Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration
Abstract
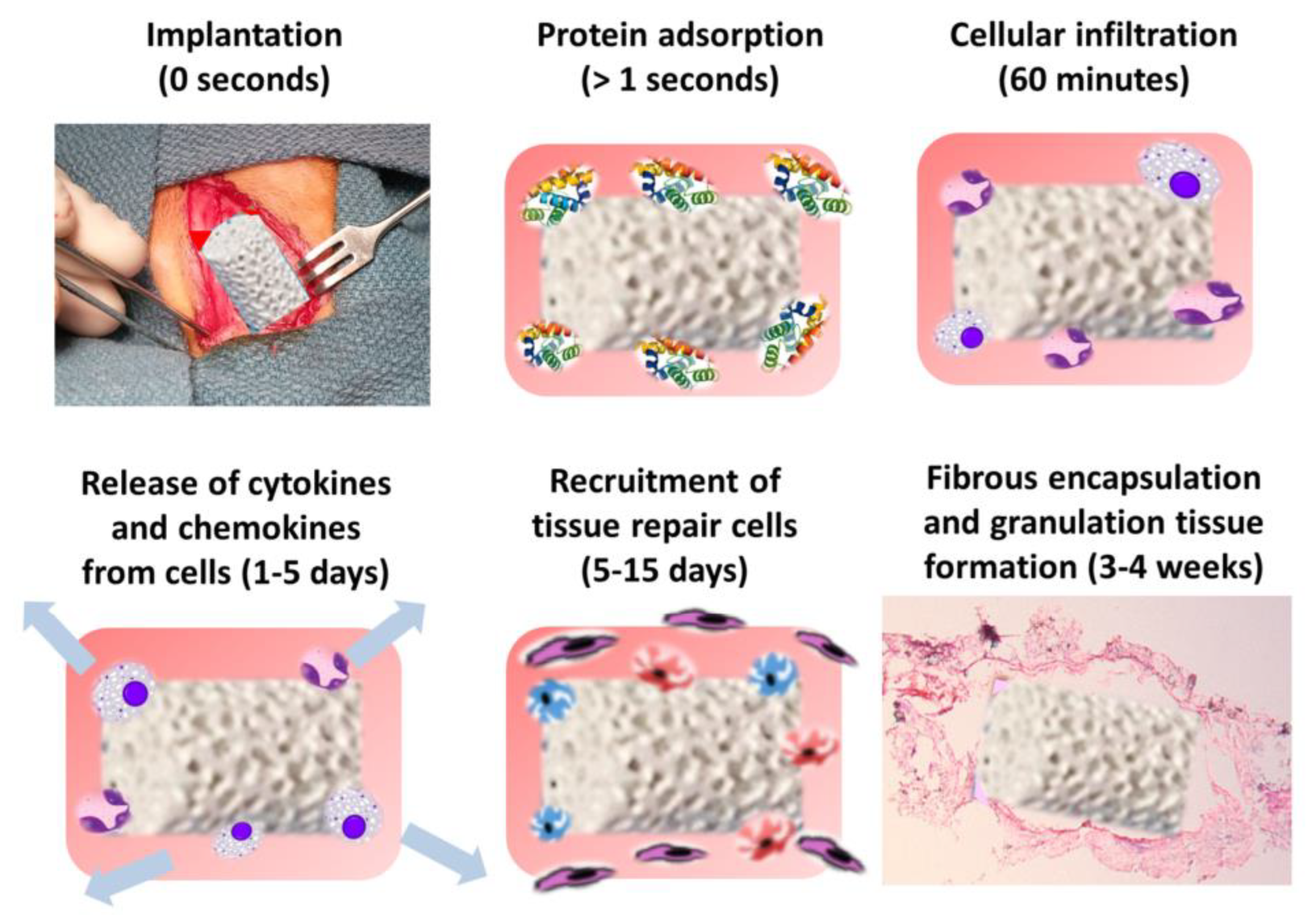
:1. Introduction
2. Signaling Pathway
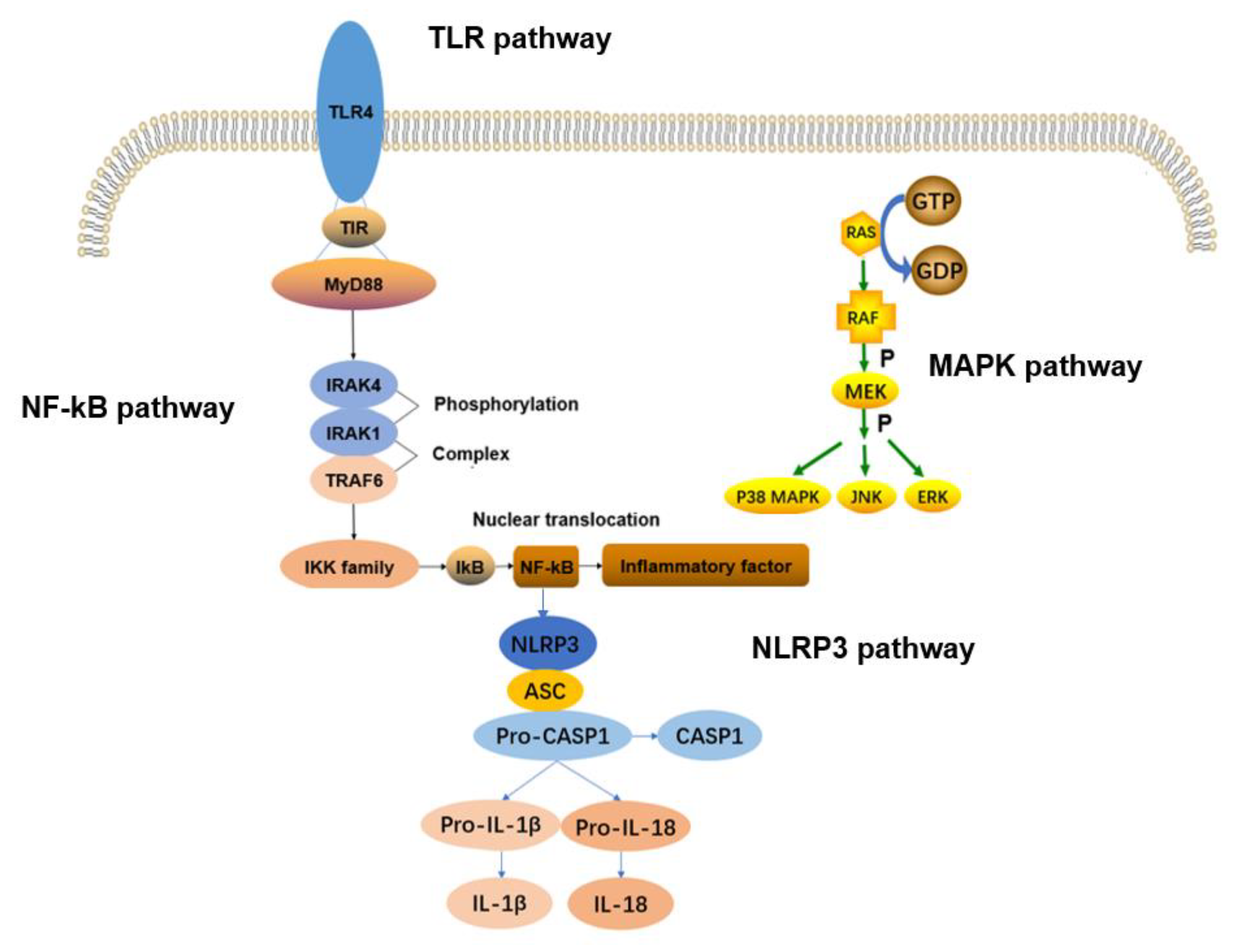
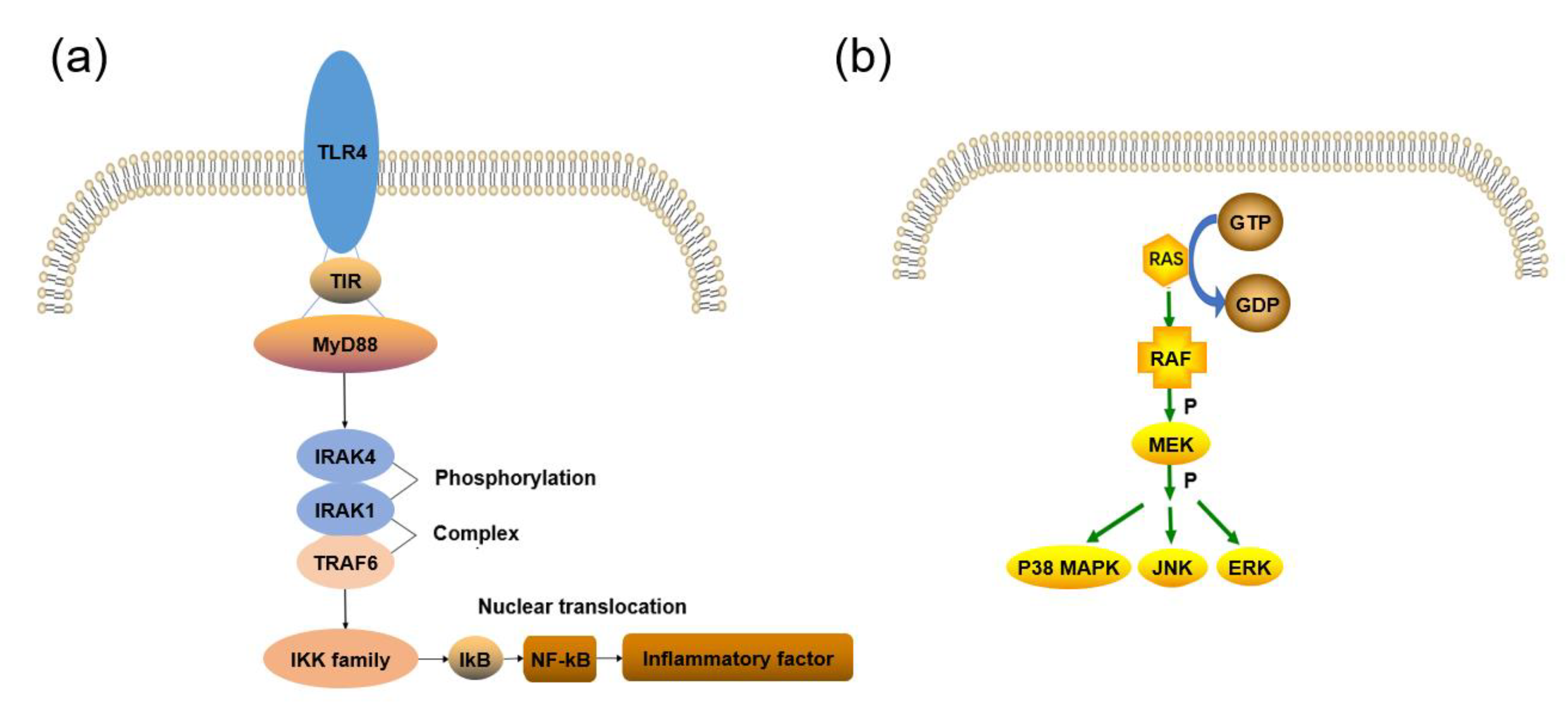
2.1. TLRs Signaling Pathway
2.2. NF–kB Signaling Pathway
2.3. MAPK Signaling Pathway
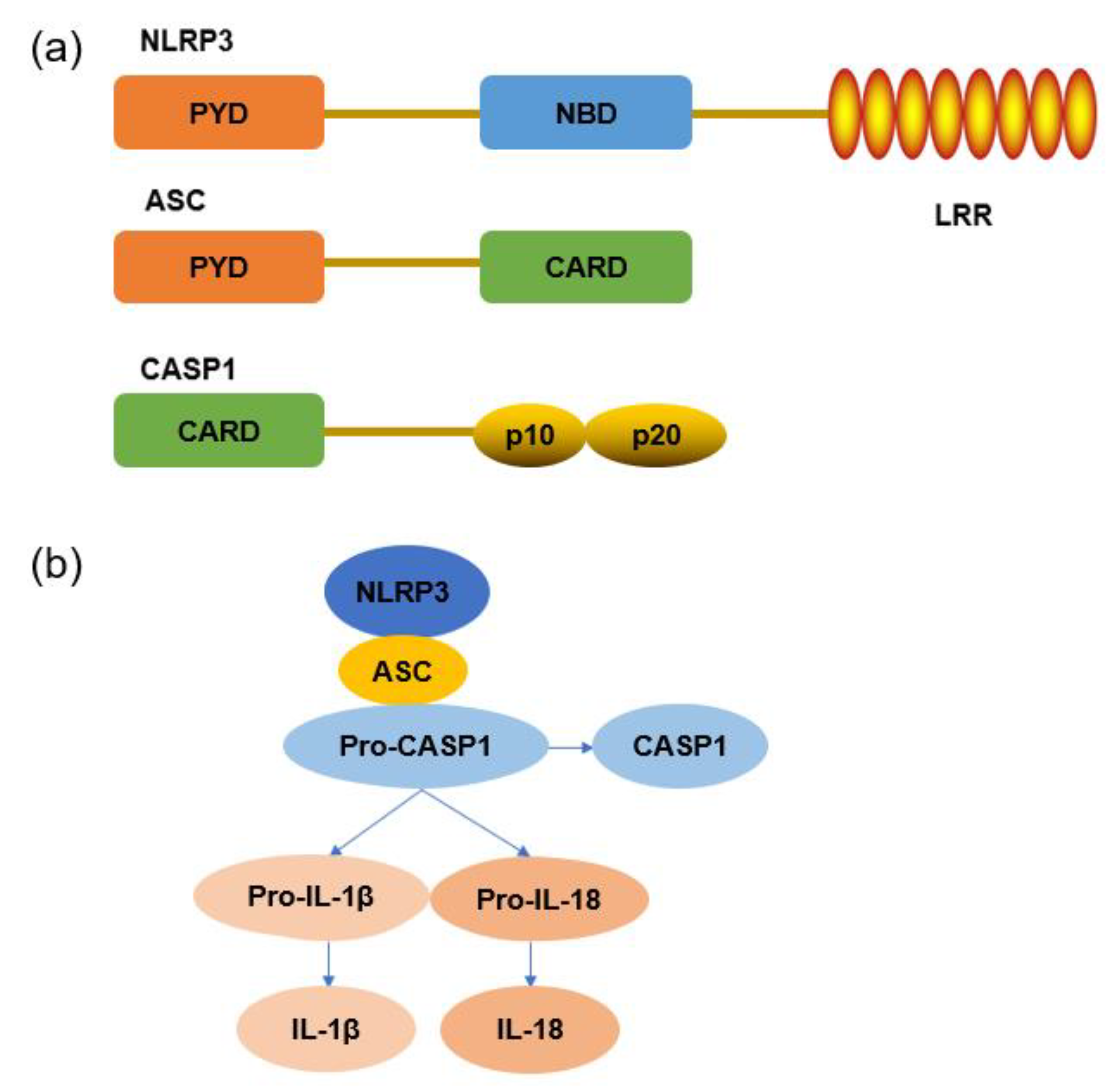
2.4. NLRP3 Inflammasome Signaling Pathway
3. Physical Stimulation in Bone Regeneration
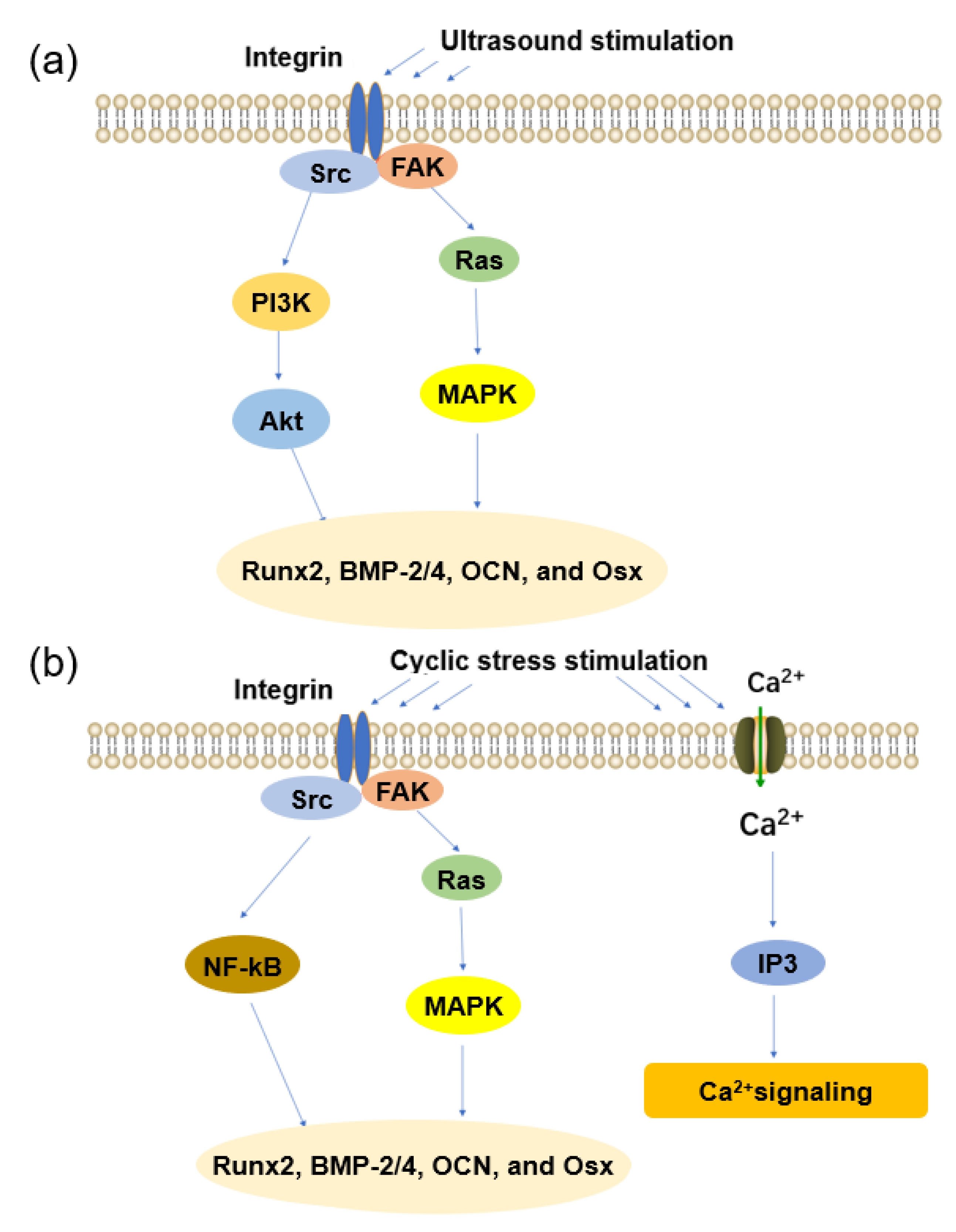
3.1. Mechanosensors in Mechanotransduction
3.2. Ultrasound
3.3. Cyclic Stress
3.4. Other Physical Stimuli
3.5. In Vivo and In Vitro Experiments
4. Inflammation under the Mechanical Environment in Bone Scaffolds
4.1. The Immune Environment of Bone Scaffolds
4.2. Regulatory Strategies for the Immune Environment of Bone Scaffolder
4.2.1. Surface Modification
4.2.2. Drug Delivery
4.2.3. Mechanical Stimulation
4.3. Effects of Physical Stimulation on Inflammatory Response
4.3.1. Ultrasound
4.3.2. Cyclic Stress
4.4. Physical Stimuli in Bone Tissue Engineering
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SA | sodium alginate | MEKK | MAPK kinase kinase |
| COL | collagen | MEK | MAPK kinase |
| GEL | gelatin | ERK | extracellular signal–regulated kinase |
| HA | hyaluronic acid | JNK | Jun N–terminal kinase |
| ECM | extracellular matrix | PYD | pyrin domain |
| PLA | polylactic acid | NBD | nucleoside–binding domain |
| PCL | polycaprolactone | LRRs | leucine–rich repeats |
| PC | polycarbonate | ATP | adenosine triphosphate |
| PEEK | polyetheretherketone | ROS | reactive oxygen species |
| PP | polypropylene | Fas | focal adhesions |
| hMSCs | human mesenchymal stem cells | FAK | focal adhesion kinase |
| FBGCs | foreign body giant cells | LIPUS | low–intensity pulsed ultrasound |
| IL | interleukin | EF | electric field |
| TNF | tumor necrosis factor | PMEF | pulsed electromagnetic field |
| IFN | Interferon | CSFs | colony–stimulating factors |
| TLRs | toll–like receptors | GSTR | silk––fibroin–gelatin porous scaffold |
| LPS | lipopolysaccharide | MEHA | methacrylated hyaluronic acid |
| IRAK | IL1RI–related protein kinase | HPMVECs | human pulmonary microvascular endothelial cells |
| IkB | Inhibitory kB | AMPK | adenosine monophosphate–activated protein kinase |
| IKK | IkB kinase | MS | mechanical stretching |
| MAPK | mitogen–activated protein kinase |
References
- Rahim, T.; Abdullah, A.M.; Akil, H.M.; Mohamad, D.; Rajion, Z.A. The improvement of mechanical and thermal properties of polyamide 12 3D printed parts by fused deposition modelling. Express Polym. Lett. 2017, 11, 963–982. [Google Scholar] [CrossRef]
- Biggemann, J.; Pezoldt, M.; Stumpf, M.; Greil, P.; Fey, T. Modular ceramic scaffolds for individual implants. Acta Biomater. 2018, 80, 390–400. [Google Scholar] [CrossRef]
- Larsen, M.; Pelzer, M.; Friedrich, P.F.; Wood, C.M.; Bishop, A.T. Living Bone Allotransplants Survive by Surgical Angiogenesis Alone: Development of a Novel Method of Composite Tissue Allotransplantation. J. Bone Jt. Surg.–Am. Vol. 2011, 93, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Buza, J.A., 3rd; Einhorn, T. Bone healing in 2016. Clin. Cases Miner. Bone Metab. 2016, 13, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Zhang, F.Y.; Zhai, W.J.; Cheng, S.J.; Li, J.H.; Wang, Y. Unraveling of Advances in 3D–Printed Polymer–Based Bone Scaffolds. Polymers 2022, 14, 566. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.W.; Jiang, F.L.; Zhang, H.B.; Yin, R.X.; Cen, L.; Zhang, W.J. Calcium Carbonate/Gelatin Methacrylate Microspheres for 3D Cell Culture in Bone Tissue Engineering. Tissue Eng. Part C–Methods 2020, 26, 418–432. [Google Scholar] [CrossRef]
- Wang, T.D.; Zheng, J.C.; Hu, T.Z.; Zhang, H.B.; Fu, K.; Yin, R.X.; Zhang, W.J. Three–Dimensional Printing of Calcium Carbonate/Hydroxyapatite Scaffolds at Low Temperature for Bone Tissue Engineering. 3D Print. Addit. Manuf. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Nagarajan, S.; Belaid, H.; Radhakrishnan, S.; Teyssier, C.; Balme, S.; Miele, P.; Cornu, D.; Subbaraya, N.K.; Cavaillès, V.; Bechelany, M. Sacrificial mold–assisted 3D printing of stable biocompatible gelatin scaffolds. Bioprinting 2021, 22, e00140. [Google Scholar] [CrossRef]
