Synthesis and Characterization of Nanostructured Oxide Layers on Ti-Nb-Zr-Ta and Ti-Nb-Zr-Fe Biomedical Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
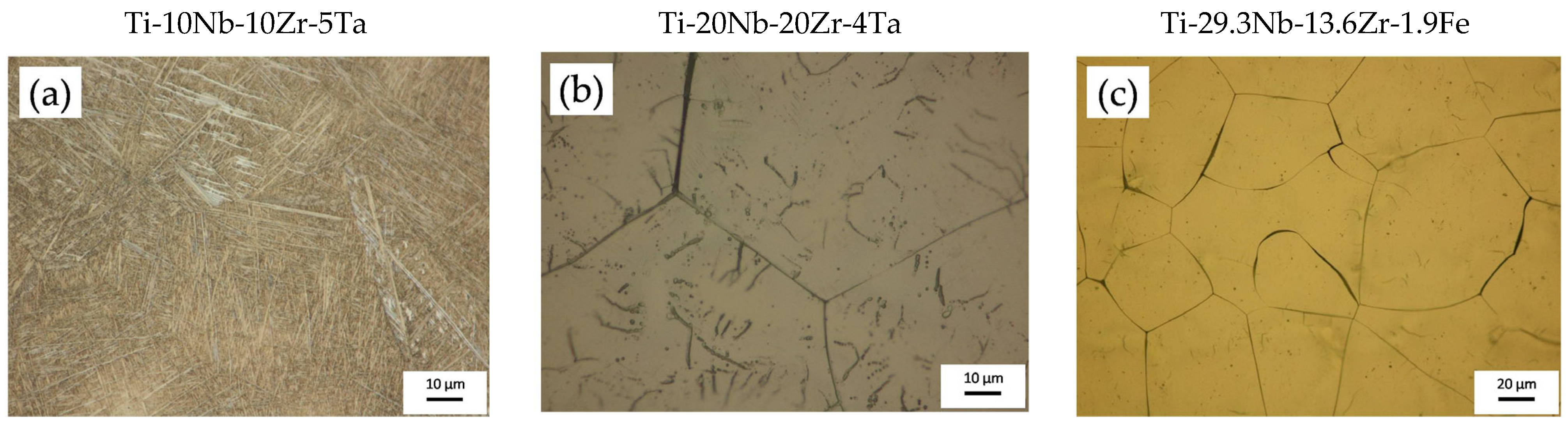
- The Ti-10Nb-10Zr-5Ta alloy contained 55% α-Ti phase and 45% β-Ti phase;
- The Ti-20Nb-20Zr-4Ta alloy contained 15% α-Ti phase and 85% β-Ti phase;
- The Ti-29.3Nb-13.6Zr-1.9Fe alloy contained 100% β-Ti phase.
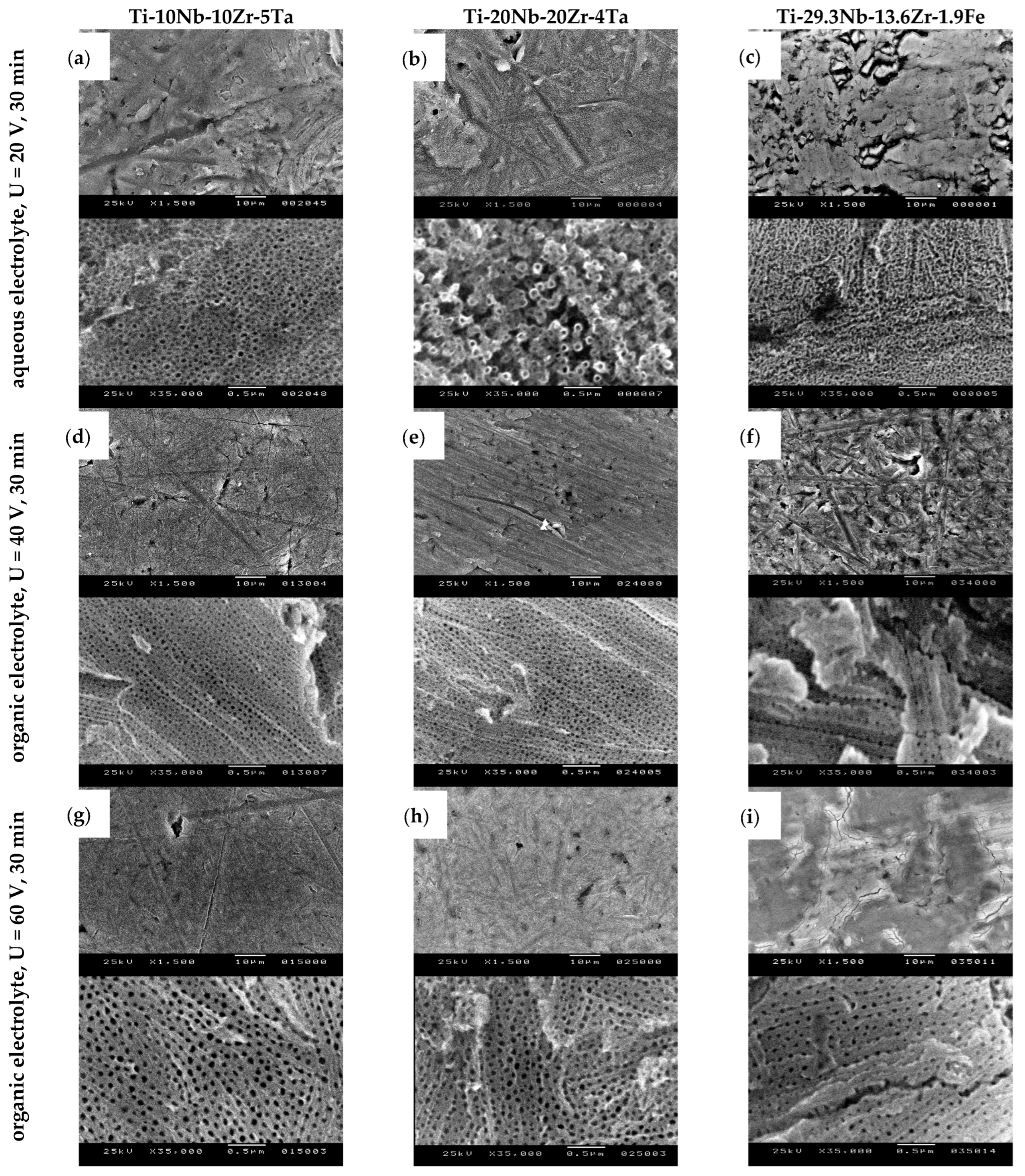
3.1. Surface Morphology and Topography Evaluation after Electrochemical Anodization
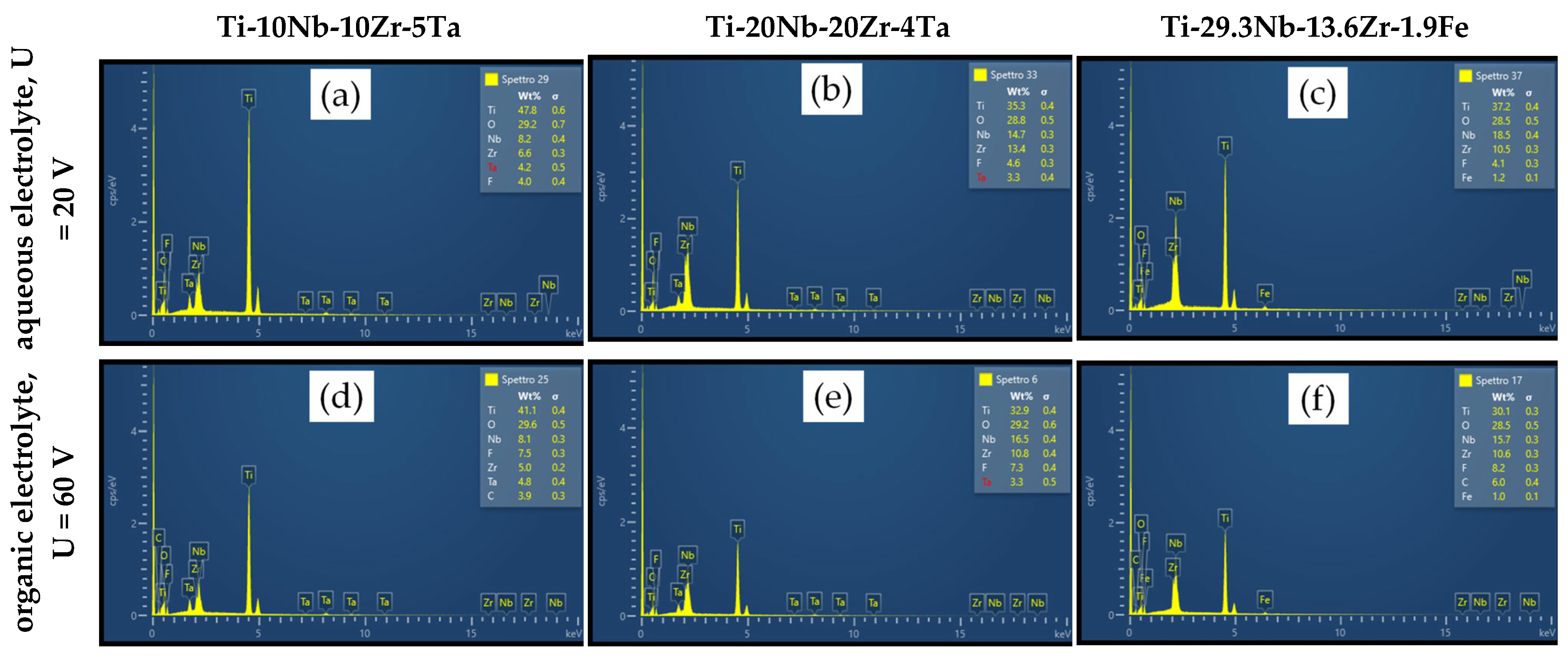
3.2. EDS Analysis
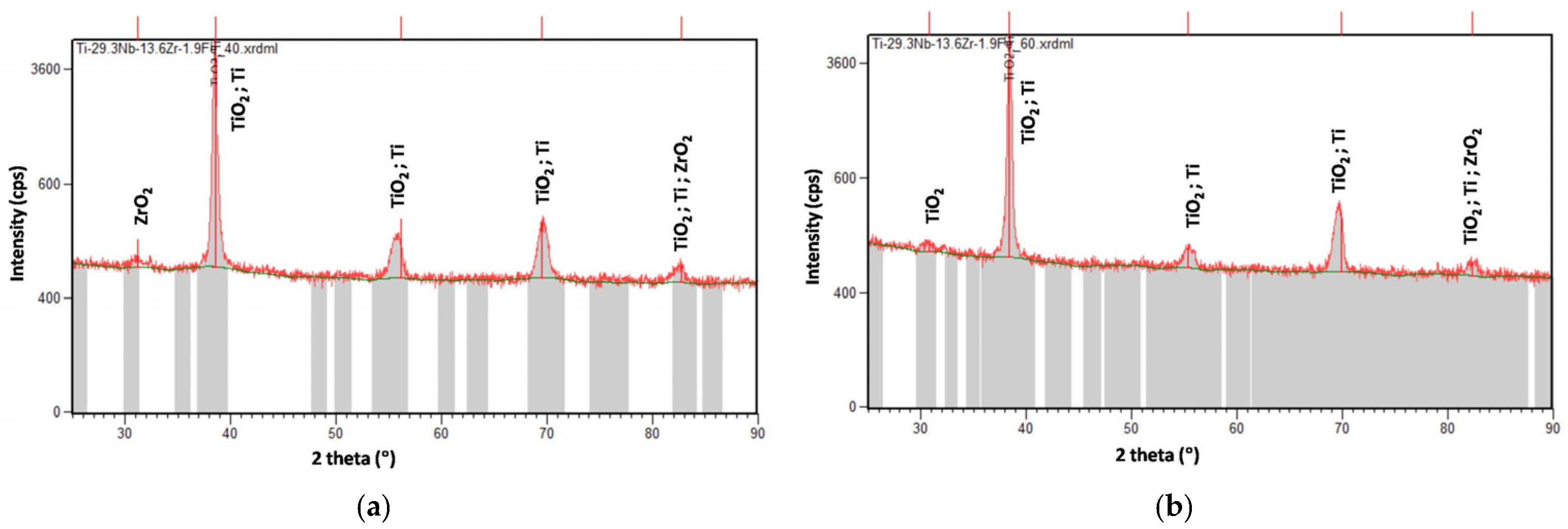
3.3. XRD Analysis
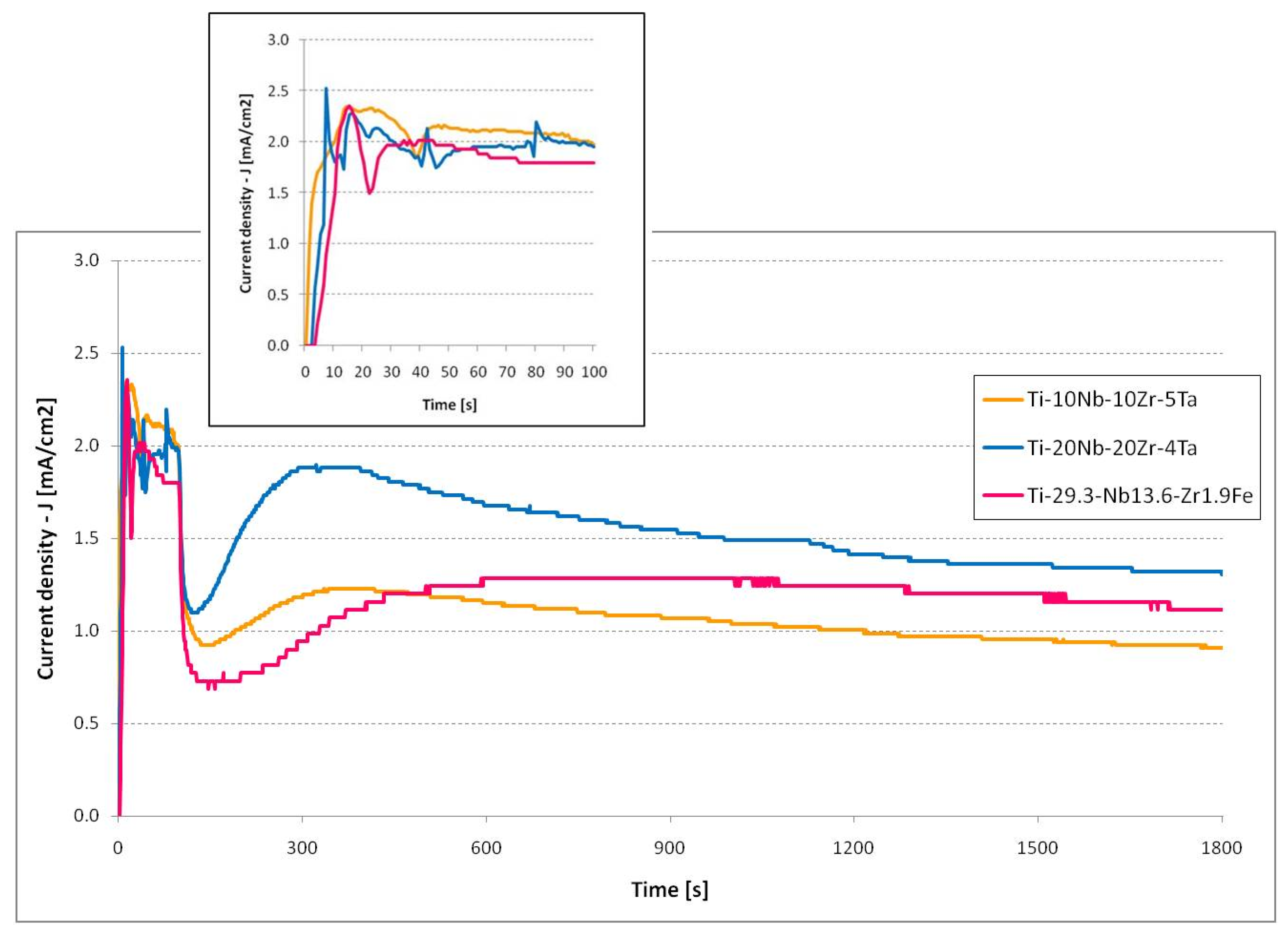
3.4. Current Evolution Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, J.D.; Briggs, R.D.; Boyer, R.R.; Tamirisakandala, S.; Russo, P.; Shchetnikov, N.; Fanning, J.C. State of the Art in Beta Titanium Alloys for Airframe Applications. JOM 2015, 67, 1281–1303. [Google Scholar] [CrossRef] [Green Version]
- Sarimov, R.M.; Glinushkin, A.P.; Sevostyanov, M.A.; Konushkin, S.V.; Serov, D.A.; Astashev, M.E.; Lednev, V.N.; Yanykin, D.V.; Sibirev, A.V.; Smirnov, A.A.; et al. Ti-20Nb-10Ta-5Zr Is Biosafe Alloy for Building of Ecofriendly Greenhouse Framework of New Generation. Metals 2022, 12, 2007. [Google Scholar] [CrossRef]
