S. Gastrointestinal Complications Following Radical Cystectomy Using Enhanced Recovery Protocol. Eur. Urol. Focus 2018, 4, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Freeman, M.R.; Vacanti, J.P.; Shepard, J.; Retik, A.B. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J. Urol. 1993, 150, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Oberpenning, F.; Meng, J.; Yoo, J.J.; Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999, 17, 149–155. [Google Scholar] [CrossRef]
- Lai, J.Y.; Yoon, C.Y.; Yoo, J.J.; Wulf, T.; Atala, A.; Kropp, B. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J. Urol. 2002, 168, 1853–1858. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Chen, G.; Komuro, H.; Ushida, T.; Kaneko, S.; Tateishi, T.; Kaneko, M. Tissue-Engineered Urinary Bladder Wall Using PLGA Mesh-Collagen Hybrid Scaffolds: A Comparison Study of Collagen Sponge and Gel as a Scaffold. J. Pediatr. Surg. 2003, 38, 1781–1784. [Google Scholar] [CrossRef]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef]
- Micol, L.A.; Arenas da Silva, L.F.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.J.; Frey, P. In-Vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef]
- Sayeg, K.; Freitas-Filho, L.G.; Waitzberg, Â.F.L.; Arias, V.E.A.; Laks, M.; Egydio, F.M.; Oliveira, A.S. Integration of collagen matrices into the urethra when implanted as onlay graft. Int. Braz. J. Urol. 2013, 39, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Busch, S.; Daum, L.; Stenzl, A.; Amend, B.; et al. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: First results in a xenograft minipig model. World J. Urol. 2017, 35, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef]
- Algarrahi, K.; Franck, D.; Ghezzi, C.E.; Cristofaro, V.; Yang, X.; Sullivan, M.P.; Chung, Y.G.; Affas, S.; Jennings, R.; Kaplan, D.L.; et al. Acellular bi-layer silk fibroin scaffolds support functional tissue regeneration in a rat model of onlay esophagoplasty. Biomaterials 2015, 53, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1201–1211. [Google Scholar] [CrossRef]
- Kropp, B.P.; Rippy, M.K.; Badylak, S.F.; Adams, M.C.; Keating, M.A.; Rink, R.C.; Thor, K.B. Regenerative urinary bladder augmentation using small intestinal submucosa: Urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol. 1996, 155, 2098–2104. [Google Scholar] [CrossRef]
- Campodonico, F.; Benelli, R.; Michelazzi, A.; Ognio, E.; Toncini, C.; Maffezzini, M. Bladder cell culture on small intestinal submucosa as bioscaffold: Experimental study on engineered urothelial grafts. Eur. Urol. 2004, 46, 531–537. [Google Scholar] [CrossRef]
- Drewa, T. The Artificial Conduit for Urinary Diversion in Rats: A Preliminary Study. Transplant. Proc. 2007, 39, 1647–1651. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Liu, Y.; Bharadwaj, S.; Lee, S.J.; Atala, A.; Zhang, Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials 2009, 30, 3865–3873. [Google Scholar] [CrossRef]
- Liao, W.-B.; Song, C.; Li, Y.-W.; Yang, S.-X.; Meng, L.-C.; Li, X.-H. Tissue-engineered conduit using bladder acellular matrix and bladder epithelial cells for urinary diversion in rabbits. Chin. Med. J. 2013, 126, 335–339. [Google Scholar] [PubMed]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Smith, Z.L.; Sack, B.S.; Steinberg, G.D. Tissue Engineering and Conduit Substitution. Urol. Clin. N. Am. 2018, 45, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A.; et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. Int. J. Mol. Sci. 2022, 23, 2826. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Todesco, M.; Sandrin, D.; Romanato, F.; Bagno, A.; Morlacco, A.; Moro, F.D. A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers. J. Funct. Biomater. 2022, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Inclusion of Substances of Very High Concerns in the Candidate List (Decision of the European Chemicals Agency); European Chemicals Agency: Helsinki, Finland, 2012; pp. 1–9.