- Su, C.Y.; Chen, Y.T.; Tian, S.J.; Lu, C.X.; Lv, Q.Z. Natural Materials for 3D Printing and Their Applications. Gels 2022, 8, 748. [Google Scholar] [CrossRef]
- Raszewski, Z.; Chojnacka, K.; Kulbacka, J.; Mikulewicz, M. Mechanical Properties and Biocompatibility of 3D Printing Acrylic Material with Bioactive Components. J. Funct. Biomater. 2023, 14, 13. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2021, 31, 2006967. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Kim, G. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D–structural flexibility using a 3D–printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Wang, L.; Gramlich, W.; Gardner, D.; Han, Y.; Tajvidi, M. Spray–Dried Cellulose Nanofibril–Reinforced Polypropylene Composites for Extrusion–Based Additive Manufacturing: Nonisothermal Crystallization Kinetics and Thermal Expansion. J. Compos. Sci. 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.; Wang, Y.; Fu, H.; He, J. Hydroxyapatite/polyetheretherketone nanocomposites for selective laser sintering: Thermal and mechanical performances. E–Polymers 2020, 20, 542–549. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Miyamoto, I.; Xue, Y.; Andersson, M.; Mustafa, K.; Wennerberg, A.; Jimbo, R. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri–implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014, 10, 5193–5201. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg–Cu alloys with enhanced osteogenesis, angiogenesis, and long–lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef] [Green Version]
- Yusa, K.; Yamamoto, O.; Takano, H.; Fukuda, M.; Iino, M. Zinc–modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci. Rep. 2016, 6, 29462. [Google Scholar] [CrossRef] [Green Version]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Jorda–Vilaplana, A.; Fombuena, V.; Garcia–Garcia, D.; Samper, M.D.; Sanchez–Nacher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.I.; Rutkowski, S.; Frueh, J.; Gogolev, A.S.S.; Chistyakov, S.G.G.; Gnedenkov, S.V.V.; Sinebryukhov, S.L.L.; Frueh, A.; Egorkin, V.S.S.; Choynzonov, E.L.L.; et al. Surface Modification of Additively Fabricated Titanium–Based Implants by Means of Bioactive Micro–Arc Oxidation Coatings for Bone Replacement. J. Funct. Biomater. 2022, 13, 285. [Google Scholar] [CrossRef]
- Major, M.R.; Wong, V.W.; Nelson, E.R.; Longaker, M.T.; Gurtner, G.C. The Foreign Body Response: At the Interface of Surgery and Bioengineering. Plast. Reconstr. Surg. 2015, 135, 1489–1498. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, B.; Sun, J.; Li, J.; Liu, S.; Song, J. Inhibitory Effect of Low–Intensity Pulsed Ultrasound on the Expression of Lipopolysaccharide–Induced Inflammatory Factors in U937 Cells. J. Ultrasound Med. 2017, 36, 2419–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.F.; Kodadek, T. The Immune System and Neuroinflammation as Potential Sources of Blood–Based Biomarkers for Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. ACS Chem. Neurosci. 2016, 7, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakamura, T.; Fujihara, S.; Tanaka, E. Ultrasound Modulates the Inflammatory Response and Promotes Muscle Regeneration in Injured Muscles. Ann. Biomed. Eng. 2013, 41, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yan, C.; An, F.; Li, S. Research progress of the regulation mechanism of inflammatory factors and signaling pathways in osteoarthritis of the knee. J. Chin. Pharm. Sci 2019, 35, 1308–1311. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF–κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Laurencin, C.T.; Khan, Y. Regenerative Engineering. Sci. Transl. Med. 2012, 4, 160ed169. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.H.; Jiang, T.; Gagnon, K.A.; Nelson, C.; Laurencin, C.T. Small–molecule based musculoskeletal regenerative engineering. Trends Biotechnol. 2014, 32, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Kohavi, D.; Pollack, S.R.; Brighton, C. Short–term effect of guided bone regeneration and electrical stimulation on bone growth in a surgically modelled resorbed dog mandibular ridge. Biomater. Artif. Cells Immobil. Biotechnol. 1992, 20, 131–138. [Google Scholar] [CrossRef]