- Vijayaram, D.T.; Natarajan, M.; Ramarao, M.; Ananthapadmanaban, D. Titanium and Titanium Alloys: Advanced Materials for Engineering Industries. Compliance Eng. J. 2021, 12, 116–121. [Google Scholar]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Dostayeva, A.; Toleuova, A. Physical and chemical interaction of aluminum with titanium and nickel for further use in parts of agricultural machinery. Engineering for Rural Development. Proc. Int. Sci. Conf. 2021, 105, 811–816. [Google Scholar]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.G.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [Green Version]
- Barbint, A.C.; Earar, K.; Crimu, C.I.; Dragan, L.A.; Munteanu, C. In vitro evaluation of the cytotoxicity of some new titanium alloys. Key Eng. Mater. 2014, 587, 303–308. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Ma, J.U.N.; Gao, Y.; Yang, L.E.I. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef]
- You, R.; Liu, Y.-Q.; Hao, Y.-L.; Han, D.-D.; Zhang, Y.-L.; You, Z. Laser Fabrication of Graphene-Based Flexible Electronics. Adv. Mater. 2020, 32, 1901981. [Google Scholar] [CrossRef]
- Peter, I. Investigations into Ti-Based Metallic Alloys for Biomedical Purposes. Metals 2021, 11, 1626. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 539–1560. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglič, V.; Milosev, I.; Schmuki, P.; Iglic, A.; Mozetic, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhou, Z.; Zhou, S.; Hu, Z. Micro-/Nano-Scales Direct Cell Behavior on Biomaterial Surfaces. Molecules 2019, 24, 75. [Google Scholar] [CrossRef] [Green Version]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef]
- Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved osteoblast and chondrocyte adhesion and viability by surface-modified Ti6Al4V alloy with anodized TiO2 nanotubes using a super-oxidative solution. Materials 2015, 8, 867–888. [Google Scholar] [CrossRef] [Green Version]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.S.; Yoriya, S.; Grissom, L.; Grimes, C.A.; Popat, K.C. Hemocompatibility of titania nanotube arrays. J. Med. Res. A 2010, 95, 350–360. [Google Scholar] [CrossRef]
- Mazare, A. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Shen, X.; Al-Baadani, M.A.; He, H.; Cai, L.; Wu, Z.; Yao, L.; Wu, X.; Wu, S.; Chen, M.; Zhang, H.; et al. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3043–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karazisis, D.; Ballo, A.M.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1381. [Google Scholar]
- Ding, X.; Zhou, L.; Wang, J.; Zhao, Q.; Lin, X.; Gao, Y.; Li, S.; Wu, J.; Rong, M.; Guo, Z.; et al. The effects of hierarchical micro/nanosurfaces decorated with TiO2 nanotubes on the bioactivity of titanium implants in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6955–6973. [Google Scholar]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone-implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef] [Green Version]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100-B, 9–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental Implant Nano-Engineering: Advances, Limitations and Future Directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.J.; Li, T.; Sudheesh Kumar, P.T.; Ivanovski, S. Titania nanopores with dual