- Iop, L.; Bonetti, A.; Naso, F.; Rizzo, S.; Cagnin, S.; Bianco, R.; Dal Lin, C.; Martini, P.; Poser, H.; Franci, P.; et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS ONE 2014, 9, e99593. [Google Scholar] [CrossRef] [PubMed]
- Iop, L.; Paolin, A.; Aguiari, P.; Trojan, D.; Cogliati, E.; Gerosa, G. Decellularized Cryopreserved Allografts as Off-the-Shelf Allogeneic Alternative for Heart Valve Replacement: In Vitro Assessment before Clinical Translation. J. Cardiovasc. Transl. Res. 2017, 10, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, M.; Moro, A.; Butt, S.; Todesco, M.; Sandrin, D.; Borile, G.; Bagno, A.; Fabozzo, A.; Romanato, F.; Marchesan, M.; et al. A New Decellularization Protocol of Porcine Aortic Valves Using Tergitol to Characterize the Scaffold with the Biocompatibility Profile Using Human Bone Marrow Mesenchymal Stem Cells. Polymers 2022, 14, 1226. [Google Scholar] [CrossRef]
- Zhao, S.; Todorov, M.I.; Cai, R.; -Maskari, R.A.; Steinke, H.; Kemter, E.; Mai, H.; Rong, Z.; Warmer, M.; Stanic, K.; et al. Cellular and Molecular Probing of Intact Human Organs. Cell 2020, 180, 796–812. [Google Scholar] [CrossRef]
- Filippi, A.; Gintoli, M.; Filippi, A.; Sasso, E.D.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; et al. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borile, G.; Sandrin, D.; Filippi, A.; Anderson, K.I.; Romanato, F. Label-Free Multiphoton Microscopy: Much more than Fancy Images. Int. J. Mol. Sci. 2021, 22, 2657. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; Van De Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A comprehensive comparison of bovine and porcine decellularized pericardia: New insights for surgical applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Todesco, M.; Zardin, C.; Iop, L.; Palmosi, T.; Capaldo, P.; Romanato, F.; Gerosa, G.; Bagno, A. Hybrid membranes for the production of blood contacting surfaces: Physicochemical, structural and biomechanical characterization. Biomater. Res. 2021, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Bagno, A.; Aguiari, P.; Fiorese, M.; Iop, L.; Spina, M.; Gerosa, G. Native Bovine and Porcine Pericardia Respond to Load with Additive Recruitment of Collagen Fibers. Artif. Organs 2018, 42, 540–548. [Google Scholar] [CrossRef]
- Pei, M.; Zou, D.; Gao, Y.; Zhang, J.; Huang, P.; Wang, J.; Huang, J.; Li, Z.; Chen, Y.; Li, Z.; et al. The influence of sample geometry and size on porcine aortic material properties from uniaxial tensile tests using custom-designed tissue cutters, clamps and molds. PLoS ONE 2021, 16, e0244390. [Google Scholar] [CrossRef]
- Brauner, J.W.; Flach, C.R.; Mendelsohn, R. A quantitative reconstruction of the amide I contour in the IR spectra of globular proteins: From structure to spectrum. J. Am. Chem. Soc. 2005, 127, 100–109. [Google Scholar] [CrossRef]
- Todesco, M.; Imran, S.J.; Fortunato, T.M.; Sandrin, D.; Borile, G.; Romanato, F.; Casarin, M.; Giuggioli, G.; Conte, F.; Marchesan, M.; et al. A New Detergent for the Effective Decellularization of Bovine and Porcine Pericardia. Biomimetics 2022, 7, 104. [Google Scholar] [CrossRef]
- Oldenburg, K. LoadSpectra. Available online: https://www.mathworks.com/matlabcentral/fileexchange/57904-loadspectra (accessed on 23 May 2022).
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe 2.6.1. Sterility. Eur. Pharmacopoeia 2005, 5, 145–149.