- Bonassar, L.J.; Grodzinsky, A.J.; Frank, E.H.; Davila, S.G.; Bhaktav, N.R.; Trippel, S.B. The effect of dynamic compression on the response of articular cartilage to insulin–like growth factor–I. J. Orth. Res. 2001, 19, 11–17. [Google Scholar] [CrossRef]
- Parvizi, J.; Parpura, V.; Greenleaf, J.F.; Bolander, M.E. Calcium signaling is required for ultrasound–stimulated aggrecan synthesis by rat chondrocytes. J. Orth. Res. 2002, 20, 51–57. [Google Scholar] [CrossRef]
- Wang, M. –N.; Liu, L.; Zhao, L.–P.; Yuan, F.; Fu, Y.–B.; Xu, X.–B.; Li, B. Research of inflammatory factors and signaling pathways in knee osteoarthritis. Zhongguo Gu Shang 2020, 33, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti–inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF–kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular mechanisms underlying chronic inflammation–associated cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Mi, Y.Q.; Gang, J.H. Experimental progress of signal transduction pathways in knee osteoarthritis. CJTER 2016, 20, 267–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Pizzute, T.; Pei, M. A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration. Cell Tissue Res. 2014, 358, 633–649. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL–beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Cerretti, D.P.; Kozlosky, C.J.; Mosley, B.; Nelson, N.; Van Ness, K.; Greenstreet, T.A.; March, C.J.; Kronheim, S.R.; Druck, T.; Cannizzaro, L.A.; et al. Molecular cloning of the interleukin–1 beta converting enzyme. Science 1992, 256, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Bull, H.G.; Calaycay, J.R.; Chapman, K.T.; Howard, A.D.; Kostura, M.J.; Miller, D.K.; Molineaux, S.M.; Weidner, J.R.; Aunins, J.; et al. A novel heterodimeric cysteine protease is required for interleukin–1 beta processing in monocytes. Nature 1992, 356, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Moltke, J.; Ayres, J.S.; Kofoed, E.M.; Chavarria–Smith, J.; Vance, R.E. Recognition of Bacteria by Inflammasomes. Annu. Rev. Immunol. 2013, 31, 73–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.J.; McDermott, M.F.; Kanneganti, T.-D. Inflammasomes and autoimmunity. Trends Mol. Med. 2011, 17, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes–Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF–kappa B Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, J.K.; O’Neill, L.A.J. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 424–443. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL–1β and IL–18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, W.J.; Xu, Q.; Sun, Y. Advances in mechanisms for NLRP3 inflammasomes regulation. Yao Xue Xue Bao 2016, 51, 1505–1512. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Ding, Y.; Zhou, W.; Tao, L.; Lu, P.; Wang, Y.; Hu, R. Nuclear Factor E2–Related Factor–2 Negatively Regulates NLRP3 Inflammasome Activity by Inhibiting Reactive Oxygen Species–Induced NLRP3 Priming. Antioxid. Redox Signal. 2017, 26, 28–43. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, C.; Camci–Unal, G. Low Intensity Pulsed Ultrasound for Bone Tissue Engineering. Micromachines 2021, 12, 1488. [Google Scholar] [CrossRef]
- Huang, X.; Das, R.; Patel, A.; Nguyen, T.D. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef]