micro-/nano-topography for selective cellular bioactivity. Mater. Sci. Eng. C 2017, 91, 624–630. [Google Scholar] [CrossRef]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef] [Green Version]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Cui, H.; Ai, F.; Jian, L.; Kong, J.; Zhu, X. Terminated nanotubes: Evidence against the dissolution equilibrium theory. Electrochem. Commun. 2018, 86, 80–84. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Liu, L.; Ma, J.; Ni, Y.; Wang, H.; Song, Y. The effect of atmospheric pressure on the growth rate of TiO2 nanotubes: Evidence against the field-assisted dissolution theory. Electrochem. Commun. 2021, 132, 107146. [Google Scholar] [CrossRef]
- Wang, A.; Li, C.; Jiang, L.; Chen, B.; Zhang, S.; Xu, X.; Zhu, X. Preparation and formation mechanism of fast-growing ZrO2 nanotubes and slow-growing TiO2 nanotubes. Ceram. Int. 2022, 48, 27703–27711. [Google Scholar] [CrossRef]
- Li, Z.; Pan, J.; Bia, H.; Lu, J.; Li, Y.Y. New explanation on formation mechanism of anodic TiO2 nanotubes. Mater. Sci. Eng. B 2022, 286, 115985. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Dan, Y.; Sun, M.; Gong, T.; Li, X.; Zhu, X. Growth of porous anodic TiO2 in silver nitrate solution without fluoride: Evidence against the field-assisted dissolution reactions of fluoride ions. Electrochem. Commun. 2021, 126, 107022. [Google Scholar] [CrossRef]
- Strnad, G.; Portan, D.; Jakab-Farkas, L.; Petrovan, C.; Russu, O. Morphology of TiO2 surfaces for biomedical implants developed by electrochemical anodization. Mater. Sci. Forum 2017, 907, 91–98. [Google Scholar] [CrossRef]