- C.S.A. ISO 10993-5 in vitro cytotoxicity. Int. Organ. 2009, 2007, 1–11. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole organ decellularization processes. Biomaterials 2012, 32, 3233–3243. [Google Scholar] [CrossRef]
- Gallagher, W. FTIR Analysis of Protein Structure. Course Man. Chem. 2009, 455, 1–8. [Google Scholar]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Lazarev, Y.A.; Grishkovsky, B.A.; Khromova, T.B. Amide I band of IR spectrum and structure of collagen and related polypeptides. Biopolymers 1985, 24, 1449–1478. [Google Scholar] [CrossRef]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–176. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Nielsen, M.; Smith, A.; Nix, J.; Schultz, H.; Wallen, E.M. Fast Track Program in Patients Undergoing Radical Cystectomy: Results in 362 Consecutive Patients. J. Am. Coll. Surg. 2010, 210, 93–99. [Google Scholar] [CrossRef]
- Ramirez, J.A.; McIntosh, A.G.; Strehlow, R.; Lawrence, V.A.; Parekh, D.J.; Svatek, R.S. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: A systematic review. Eur. Urol. 2013, 64, 588–597. [Google Scholar] [CrossRef]
- Faba, O.R.; Moreno, R.P.; Malca, L.; Martínez, A.P.; Nervo, N.; Breda, A.; Esquinas, C.; Palou, J. Postoperative management of radical cystectomy. Review of the evidence on the prevention and treatment of urological complications. Actas Urológicas Españolas 2018, 42, 143–151. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Morlacco, A.; Dal Moro, F. Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes 2021, 9, 1643. [Google Scholar] [CrossRef]
- Casarin, M.; Morlacco, A.; Moro, F.D. Tissue Engineering and Regenerative Medicine in Pediatric Urology: Urethral and Urinary Bladder Reconstruction. Int. J. Mol. Sci. 2022, 23, 6360. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.L.; Dahlen, G.A. Autogenous vein grafts and venous valves in ureteral surgery; an experimental study. J. Urol. 1953, 70, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.J.; Sanders, A.R.; Hurwitt, E.S. The fate of fresh autogenous arterial grafts embedded in submucosal intestinal tunnels as applied to the bridging of ureteral defects. Ann. Surg. 1955, 142, 266–273. [Google Scholar] [CrossRef]
- Sewell, W.H. Failure of freeze-dried homologous arteries used as ureteral grafts. J. Urol. 1955, 74, 600–602. [Google Scholar] [CrossRef]
- Albert, P.S.; Vitolo, R.V.; Friedenberg, R.; Davis, J.E. Bovine carotid heterograft for segmental ureteral substitution. Am. J. Surg. 1976, 131, 556–559. [Google Scholar] [CrossRef]
- Kloskowski, T.; Jundziłł, A.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Pokrywczyńska, M.; Frontczak-Baniewicz, M.; Kowalewski, T.A.; Chłosta, P.; et al. Ureter regeneration-The proper scaffold has to be defined. PLoS ONE 2014, 9, e106023. [Google Scholar] [CrossRef]
- Kloskowski, T.; PokrywczyŃska, M.; Drewa, T. Artificial urinary conduit construction using tissue engineering methods. Cent. Eur. J. Urol. 2015, 68, 109–114. [Google Scholar] [CrossRef]
- Guler, S.; Aydin, H.M.; Lü, L.X.; Yang, Y. Improvement of Decellularization Efficiency of Porcine Aorta Using Dimethyl Sulfoxide as a Penetration Enhancer. Artif. Organs 2018, 42, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Walawalkar, S.; Almelkar, S. Fabrication of aortic bioprosthesis by decellularization, fibrin glue coating and re-endothelization: A cell scaffold approach. Prog. Biomater. 2019, 8, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Ando, D.; Hoshino, R.; Yamamoto, T.; Wahyudiono; Suzuki, S.; Shinohara, S.; Goto, M. Surfactant-Free Decellularization of Porcine Aortic Tissue by Subcritical Dimethyl Ether. ACS Omega 2021, 6, 13417–13425. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Basso, C.; Della Barbera, M. Pathology of the aorta and aorta as homograft. J. Cardiovasc. Dev. Dis. 2021, 8, 76. [Google Scholar] [CrossRef]