- Chao, E.Y.S.; Inoue, N. Biophysical stimulation of bone fracture repair, regeneration and remodelling. Eur. Cells Mater. 2003, 6, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Darwood, A.; Masouros, S.; Higgins, C.; Ramasamy, A. Mechanotransduction in osteogenesis. Bone Jt. Res. 2020, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yellowley, C.E.; Li, Z.Y.; Zhou, Z.Y.; Jacobs, C.R.; Donahue, H.J. Functional gap junctions between osteocytic and osteoblastic cells. J. Bone Miner. Res. 2000, 15, 209–217. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.X.; Li, J.L. The role of mechanosensor Piezo1 in bone homeostasis and mechanobiology. Dev. Biol. 2023, 493, 80–88. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. eLife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Claes, L.; Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef]
- Saito, M.; Fujii, K.; Tanaka, T.; Soshi, S. Effect of low– and high–intensity pulsed ultrasound on collagen post–translational modifications in MC3T3–E1 osteoblasts. Calcif. Tissue Int. 2004, 75, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low–Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.; Wu, S.; Dong, Y.; Chen, X.; Wang, S.; Li, X.; Zou, C. Review on experimental study and clinical application of low–intensity pulsed ultrasound in inflammation. Quant. Imaging Med. Surg. 2021, 11, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Sun, S.; Xu, Z.; Ta, D. Finite element simulation of local sound field distribution when LIPUS irradiates bone cells. Tech. Acoust. 2017, 36, 549–555. [Google Scholar] [CrossRef]
- Angle, S.R.; Sena, K.; Sumner, D.R.; Virdi, A.S. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low–intensity pulsed ultrasound. Ultrasonics 2011, 51, 281–288. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.X.; Schmelz, A.; Seufferlein, T.; Li, Y.P.; Zhao, J.S.; Bachem, M.G. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J. Biol. Chem. 2004, 279, 54463–54469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C. –H.; Yang, R.–S.; Huang, T.–H.; Lu, D.–Y.; Chuang, W.–J.; Huang, T.–F.; Fu, W.–M. Ultrasound stimulates cyclooxygenase–2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3–kinase, and akt pathway in osteoblasts. Mol. Pharmacol. 2006, 69, 2047–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, R.; Ryo, A.; Komitsu, N.; Mikuni–Takagaki, Y.; Fukui, A.; Takagi, Y.; Shiraishi, T.; Morishita, S.; Yamazaki, Y.; Kumagai, K.; et al. Low–intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three–dimensional cultures: A basic science study. Arthrit. Res. Ther. 2008, 10, R77. [Google Scholar] [CrossRef] [Green Version]
- Whitney, N.P.; Lamb, A.C.; Louw, T.M.; Subramanian, A. Integrin–mediated mechanotransduction pathway of low–intensity continuous ultrasound in human chondrocytes. Ultrasound Med. Biol. 2012, 38, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Carina, V.; Costa, V.; Raimondi, L.; Pagani, S.; Sartori, M.; Figallo, E.; Setti, S.; Alessandro, R.; Fini, M.; Giavaresi, G. Effect of low–intensity pulsed ultrasound on osteogenic human mesenchymal stem cells commitment in a new bone scaffold. J. Appl. Biomater. Funct. Mater. 2017, 15, E215–E222. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Marvel, S.W.; Bernacki, S.H.; Banes, A.J.; van Aalst, J.; Loboa, E.G. Osteogenic Effects of Rest Inserted and Continuous Cyclic Tensile Strain on hASC Lines with Disparate Osteodifferentiation Capabilities. Ann. Biomed. Eng. 2009, 37, 955–965. [Google Scholar] [CrossRef]
- Kearney, E.M.; Farrell, E.; Prendergast, P.J.; Campbell, V.A. Tensile Strain as a Regulator of Mesenchymal Stem Cell Osteogenesis. Ann. Biomed. Eng. 2010, 38, 1767–1779. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: A family of cell surface receptors. Cell 1987, 48, 549–554. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, W.R.; Guilluy, C.; Xie, Z.; Sen, B.; Brobst, K.E.; Yen, S.S.; Uzer, G.; Styner, M.; Case, N.; Burridge, K.; et al. Mechanically Activated Fyn Utilizes mTORC2 to Regulate RhoA and Adipogenesis in Mesenchymal Stem Cells. Stem Cells 2013, 31, 2528–2537. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Watanabe, S.; Ju, Y.; Xu, B. Determination of optimal cyclic uniaxial stretches for stem cell–to–tenocyte differentiation under a wide range of mechanical stretch conditions by evaluating gene expression and protein synthesis levels. Acta Bioeng. Biomech. 2013, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Okabe, K.; Kajiya, H.; Habu, T. Osmotic membrane stretch increases cytosolic Ca2+ and inhibits bone resorption activity in rat osteoclasts. Jap. J. Physiol. 2000, 50, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Pingguan–Murphy, B.; El–Azzeh, M.; Bader, D.L.; Knight, M.M. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate– and frequency–dependent manner. J. Cell. Physiol. 2006, 209, 389–397. [Google Scholar] [CrossRef]