- Russu, O.; Strnad, G.; Jakab-Farkas, L.; Cazacu, R.; Feier, A.; Gergely, I.; Trambitas, C.; Petrovan, C. Electrochemical synthesis of nanostructured oxide layers on threaded surfaces of medical implants. Rev. Chim. 2018, 69, 1636–1639. [Google Scholar] [CrossRef]
- Strnad, G.; Jakab-Farkas, L.; Petrovan, C.; Russu, O. Influence of surface preparation on morphology of self-organized nanotubular oxide layers developed on Ti6Al4V alloy. Procedia Eng. 2017, 181, 242–248. [Google Scholar] [CrossRef]
- Sarraf, M.; Nasiri-Tabrizi, B.; Yeong, C.H.; Hosseini, H.R.M.; Saber-Samandari, S.; Basirun, W.J.; Tsuzuki, T. Mixed oxide nanotubes in nanomedicine: A dead-end or a bridge to the future? Ceram. Int. 2021, 47, 2917–2948. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-H.; Kim, W.-G.; Choe, H.-C.; William, A. Brantley. Control of nanotube shape and morphology on Ti-Nb(Ta)-Zr alloys by varying anodizing potential. Thin Solid Film. 2014, 572, 105–112. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, D.; Ning, C. Anodic Fabrication of Ti-Nb-Zr-O Nanotube Arrays. J. Nanomater. 2014, 2014, 240346. [Google Scholar] [CrossRef] [Green Version]
- Fatichi, A.Z.; Mello, M.G.; Caram, R.; Cremasco, A. Self-organized TiO2 nanotube layer on Ti–Nb–Zr alloys: Growth, characterization, and effect on corrosion behavior. J. Appl. Electrochem. 2019, 49, 1079–1089. [Google Scholar] [CrossRef]
