Development of a COX-2-Selective Fluorescent Probe for the Observation of Early Intervertebral Disc Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
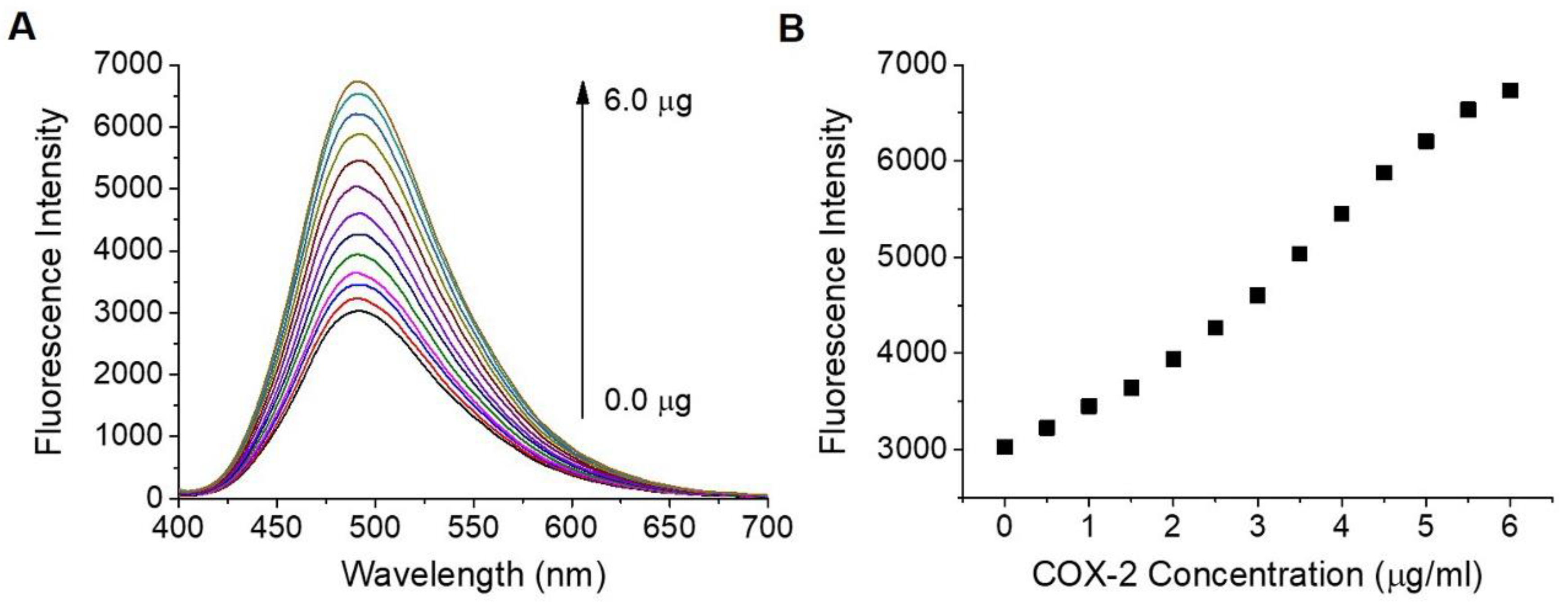
2.2. Spectroscopic Measurements
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Confocal Microscope Imaging
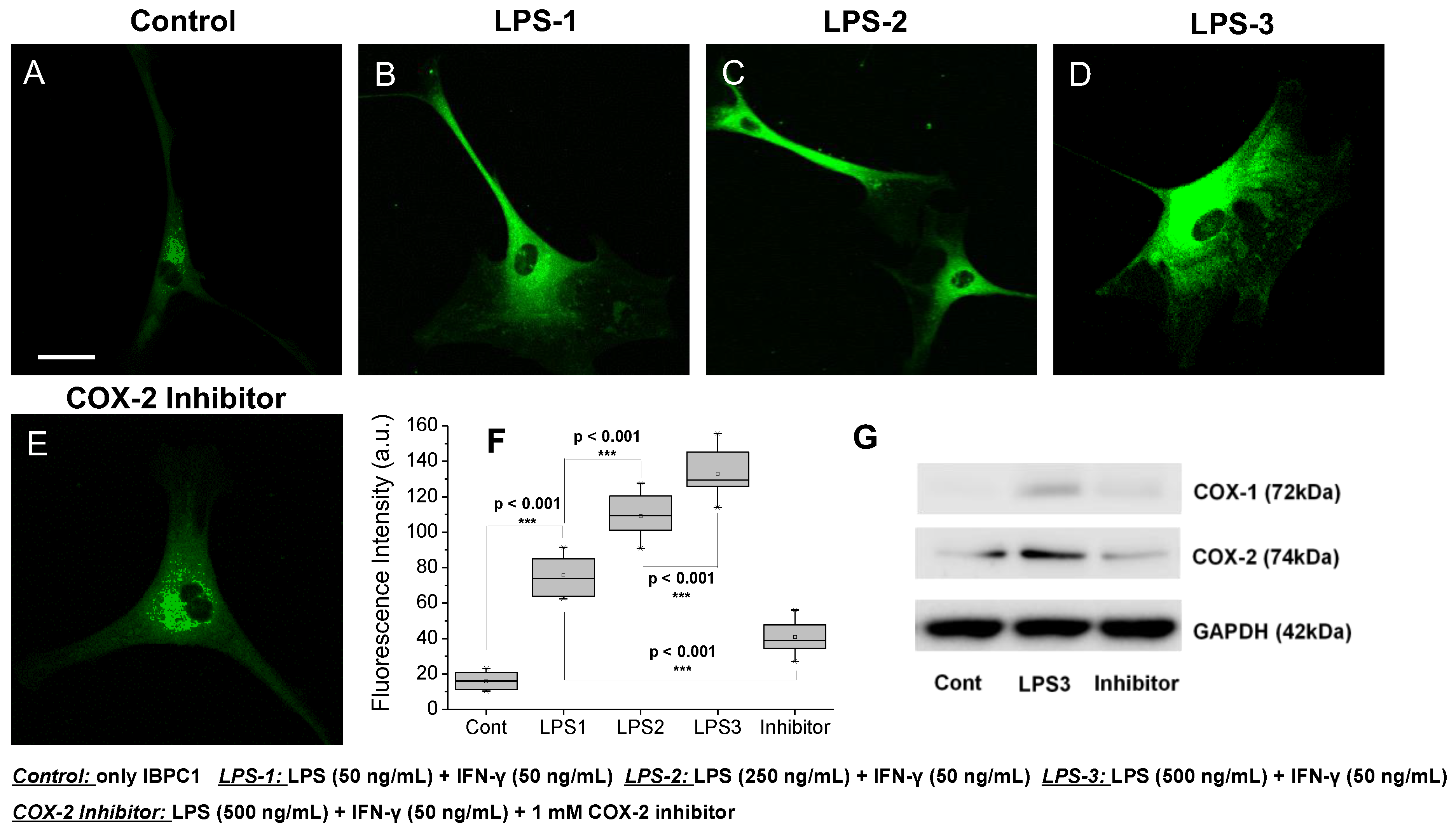
2.6. Western Blot
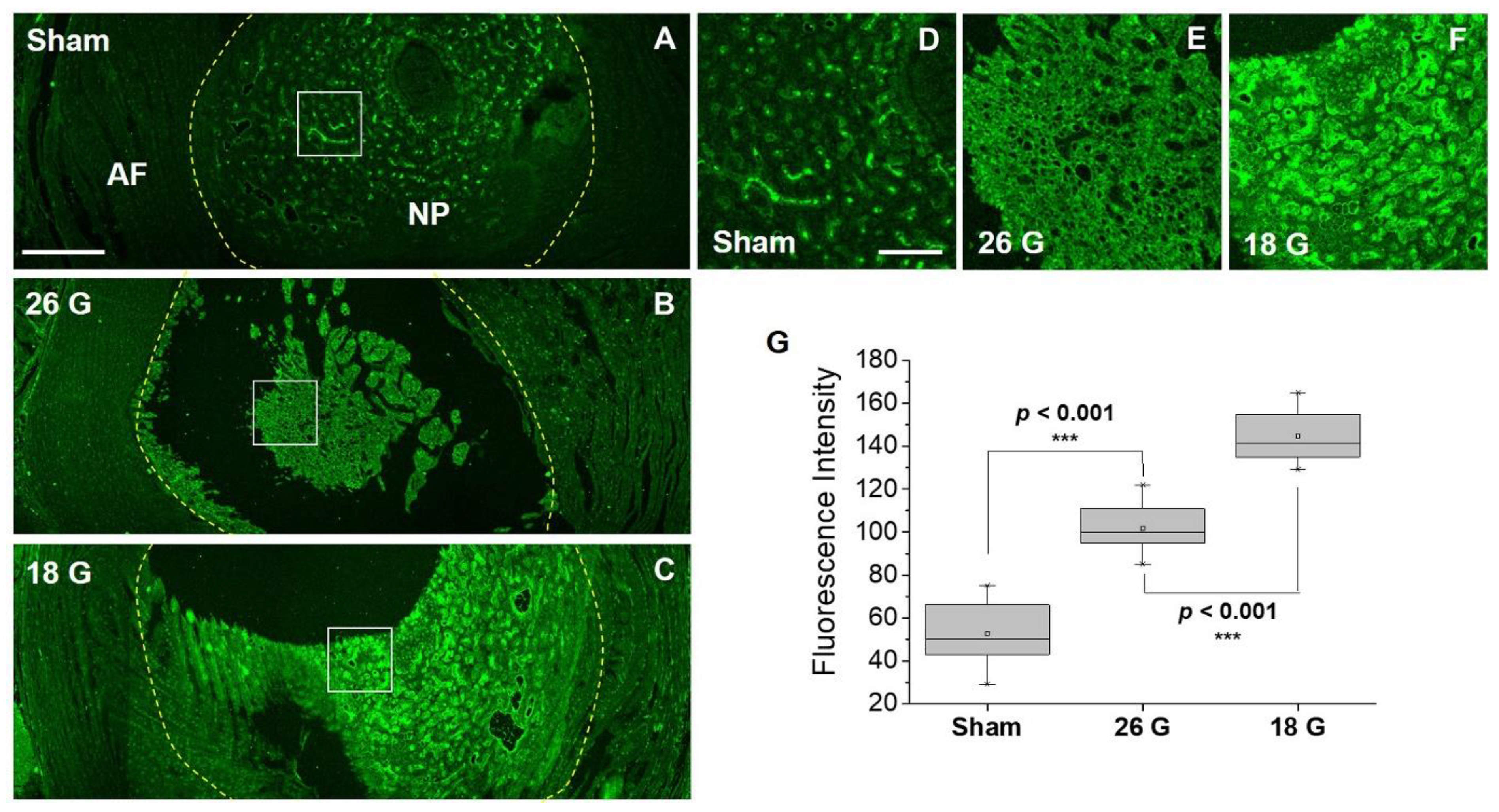
2.7. Animal Experimental Procedure and Tail-Puncture IVD Degeneration Modeling