- Roberts, S.R.; Knight, M.M.; Lee, D.A.; Bader, D.L. Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs. J. Appl. Physiol. 2001, 90, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaacson, B.M.; Bloebaum, R.D. Bone bioelectricity: What have we learned in the past 160 years? J. Biomed. Mater. Res. Part A 2010, 95, 1270–1279. [Google Scholar] [CrossRef]
- Foulds, I.S.; Barker, A.T. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol 1983, 109, 515–522. [Google Scholar] [CrossRef]
- Song, B.; Zhao, M.; Forrester, J.V.; McCaig, C.D. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 13577–13582. [Google Scholar] [CrossRef] [Green Version]
- Ryaby, J.T. Clinical effects of electromagnetic and electric fields on fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S205–S215. [Google Scholar] [CrossRef]
- Manjhi, J.; Mathur, R.; Behari, J. Effect of Low Level Capacitive–Coupled Pulsed Electric Field Stimulation on Mineral Profile of Weight–Bearing Bones in Ovariectomized Rats. J. Biomed. Mater. Res. Part B 2010, 92, 189–195. [Google Scholar] [CrossRef]
- Itoh, S.; Nakamura, S.; Nakamura, M.; Shinomiya, K.; Yamashita, K. Enhanced bone ingrowth into hydroxyapatite with interconnected pores by electrical polarization. Biomaterials 2006, 27, 5572–5579. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagai, A.; Tanaka, Y.; Sekijima, Y.; Yamashita, K. Polarized hydroxyapatite promotes spread and motility of osteoblastic cells. J. Biomed. Mater. Res. Part A 2010, 92, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Nerucci, F.; Collodel, G.; Markoll, R.; Marcolongo, R. Biochemical and morphological study of human articular chondrocytes cultivated in the presence of pulsed signal therapy. Ann. Rheum. Dis. 2002, 61, 1032–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerick, K.E.; James, A.W.; Huang, Z.; Prinz, F.B.; Longaker, M.T. Pulsed Direct Current Electric Fields Enhance Osteogenesis in Adipose–Derived Stromal Cells. Tissue Eng. Part A 2010, 16, 917–931. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Wang, W.; Seldes, R.M.; Tahernia, A.D.; Fan, H.; Brighton, C.T. Electrical stimulation induces the level of TGF–beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem. Biophys. Res. Commun. 1997, 237, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Bodamyali, T.; Bhatt, B.; Hughes, F.J.; Winrow, V.R.; Kanczler, J.M.; Simon, B.; Abbott, J.; Blake, D.R.; Stevens, C.R. Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem. Biophys. Res. Commun. 1998, 250, 458–461. [Google Scholar] [CrossRef]
- Tong, J.; Sun, L.; Zhu, B.; Fan, Y.; Ma, X.; Yu, L.; Zhang, J. Pulsed electromagnetic fields promote the proliferation and differentiation of osteoblasts by reinforcing intracellular calcium transients. Bioelectromagnetics 2017, 38, 541–549. [Google Scholar] [CrossRef]
- Suzuki, A.; Takayama, T.; Suzuki, N.; Sato, M.; Fukuda, T.; Ito, K. Daily low–intensity pulsed ultrasound–mediated osteogenic differentiation in rat osteoblasts. Acta Biochim. Biophys. Sin. 2009, 41, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant’Anna, E.F.; Leven, R.M.; Virdi, A.S.; Sumner, D.R. Effect of low intensity pulsed ultrasound and BMP–2 on rat bone marrow stromal cell gene expression. J. Orth. Res. 2005, 23, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Miwa, M.; Sakai, Y.; Niikura, T.; Kurosaka, M.; Komori, T. Osteogenic activity of human fracture haematoma–derived progenitor cells is stimulated by low–intensity pulsed ultrasound in vitro. J. Bone Jt. Surg.–Br. Vol. 2009, 91, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Gleizal, A.; Li, S.; Pialat, J.-B.; Beziat, J.-L. Transcriptional expression of calvarial bone after treatment with low–intensity ultrasound: An in vitro study. Ultrasound Med. Biol. 2006, 32, 1569–1574. [Google Scholar] [CrossRef]
