Characterization of Cyclic Olefin Copolymers for Insulin Reservoir in an Artificial Pancreas
Abstract
:1. Introduction
2. Materials and Methods
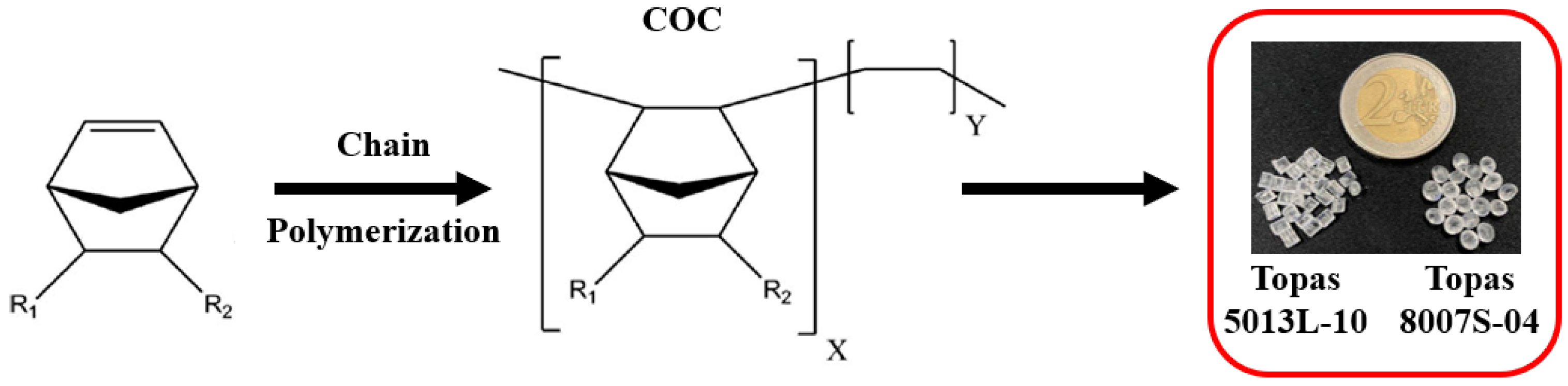
2.1. Materials
2.2. Mechanical Characterization
2.3. Thermal Characterization
2.4. Design and Fabrication of Reservoir
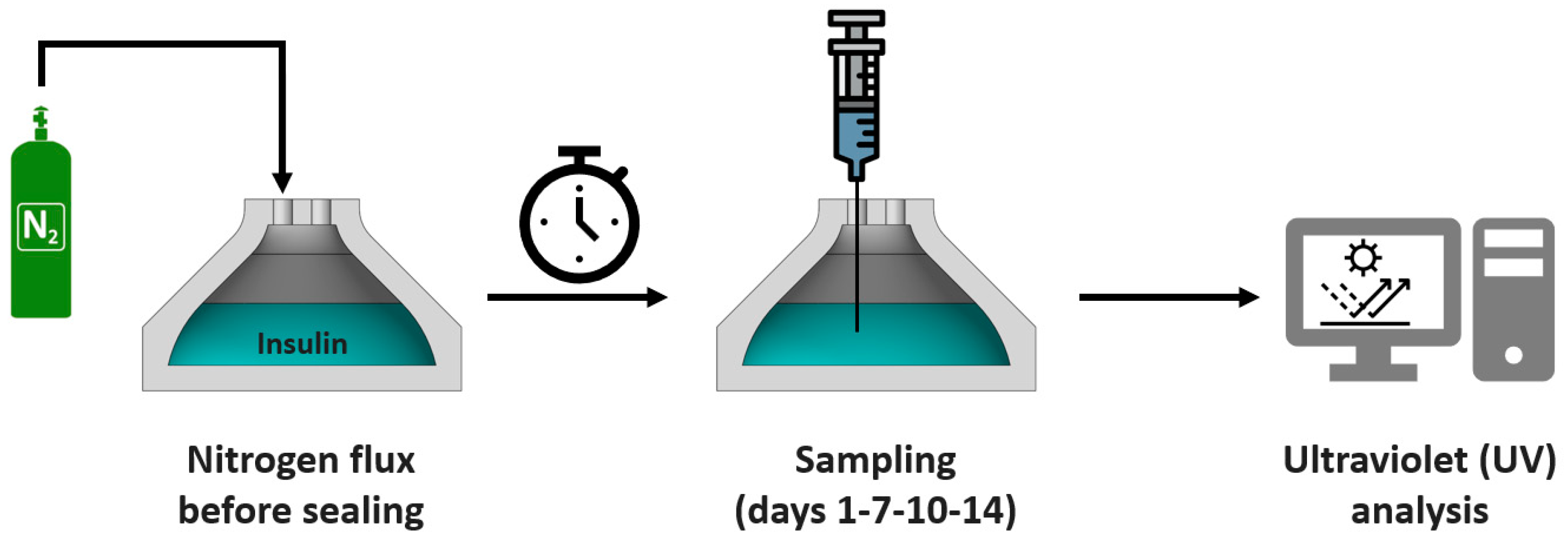
2.5. Insulin Aggregation Tests
2.6. Statistical Analysis
3. Results
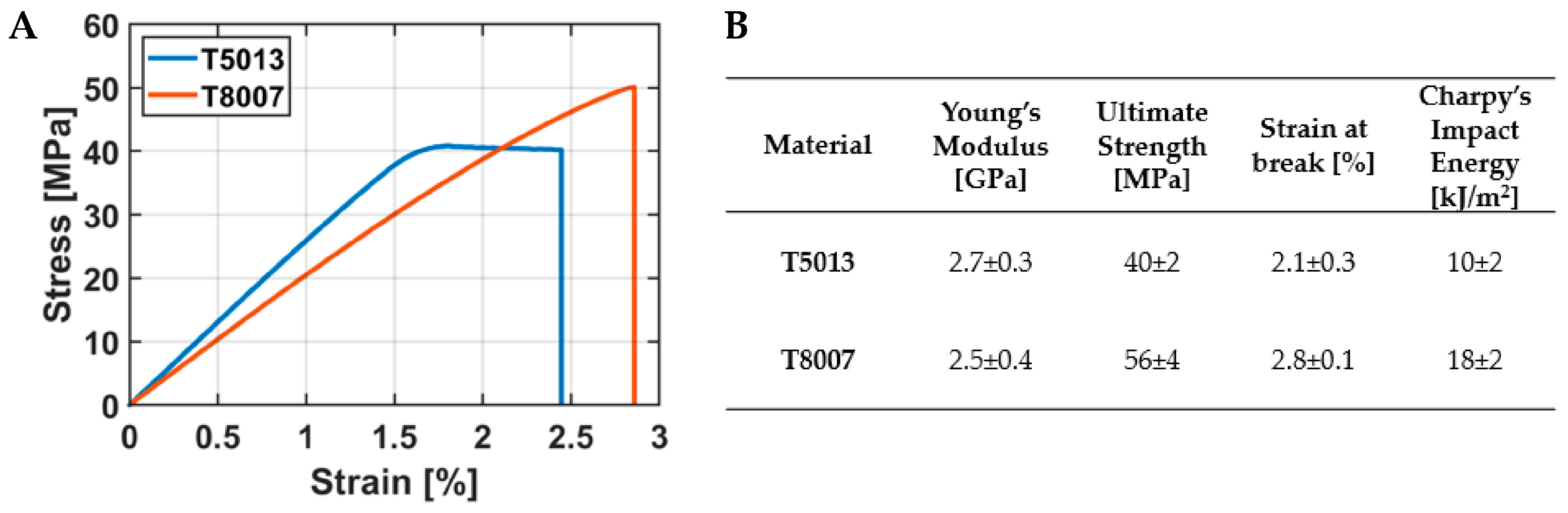
3.1. Mechanical Characterization
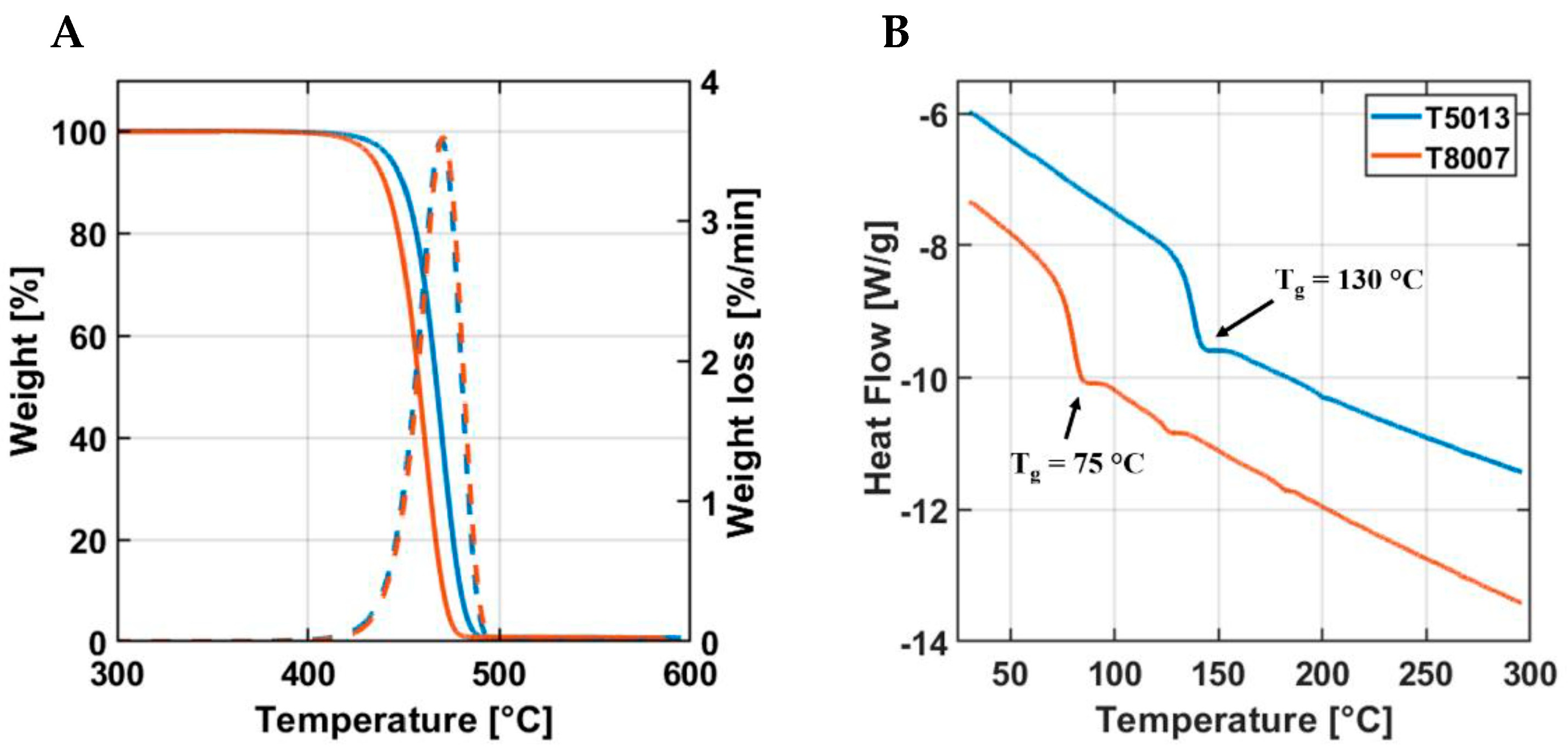
3.2. Thermal Characterization
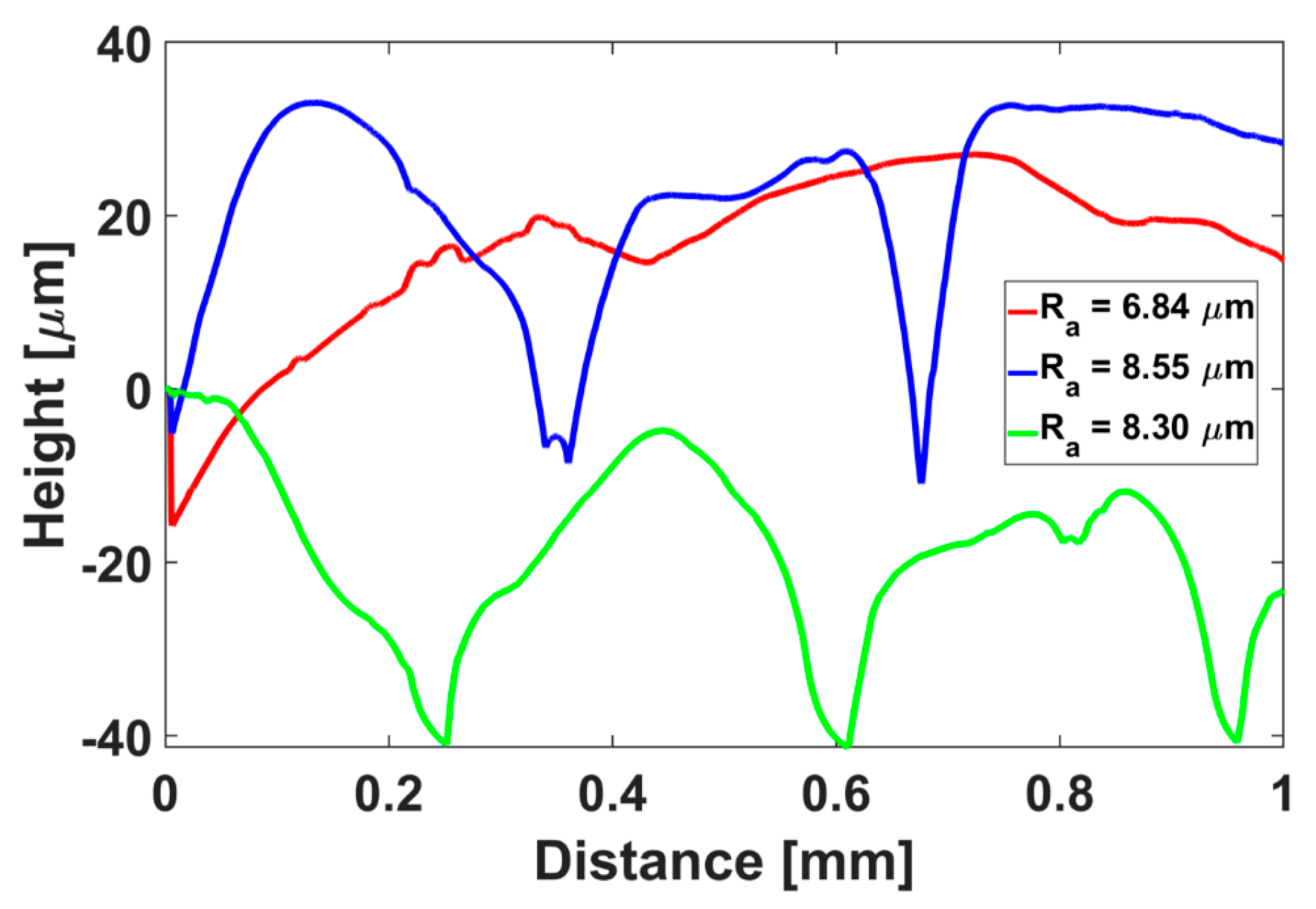
3.3. Fabrication of the Insulin Reservoir
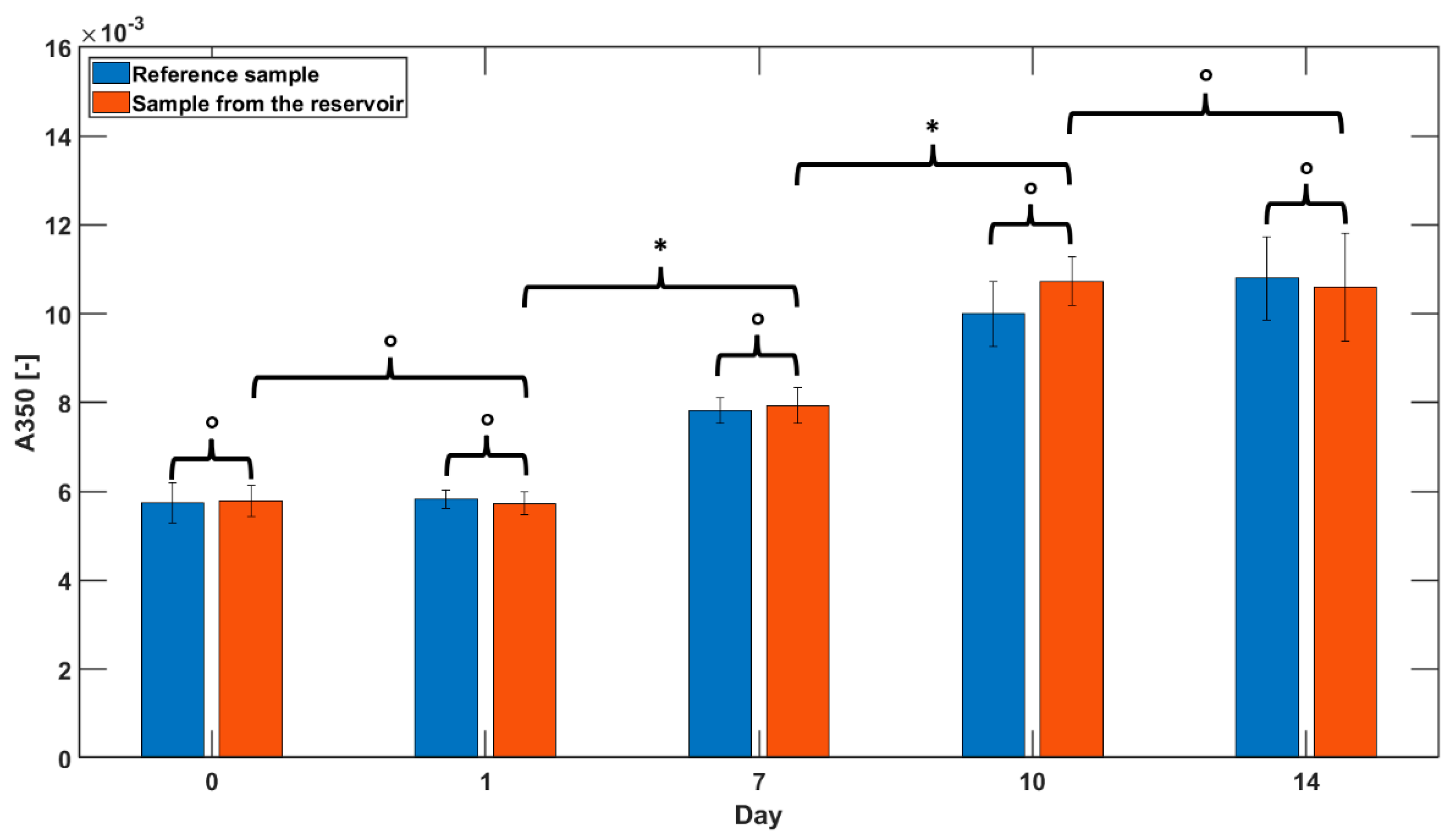
3.4. Insulin Aggregation Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2006, 29, S43. [Google Scholar]
- Peyser, T.; Dassau, E.; Breton, M.; Skyler, J.S. The Artificial Pancreas: Current Status and Future Prospects in the Management of Diabetes. Ann. N. Y. Acad. Sci. 2014, 1311, 102–123. [Google Scholar] [CrossRef]
- Harpstead, S.D. Effect of Laminin on the Interaction Between Islets and an Implantable Immunoisolation Polyurethane Membrane. J. Med. Devices 2009, 3, 027521. [Google Scholar] [CrossRef]