- Perez, D.A.G.; Junior, A.M.J.; Asato, G.H.; Lepretre, J.-C.; Roche, V.; Bolfarini, C.; Botta, W.J. Surface anodization of the biphasic Ti13Nb13Zr biocompatible alloy: Influence of phases on the formation of TiO2 nanostructures. J. Alloys Compd. 2019, 796, 93–102. [Google Scholar] [CrossRef]
- Supernak-Marczewska, M.; Ossowska, A.; Strąkowska, P.; Zieliński, A. Nanotubular oxide layers and hydroxyapatite coatings on porous titanium alloy Ti13Nb13Zr. Adv. Mater. Sci. 2018, 18, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Matras, A.; Roguska, A.; Lewandowska, M. Fabrication of nanotubular oxide layer on Ti-24Nb-4Zr-8Sn alloy by electrochemical anodization. Inz. Mater. 2017, 1, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.-H.; Lai, T.-H.; Chen, C.-Y.; Hsieh, P.-Y.; Ozas, K.; Niinomi, M.; Okada, K.; Chang, T.-F.M.; Matsushita, N.; Sone, M.; et al. Fully Depleted Ti-Nb-Ta-Zr-O Nanotubes: Interfacial Charge Dynamics and Solar Hydrogen Production. ACS Appl. Mater. Interfaces 2018, 10, 22997–23008. [Google Scholar] [CrossRef]
- Rios, J.; Santini, V.N.; Pereira, K.D.; Luchessi, A.D.; Lopes, É.S.; Caram, R.; Cremasco, A. Self-organized TiO2 nanotubes on Ti-Nb-Fe alloys for biomedical applications: Synthesis and characterization. Electrochem. Commun. 2022, 138, 107280. [Google Scholar] [CrossRef]

| Process Parameters | Material | Mass Loss, Δm (mg) | Nanostructure Diameter, D (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Electrolyte | Potential, U (V) | Time, T (min) | Average | Standard Deviation | Range | Median | Mode | ||
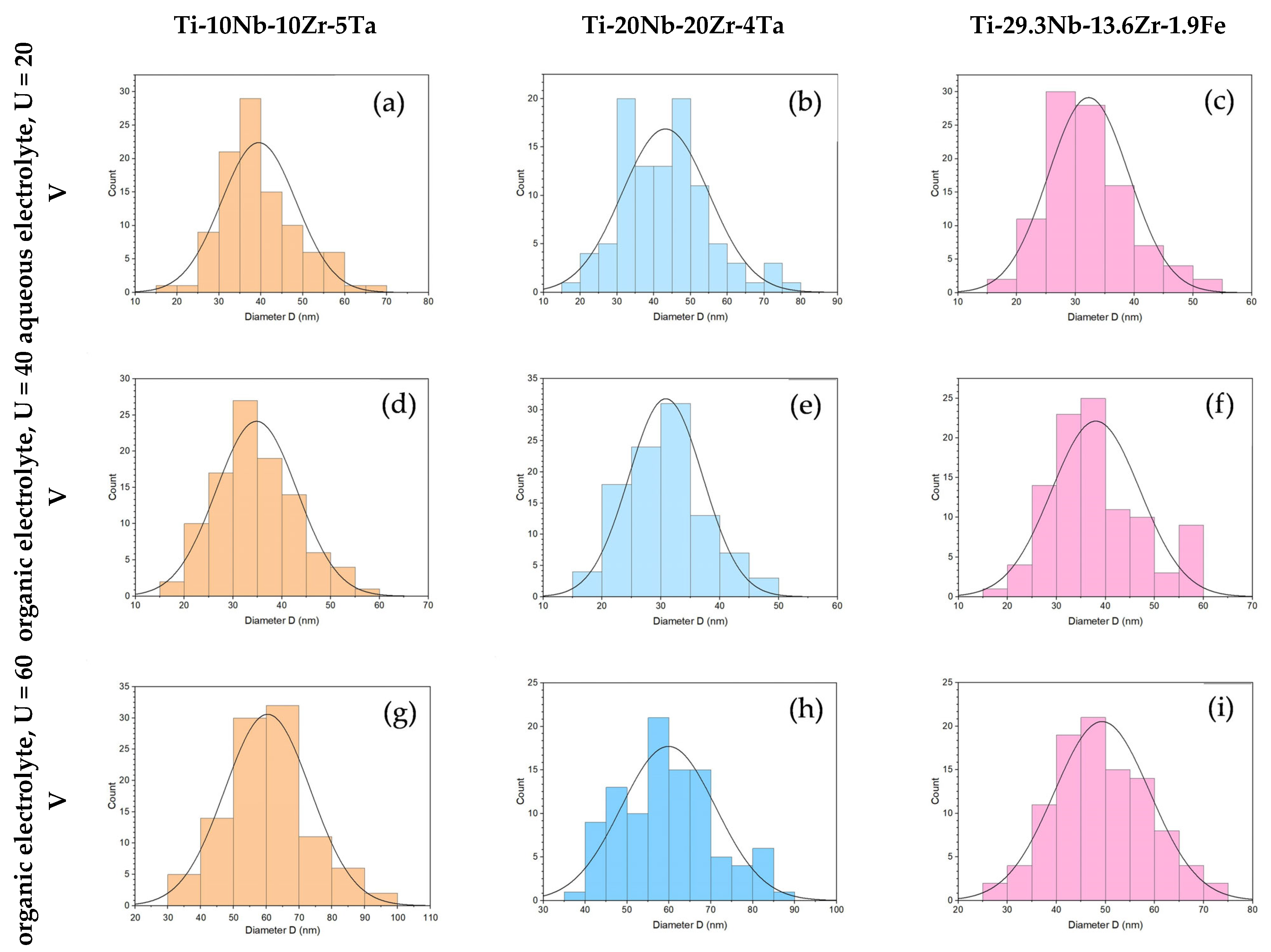