- Pomini, K.T.; Andreo, J.C.; Rodrigues Ade, C.; de O Gonçalves, J.B.; Daré, L.R.; German, I.J.; Rosa, G.M., Jr.; Buchaim, R.L. Effect of low–intensity pulsed ultrasound on bone regeneration: Biochemical and radiologic analyses. J. Ultrasound Med. 2014, 33, 713–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Mao, J.J. Chondrocyte proliferation of the cranial base cartilage upon in vivo mechanical stresses. J. Dent. Res. 2002, 81, 701–705. [Google Scholar] [CrossRef]
- van der Meulen, M.C.H.; Yang, X.; Morgan, T.G.; Bostrom, M.P.G. The Effects of Loading on Cancellous Bone in the Rabbit. Clin. Orthop. Relat. Res. 2009, 467, 2000–2006. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial–Mediated Fibrosis. Adv. Healthc. Mater. 2019, 8, 1801451. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Colony–stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Reeve, J.L.; Liu, Y.; Teitelbaum, S.L.; Ross, F.P. DAP12 couples c–Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol. Cell 2008, 31, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C–Mater. Biol. Appl. 2020, 108, 110411. [Google Scholar] [CrossRef]
- Humbert, P.; Brennan, M.A.; Davison, N.; Rosset, P.; Trichet, V.; Blanchard, F.; Layrolle, P. Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration. Front. Immunol. 2019, 10, 663. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Liu, H. Macrophage Polarization in Response to Biomaterials for Vascularization. Ann. Biomed. Eng. 2021, 49, 1992–2005. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Zhou, P.; Liu, X.; Zhao, H.; Zhou, X.; Gu, Q.; Li, B.; Zhu, X.; Shi, Q. Substrate stiffness modulates bone marrow–derived macrophage polarization through NF–kappa B signaling pathway. Bioact. Mater. 2020, 5, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares–Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jansen, J.A.; Yang, F.; van den Beucken, J. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C—Mater. Biol. Appl. 2019, 95, 143–151. [Google Scholar] [CrossRef]
- Park, J. –W.; Han, S.–H.; Hanawa, T. Effects of Surface Nanotopography and Calcium Chemistry of Titanium Bone Implants on Early Blood Platelet and Macrophage Cell Function. Biomed. Res. Int. 2018, 2018, 1362958. [Google Scholar] [CrossRef] [PubMed]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Hamlet, S.; Alfarsi, M.; George, R.; Ivanovski, S. The effect of hydrophilic titanium surface modification on macrophage inflammatory cytokine gene expression. Clin. Oral Implant. Res. 2012, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, Y.; Liu, W.; Tong, K.L.; Suen, C.W.; Huang, S.; Hou, H.; She, G.; Zhang, H.; Zheng, X.; et al. Ginsenoside Rb1/TGF–β1 loaded biodegradable silk fibroin–gelatin porous scaffolds for inflammation inhibition and cartilage regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110757. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, L.; Li, L.; Huang, C.; Shi, K.; Meng, X.; Wang, P.; Wu, M.; Li, L.; Cao, H.; et al. 3D–bioprinted BMSC–laden biomimetic multiphasic scaffolds for efficient repair of osteochondral defects in an osteoarthritic rat model. Biomaterials 2021, 279, 121216. [Google Scholar] [CrossRef]
- Shui, H.; Shi, Q.; Pugno, N.M.; Chen, Q.; Li, Z. Effect of mechanical stimulation on the degradation of poly(lactic acid) scaffolds with different designed structures. J. Mech. Behav. Biomed. Mater. 2019, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Guo, P.; Li, X.; He, Z.; Li, Z.; Stoddart, M.J.; Grad, S.; Tian, W.; Chen, D.; et al. Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration. Bioact. Mater. 2021, 6, 3097–3108. [Google Scholar] [CrossRef]