- Iacovacci, V.; Ricotti, L.; Menciassi, A.; Dario, P. The Bioartificial Pancreas (BAP): Biological, Chemical and Engineering Challenges. Biochem. Pharmacol. 2016, 100, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Kinoshita, Y.; Kitagawa, H.; Munekage, M.; Munekage, E.; Takezaki, Y.; Yatabe, T.; Yamashita, K.; Yamazaki, R.; Okabayashi, T.; et al. Evaluation of a Novel Artificial Pancreas: Closed Loop Glycemic Control System with Continuous Blood Glucose Monitoring. Artif. Organs 2013, 37, E67–E73. [Google Scholar] [CrossRef]
- Hoadley, D.; Ananthan, A. Using Modeling and Simulation in the Design of Closed-Loop Insulin Delivery System. J. Med. Devices 2013, 7, 020923. [Google Scholar] [CrossRef]
- Lee, S.W.; Welsh, J.B. Upcoming Devices for Diabetes Management: The Artificial Pancreas as the Hallmark Device. Diabetes Technol. Ther. 2015, 17, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Assaf, T.; Dario, P.; Menciassi, A. Wearable and Implantable Pancreas Substitutes. J. Artif. Organs 2013, 16, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Dassau, E.; Renard, E.; Place, J.; Farret, A.; Pelletier, M.-J.; Lee, J.; Huyett, L.M.; Chakrabarty, A.; Doyle III, F.J.; Zisser, H.C. Intraperitoneal Insulin Delivery Provides Superior Glycaemic Regulation to Subcutaneous Insulin Delivery in Model Predictive Control-Based Fully-Automated Artificial Pancreas in Patients with Type 1 Diabetes: A Pilot Study. Diabetes Obes. Metab. 2017, 19, 1698–1705. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.B.; Kim, B.H.; Lee, C.; Cho, Y.M.; Kim, S.-N.; Park, C.G.; Cho, Y.-C.; Choy, Y. Bin Implantable Batteryless Device for On-Demand and Pulsatile Insulin Administration. Nat. Commun. 2017, 8, 15032. [Google Scholar] [CrossRef] [PubMed]