| 1M H3PO4 + 0.5 wt% HF | 20 | 30 | Ti-10Nb-10Zr-5Ta | 1.5 | 39 | 8.9 | 20–69 | 38 | 38 |
| Ti-20Nb-20Zr-4Ta | 1.0 | 43 | 11.8 | 19–77 | 43 | 45 | |||
| Ti-29.3Nb-13.6Zr-1.9Fe | 1.0 | 32 | 6.8 | 17–52 | 31 | 29 | |||
| 0.5 wt% NH4F + 2wt% H20 + EG | 40 | 30 | Ti-10Nb-10Zr-5Ta | 0.8 | 35 | 8.3 | 18–57 | 34 | 31 |
| Ti-20Nb-20Zr-4Ta | 0.4 | 31 | 6.3 | 17–46 | 31 | 28 | |||
| Ti-29.3Nb-13.6Zr-1.9Fe | 0.7 | 38 | 9.0 | 17–58 | 37 | 35 | |||
| 0.5 wt% NH4F + 2wt% H20 + EG | 60 | 30 | Ti-10Nb-10Zr-5Ta | 1.4 | 60 | 13.0 | 31–92 | 60 | 67 |
| Ti-20Nb-20Zr-4Ta | 2.1 | 60 | 11.3 | 40–89 | 58 | 49 | |||
| Ti-29.3Nb-13.6Zr-1.9Fe | 3.2 | 49 | 9.7 | 25–72 | 48 | 47 | |||
| Element | Ti-10Nb-10Zr-5Ta | Ti-20Nb-20Zr-4Ta | Ti-29.3Nb-13.6Zr-1.9Fe | ||||
|---|---|---|---|---|---|---|---|
| (wt%) | Whole Area | Average on Selected Areas | Whole Area | Average on Selected Areas | Whole Area | Average on Selected Areas | |
| Aqueous Electrolyte | Ti | 47.80 | 48.73 ± 1.21 | 35.30 | 36.20 ± 1.06 | 37.20 | 37.20 ± 1.60 |
| O | 29.20 | 29.53 ± 1.16 | 28.80 | 27.67 ± 0.67 | 28.50 | 27.90 ± 0.92 | |
| Nb | 8.20 | 7.97 ± 0.23 | 14.70 | 15.03 ± 0.47 | 18.50 | 18.50 ± 0.64 | |
| Zr | 6.60 | 6.73 ± 0.21 | 13.40 | 13.67 ± 0.60 | 10.50 | 10.80 ± 0.57 | |
| Ta | 4.20 | 3.23 ± 0.15 | 3.30 | 2.83 ± 0.35 | - | - | |
| Fe | - | - | - | - | 1.20 | 1.20 ± 0.12 | |
| F | 4.00 | 3.77 ± 0.31 | 4.60 | 4.53 ± 0.42 | 4.10 | 4.40 ± 0.32 | |
| Organic Electrolyte | Ti | 41.10 | 42.57 ± 0.83 | 32.90 | 32.95 ± 1.81 | 30.10 | 31.17 ± 2.27 |