- Hu, M.; Wazir, J.; Ullah, R.; Wang, W.; Cui, X.; Tang, M.; Zhou, X. Phytotherapy and physical therapy in the management of chronic prostatitis–chronic pelvic pain syndrome. Int. Urol. Nephrol. 2019, 51, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Holfeld, J.; Tepekoeylue, C.; Kozaryn, R.; Urbschat, A.; Zacharowski, K.; Grimm, M.; Paulus, P. Shockwave Therapy Differentially Stimulates Endothelial Cells: Implications on the Control of Inflammation via Toll–Like Receptor 3. Inflammation 2014, 37, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, P.; Barbanti, P.; Grazzi, L.; Pierangeli, G.; Rainero, I.; Geppetti, P.; Ambrosini, A.; Sarchielli, P.; Tassorelli, C.; Liebler, E.; et al. Consistent effects of non–invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: Additional findings from the randomized, sham–controlled, double–blind PRESTO trial. J. Headache Pain 2018, 19, 101. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.L.; Chen, H.Y.; Yang, C.C. Early Intervention with Therapeutic Low–Intensity Pulsed Ultrasound in Halting the Progression of Post–traumatic Osteoarthritis in a Rat Model. Ultrasound Med. Biol. 2018, 44, 2637–2645. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, S. –M.; Lian, H.; Lin, Y.–Z.; Zhuang, R.; Thapa, S.; Chen, Q.–Z.; Chen, Y.–F.; Lin, J.–F. Low–intensity pulsed ultrasound attenuates cardiac inflammation of CVB3–induced viral myocarditis via regulation of caveolin–1 and MAPK pathways. J. Cell. Mol. Med. 2019, 23, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, W.; Ma, L.; Zhang, L.; Yang, D. Prevention of Proximal Junctional Kyphosis: Are Polyaxial Pedicle Screws Superior to Monoaxial Pedicle Screws at the Upper Instrumented Vertebrae? World Neurosurg. 2017, 101, 405–415. [Google Scholar] [CrossRef]
- Sahu, N.; Viljoen, H.J.; Subramanian, A. Continuous low–intensity ultrasound attenuates IL–6 and TNF alpha–induced catabolic effects and repairs chondral fissures in bovine osteochondral explants. BMC Musculoskel. Disord. 2019, 20, 193. [Google Scholar] [CrossRef] [Green Version]
- Chen, S. –F.; Su, W.–S.; Wu, C.–H.; Lan, T.–H.; Yang, F.–Y. Transcranial Ultrasound Stimulation Improves Long–Term Functional Outcomes and Protects Against Brain Damage in Traumatic Brain Injury. Mol. Neurobiol. 2018, 55, 7079–7089. [Google Scholar] [CrossRef] [PubMed]
- Nakao, J.; Fujii, Y.; Kusuyama, J.; Bandow, K.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. Low–intensity pulsed ultrasound (LIPUS) inhibits LPS–induced inflammatory responses of osteoblasts through TLR4–MyD88 dissociation. Bone 2014, 58, 17–25. [Google Scholar] [CrossRef]
- Ueno, M.; Maeshige, N.; Hirayama, Y.; Yamaguchi, A.; Ma, X.; Uemura, M.; Kondo, H.; Fujino, H. Pulsed ultrasound prevents lipopolysaccharide–induced muscle atrophy through inhibiting p38 MAPK phosphorylation in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2021, 570, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, B.; Xing, R.; Zhang, L.; Mao, J.; Wang, P.; Zhang, D. Low–intensity pulsed ultrasound inhibits IL–1β–induced inflammation of fibroblast–like synoviosytes via NF–κB pathway. Appl. Acoust. 2020, 167, 107384. [Google Scholar] [CrossRef]
- Liao, Q.; Li, B.J.; Li, Y.; Xiao, Y.; Zeng, H.; Liu, J.M.; Yuan, L.X.; Liu, G. Low–intensity pulsed ultrasound promotes osteoarthritic cartilage regeneration by BMSC–derived exosomes via modulating the NF–κB signaling pathway. Int. Immunopharmacol. 2021, 97, 107824. [Google Scholar] [CrossRef]
- Xia, C.; Shao, L.; Ma, Y.; Wang, X.; Zhang, Y.; Shi, C.; Li, J.; Zhang, W.; Li, H.; Wang, J. Protective action of ultrasound–guided intraparenchymal transplantation of BMSCs in adriamycin nephropathy rats through the RIPK3/MLKL and NLRP3 pathways. Acta Histochem. 2021, 123, 151773. [Google Scholar] [CrossRef]