- Renard, E. Implantable Insulin Delivery Pumps. Minim. Invasive Ther. Allied Technol. 2004, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Bellingham, J.; Dupont, P.E.; Fischer, P.; Floridi, L.; Full, R.; Jacobstein, N.; Kumar, V.; McNutt, M.; Merrifield, R.; et al. The Grand Challenges of Science Robotics. Sci. Robot. 2018, 3, eaar7650. [Google Scholar] [CrossRef] [PubMed]
- Iacovacci, V.; Tamadon, I.; Rocchi, M.; Dario, P.; Menciassi, A. Toward Dosing Precision and Insulin Stability in an Artificial Pancreas System. J. Med. Devices 2019, 13, 011008. [Google Scholar] [CrossRef]
- Schlein, M. Insulin Formulation Characterization—The Thioflavin T Assays. AAPS J. 2017, 19, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an Amyloid Dye: Fibril Quantification, Optimal Concentration and Effect on Aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [PubMed]
- Bratlie, K.M.; York, R.L.; Invernale, M.A.; Langer, R.; Anderson, D.G. Materials for Diabetes Therapeutics. Adv. Healthc. Mater. 2012, 1, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Schaepelynck, P.; Riveline, J.-P.; Renard, E.; Hanaire, H.; Guerci, B.; Baillot-Rudoni, S.; Sola-Gazagnes, A.; Catargi, B.; Fontaine, P.; Millot, L.; et al. Assessment of a New Insulin Preparation for Implanted Pumps Used in the Treatment of Type 1 Diabetes. Diabetes Technol. Ther. 2014, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Feingold, V.; Jenkins, A.B.; Kraegen, E.W. Effect of Contact Material on Vibration-Induced Insulin Aggregation. Diabetologia 1984, 27, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Melberg, S.G.; Havelund, S.; Villumsen, J.; Brange, J. Insulin Compatibility with Polymer Materials Used in External Pump Infusion Systems. Diabet. Med. 1988, 5, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Johnson, S.; Micic, M.; Orbulescu, J.; Whyte, J.; Garcia, A.R.; Leblanc, R.M. Study of the Aggregation of Human Insulin Langmuir Monolayer. Langmuir 2012, 28, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Sluzky, V.; Tamada, J.A.; Klibanov, A.M.; Langer, R. Kinetics of Insulin Aggregation in Aqueous Solutions upon Agitation in the Presence of Hydrophobic Surfaces. Proc. Natl. Acad. Sci. USA 1991, 88, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Roy, I. Probing the Mechanism of Insulin Aggregation during Agitation. Int. J. Pharm. 2011, 413, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Leblanc, R.M. Aggregation of Insulin at the Interface. J. Phys. Chem. B 2014, 118, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Park, J.Y.; Liu, C.; He, J.; Kim, S.C. Chemical Structure and Physical Properties of Cyclic Olefin Copolymers (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 801–814. [Google Scholar] [CrossRef]