- Guler, S.; Aslan, B.; Hosseinian, P.; Aydin, H.M. Supercritical Carbon Dioxide-Assisted Decellularization of Aorta and Cornea. Tissue Eng.—Part C Methods 2017, 23, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Deisseroth, A.; Rubartelli, A. Damage associated molecular pattern molecules. Clin. Immunol. 2007, 124, 1–4. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef]
- Chow, M.J.; Turcotte, R.; Lin, C.P.; Zhang, Y. Arterial extracellular matrix: A mechanobiological study of the contributions and interactions of elastin and collagen. Biophys. J. 2014, 106, 2684–2692. [Google Scholar] [CrossRef]
- Dingemans, K.P.; Teeling, P.; Lagendijk, J.H.; Becker, A.E. Extracellular matrix of the human aortic media: An ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat. Rec. 2000, 258, 1–14. [Google Scholar] [CrossRef]
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: A review. J. R. Soc. Interface 2013, 10, 20121004. [Google Scholar] [CrossRef]
- Deplano, V.; Boufi, M.; Boiron, O.; Guivier-Curien, C.; Alimi, Y.; Bertrand, E. Biaxial tensile tests of the porcine ascending aorta. J. Biomech. 2016, 49, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Zeinali-Davarani, S.; Wang, Y.; Chow, M.J.; Turcotte, R.; Zhang, Y. Contribution of collagen fiber undulation to regional biomechanical properties along porcine thoracic aorta. J. Biomech. Eng. 2015, 137, 051001. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fung, Y.C. Elastic and inelastic properties of the canine aorta and their variation along the aortic tree. Fed. Proc. 1974, 33, 861. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. Positional variations in fracture toughness, stiffness and strength of descending thoracic pig aorta. J. Biomech. 1983, 16, 947–953. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Łopianiak, I.; Butruk-Raszeja, B.A. Evaluation of sterilization/disinfection methods of fibrous polyurethane scaffolds designed for tissue engineering applications. Int. J. Mol. Sci. 2020, 21, 8092. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.M.; Kang, K.S.; Woo, H.M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Yang, M.; Zou, Y.; Liu, H.; Liang, Z.; Yin, Y.; Niu, G.; Yan, Z.; Zhang, B. Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in vitro. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 257. [Google Scholar] [CrossRef]
- Gu, W.; Hong, X.; Le Bras, A.; Nowak, W.N.; Bhaloo, S.I.; Deng, J.; Xie, Y.; Hu, Y.; Ruan, X.Z.; Xu, Q. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. J. Biol. Chem. 2018, 293, 8089–8102. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Joergensen, B.; Bornhaeuser, M.; Ehninger, G.; Werner, C. Mesenchymal Stem Cells (MSC) can be differentiated into endothelial cells in vitro. Stem Cells. 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]



| Sample Name | Turbidity within 14 Days (Yes/No) |
|---|---|
| Native | Yes |
| Decellularized | Yes |
| Decellularized + antibiotic/antimycotic/PAA | No |
| Control (only media) | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarin, M.; Fortunato, T.M.; Imran, S.J.; Todesco, M.; Sandrin, D.; Marchesan, M.; Gerosa, G.; Romanato, F.; Bagno, A.; Dal Moro, F.; et al. Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes. J. Funct. Biomater. 2023, 14, 141. https://doi.org/10.3390/jfb14030141
Casarin M, Fortunato TM, Imran SJ, Todesco M, Sandrin D, Marchesan M, Gerosa G, Romanato F, Bagno A, Dal Moro F, et al. Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes. Journal of Functional Biomaterials. 2023; 14(3):141. https://doi.org/10.3390/jfb14030141
Chicago/Turabian StyleCasarin, Martina, Tiago Moderno Fortunato, Saima Jalil Imran, Martina Todesco, Deborah Sandrin, Massimo Marchesan, Gino Gerosa, Filippo Romanato, Andrea Bagno, Fabrizio Dal Moro, and et al. 2023. "Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes" Journal of Functional Biomaterials 14, no. 3: 141. https://doi.org/10.3390/jfb14030141
APA StyleCasarin, M., Fortunato, T. M., Imran, S. J., Todesco, M., Sandrin, D., Marchesan, M., Gerosa, G., Romanato, F., Bagno, A., Dal Moro, F., & Morlacco, A. (2023). Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes. Journal of Functional Biomaterials, 14(3), 141. https://doi.org/10.3390/jfb14030141