| O | 29.60 | 28.43 ± 0.91 | 29.20 | 28.85 ± 2.29 | 28.50 | 27.67 ± 2.00 | |
| Nb | 8.10 | 8.53 ± 0.15 | 16.50 | 16.08 ± 0.62 | 15.70 | 16.20 ± 0.95 | |
| Zr | 5.00 | 5.17 ± 0.38 | 10.80 | 10.75 ± 0.64 | 10.60 | 11.07 ± 0.91 | |
| Ta | 4.80 | 4.97 ± 0.15 | 3.30 | 3.93 ± 0.47 | - | - | |
| Fe | - | - | - | - | 1.00 | 1.00 ± 0.10 | |
| F | 7.50 | 6.97 ± 0.50 | 7.30 | 7.43 ± 0.56 | 8.20 | 7.93 ± 0.90 | |
| C | 3.90 | 3.40 ± 0.26 | - | - | 6.00 | 4.97 ± 1.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strnad, G.; Jakab-Farkas, L.; Gobber, F.S.; Peter, I. Synthesis and Characterization of Nanostructured Oxide Layers on Ti-Nb-Zr-Ta and Ti-Nb-Zr-Fe Biomedical Alloys. J. Funct. Biomater. 2023, 14, 180. https://doi.org/10.3390/jfb14040180
Strnad G, Jakab-Farkas L, Gobber FS, Peter I. Synthesis and Characterization of Nanostructured Oxide Layers on Ti-Nb-Zr-Ta and Ti-Nb-Zr-Fe Biomedical Alloys. Journal of Functional Biomaterials. 2023; 14(4):180. https://doi.org/10.3390/jfb14040180
Chicago/Turabian StyleStrnad, Gabriela, Laszlo Jakab-Farkas, Federico Simone Gobber, and Ildiko Peter. 2023. "Synthesis and Characterization of Nanostructured Oxide Layers on Ti-Nb-Zr-Ta and Ti-Nb-Zr-Fe Biomedical Alloys" Journal of Functional Biomaterials 14, no. 4: 180. https://doi.org/10.3390/jfb14040180
APA StyleStrnad, G., Jakab-Farkas, L., Gobber, F. S., & Peter, I. (2023). Synthesis and Characterization of Nanostructured Oxide Layers on Ti-Nb-Zr-Ta and Ti-Nb-Zr-Fe Biomedical Alloys. Journal of Functional Biomaterials, 14(4), 180. https://doi.org/10.3390/jfb14040180