- Lamonte, R.R.; MCNALLU, D. Uses and Processing of Cyclic Olefin Copolymers. Plast. Eng. 2000, 56, 51–55. [Google Scholar]
- Khanarian, G. Optical Properties of Cyclic Olefin Copolymers. Opt. Eng. 2001, 40, 1024–1029. [Google Scholar] [CrossRef]
- Eakins, M.N. New Plastics for Old Vials. BioProcess Int. 2005, 3, 52–58. [Google Scholar]
- Woods, E.J.; Thirumala, S. Packaging Considerations for Biopreservation. Transfus. Med. Hemother. 2011, 38, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mlejnek, P. Cycloolefin Copolymers: Processing, Properties Application. Bachelor’s Thesis, Univerzita Tomase Bati ve Zline, Faculty of Technology, Zlin, Czech Republic, 2007. [Google Scholar]
- Mahmood, H.; Dorigato, A.; Pegoretti, A. Healable Carbon Fiber-Reinforced Epoxy/Cyclic Olefin Copolymer Composites. Materials 2020, 13, 2165. [Google Scholar] [CrossRef]
- Mahmood, H.; Dorigato, A.; Pegoretti, A. Thermal Mending in Novel Epoxy/Cyclic Olefin Copolymer Blends. Express Polym. Lett. 2020, 14, 368–383. [Google Scholar] [CrossRef]
- Dorigato, A.; Mahmood, H.; Pegoretti, A. Optimization of the Thermal Mending Process in Epoxy/Cyclic Olefin Copolymer Blends. J. Appl. Polym. Sci. 2021, 138, 49937. [Google Scholar] [CrossRef]