- Maruyama, K.; Nemoto, E.; Yamada, S. Mechanical regulation of macrophage function—Cyclic tensile force inhibits NLRP3 inflammasome–dependent IL–1β secretion in murine macrophages. Inflamm. Regen. 2019, 39, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Hu, J.; Zheng, Q.; Feng, X.; Zhan, F.; Wang, X.; Xu, G.; Hua, F. Piezo1 Channels as Force Sensors in Mechanical Force–Related Chronic Inflammation. Front. Immunol. 2022, 13, 816149. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Negrini, D.; Rocco, P.R.M. Mechanisms of ventilator–induced lung injury in healthy lungs. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Ito, S.; Morioka, M.; Iwata, S.; Numaguchi, Y.; Ishii, M.; Kondo, M.; Kume, H.; Naruse, K.; Sokabe, M.; et al. Mechanical stretch enhances IL–8 production in pulmonary microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2009, 389, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Oudin, S.; Pugin, M. Role of MAP kinase activation in interleukin–8 production by human BEAS–2B bronchial epithelial cells submitted to cyclic stretch. Am. J. Respir. Cell Mol. Biol. 2002, 27, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Sebag, S.C.; Bastarache, J.A.; Ware, L.B. Mechanical Stretch Inhibits Lipopolysaccharide–induced Keratinocyte–derived Chemokine and Tissue Factor Expression While Increasing Procoagulant Activity in Murine Lung Epithelial Cells. J. Biol. Chem. 2013, 288, 7875–7884. [Google Scholar] [CrossRef] [Green Version]
- Charles, P.E.; Tissières, P.; Barbar, S.D.; Croisier, D.; Dufour, J.; Dunn–Siegrist, I.; Chavanet, P.; Pugin, J. Mild–stretch mechanical ventilation upregulates toll–like receptor 2 and sensitizes the lung to bacterial lipopeptide. Crit. Care 2011, 15, R181. [Google Scholar] [CrossRef] [Green Version]

| Author | Ultrasound Parameters | Conclusion |
|---|---|---|
| Zheng et al. [121] | Frequency, 1 MHz; duty cycle, 20%; pulse repetition frequency, 100 Hz; intensity, 0.5 W/cm2; 20 min/d | LIPUS induces caveolin–1 activation and inhibits the phosphorylation of p38 and ERK, thereby inhibiting proinflammatory–factor expression. |
| Zhang et al. [122] | Frequency, 1.5 MHz, pulse repetition frequency 1 kHz, duty cycle 20%; intensity, 10, 30, 60, and 90 mW/cm2 | LIPUS reduces the expression of IL–1β, IL–6 and IL–8 by inhibiting TLR4–MyD88 and NF–kB pathways. |
| Sahu et al. [123] | Frequency, 5 MHz; continuous ultrasound; intensity, 0.528 W/cm2 | cLIUS enhances cartilage phenotype and cell migration by inhibiting TNF–α–induced NF–kB pathway. |
| Chen et al. [124] | Frequency, 1 MHz; intensity, 0.528 W/cm2 | LIPUS inhibits the expression of related proteins in the TLR4 and NF–kB pathways. |
| Nakao et al. [125] | Frequency, 1.5 MHz; pulse repetition frequency, 1 kHz; intensity, 30 mW/cm2 | TLR4/MyD88 complex inhibits p38 and ERK1/2 phosphorylation in downstream pathways in addition to NF–kB pathway activation. |
| Mizuki et al. [126] | Frequency, 3 MHz; 20% duty cycle; 0.5 W/cm2 intensity | LIPUS inhibits LPS–induced p38 MAPK phosphorylation and muscle atrophy. |
| Zhang et al. [127] | Frequency, 1 MHz; pulse repetition frequency, 250 Hz; sound pressure, 0.1, 0.2, 0.3, and 0.4 MPa; time 1, 2, 3, 4, and 5 min/d | LIPUS inhibits NF–kB pathway activation. |
| Xia et al. [129] | Frequency, 8–18 MHz | The expression of NLRP3 protein was significantly inhibited, and the secretion of pro–inflammatory factors such as IL–1β and IL–18 was reduced. |
| Liao et al. [128] | Frequency, 1.5 MHz; duty cycle, 20%; intensity, 30 mW/cm2; 20 min/d | LIPUS promotes ECM synthesis, suppresses inflammatory responses, and inhibits NF–kB pathway activation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yuan, B.; Yin, R.; Zhang, H. Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration. J. Funct. Biomater. 2023, 14, 169. https://doi.org/10.3390/jfb14030169
Wang J, Yuan B, Yin R, Zhang H. Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration. Journal of Functional Biomaterials. 2023; 14(3):169. https://doi.org/10.3390/jfb14030169
Chicago/Turabian StyleWang, Junjie, Bo Yuan, Ruixue Yin, and Hongbo Zhang. 2023. "Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration" Journal of Functional Biomaterials 14, no. 3: 169. https://doi.org/10.3390/jfb14030169
APA StyleWang, J., Yuan, B., Yin, R., & Zhang, H. (2023). Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration. Journal of Functional Biomaterials, 14(3), 169. https://doi.org/10.3390/jfb14030169