- Limam, M.; Tighzert, L.; Fricoteaux, F.; Bureau, G. Sorption of Organic Solvents by Packaging Materials: Polyethylene Terephthalate and TOPAS®. Polym. Test. 2005, 24, 395–402. [Google Scholar] [CrossRef]
- Eakins, M.N. INJECTABLES: Plastic Pre-Fillable Syringes and Vials: Progress Towards a Wider Acceptance. Am. Pharm. Rev. 2010, 13, 12. [Google Scholar]
- Qadry, S.S.; Roshdy, T.H.; Char, H.; Del Terzo, S.; Tarantino, R.; Moschera, J. Evaluation of CZ-Resin Vials for Packaging Protein-Based Parenteral Formulations. Int. J. Pharm. 2003, 252, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Petrtyl, M.; Bastl, Z.; Krulis, Z.; Hulejova, H.; Polanska, M.; Lisal, J.; Danesova, J.; Cerny, P. Cycloolefin-Copolymer/Polyethylene (COC/PE) Blend Assists with the Creation of New Articular Cartilage. In Macromolecular Symposia; Wiley: Weinheim, Germany, 2010; Volume 294, pp. 120–132. [Google Scholar]
- Leech, P.W.; Zhang, X.; Zhu, Y. Effect of Norbornene Content on Deformation Properties and Hot Embossing of Cyclic Olefin Copolymers. J. Mater. Sci. 2010, 45, 5364–5369. [Google Scholar] [CrossRef]
- Cristallini, C.; Danti, S.; Azimi, B.; Tempesti, V.; Ricci, C.; Ventrelli, L.; Cinelli, P.; Barbani, N.; Lazzeri, A. Multifunctional Coatings for Robotic Implanted Device. Int. J. Mol. Sci. 2019, 20, 5126. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Scrivani, T.; Benavente, R.; Pérez, E.; Pereña, J.M. Stress-Strain Behaviour, Microhardness, and Dynamic Mechanical Properties of a Series of Ethylene-Norbornene Copolymers. Macromol. Chem. Phys. 2001, 202, 2547–2553. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Sun, X.; Zhang, J.; He, J. Thermal Degradation Studies of Cyclic Olefin Copolymers. Polym. Degrad. Stab. 2003, 81, 197–205. [Google Scholar] [CrossRef]
- Stivala, S.; Kimura, J.; Gabbay, S. Degradation and Stabilization of Polyolefins; Allen, N., Ed.; Applied Science Publishers: New York, NY, USA, 1983. [Google Scholar]
- McNally, D. Encyclopedia of Polymer Science and Technology, 3rd ed.; Herman, F., Ed.; Wiley–Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar]
- Nielsen, L.; Khurana, R.; Coats, A.; Frokjaer, S.; Brange, J.; Vyas, S.; Uversky, V.N.; Fink, A.L. Effect of Environmental Factors on the Kinetics of Insulin Fibril Formation: Elucidation of the Molecular Mechanism. Biochemistry 2001, 40, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, T.B.; Mair, D.A.; Holden, T.G.; Lee, L.J.; Svec, F.; Fréchet, J.M.J. Hydrophilic Surface Modification of Cyclic Olefin Copolymer Microfluidic Chips Using Sequential Photografting. J. Sep. Sci. 2007, 30, 1088–1093. [Google Scholar] [CrossRef] [PubMed]



| Material | Extrusion Temperature (°C) | Screw Speed (rpm) | Cycle Time (s) | Injection Temperature (°C) | Injection Pressure (bar) | Molding Time (s) | Mold Temperature (°C) |
|---|---|---|---|---|---|---|---|
| T5013 | 250 | 100 | 60 | 250 | 700 | 20 | 140 |
| T8007 | 230 | 100 | 60 | 230 | 650 | 20 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallegni, N.; Milazzo, M.; Cristallini, C.; Barbani, N.; Fredi, G.; Dorigato, A.; Cinelli, P.; Danti, S. Characterization of Cyclic Olefin Copolymers for Insulin Reservoir in an Artificial Pancreas. J. Funct. Biomater. 2023, 14, 145. https://doi.org/10.3390/jfb14030145
Mallegni N, Milazzo M, Cristallini C, Barbani N, Fredi G, Dorigato A, Cinelli P, Danti S. Characterization of Cyclic Olefin Copolymers for Insulin Reservoir in an Artificial Pancreas. Journal of Functional Biomaterials. 2023; 14(3):145. https://doi.org/10.3390/jfb14030145
Chicago/Turabian StyleMallegni, Norma, Mario Milazzo, Caterina Cristallini, Niccoletta Barbani, Giulia Fredi, Andrea Dorigato, Patrizia Cinelli, and Serena Danti. 2023. "Characterization of Cyclic Olefin Copolymers for Insulin Reservoir in an Artificial Pancreas" Journal of Functional Biomaterials 14, no. 3: 145. https://doi.org/10.3390/jfb14030145
APA StyleMallegni, N., Milazzo, M., Cristallini, C., Barbani, N., Fredi, G., Dorigato, A., Cinelli, P., & Danti, S. (2023). Characterization of Cyclic Olefin Copolymers for Insulin Reservoir in an Artificial Pancreas. Journal of Functional Biomaterials, 14(3), 145. https://doi.org/10.3390/jfb14030145