2.8. Tissue Processing and Sectioning
2.9. Fluorescent Probe Staining and Immunofluorescence
3. Results and Discussion
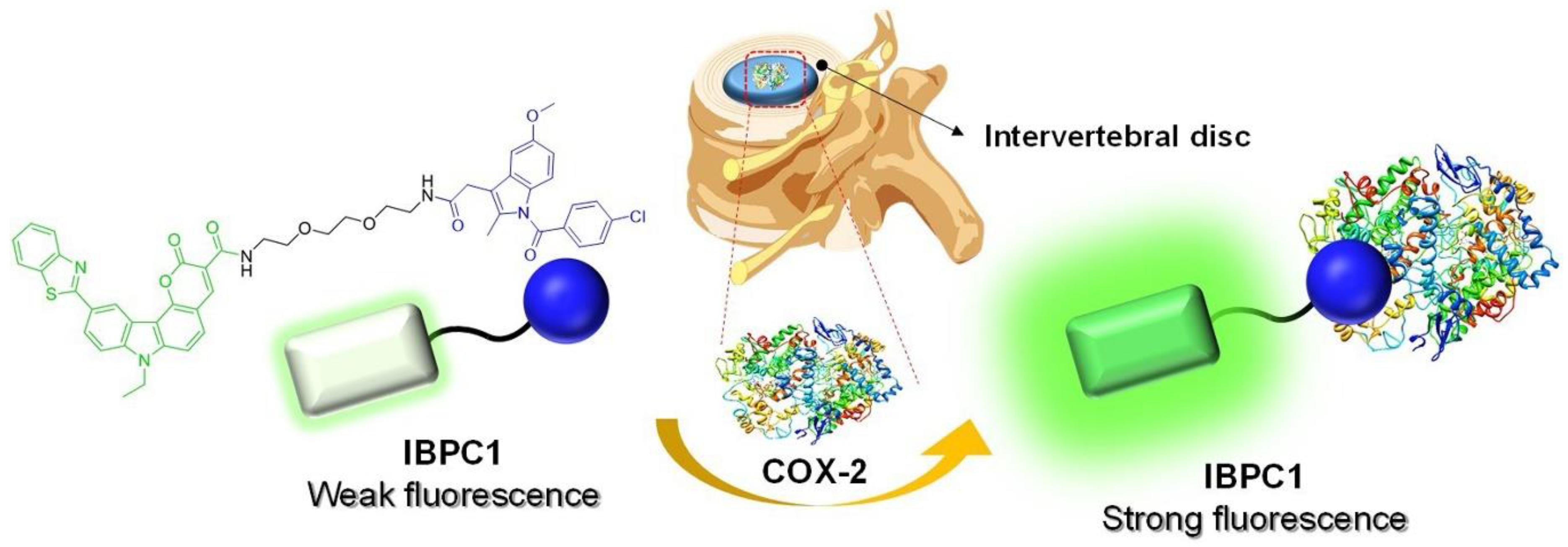
3.1. Design and Synthesis of the COX-2–Selective Fluorescence Probe (IBPC1)
3.2. Photophysical Properties of IBPC1
3.3. Evaluation of COX-2 Detection Ability in Cells
3.4. Efficacy of IBPC1 in an Animal Model of IVD Degeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Kumar, N.; Pathak, Z.; Kumar, H. Extra Cellular Matrix Remodeling: An Adjunctive Target for Spinal Cord Injury and Intervertebral Disc Degeneration. Neurospine 2022, 19, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.J.; Cui, H.; Pan, H.; Mc Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Han, I.B. Moving Forward: Gene Therapy for Intervertebral Disc Degeneration. Neurospine 2020, 17, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, I.L.; Mokhtar, S.A.; Abbah, S.A.; Fauzi, M.B.; Devitt, A.; Pandit, A. Intervertebral Disc Degeneration: Biomaterials and Tissue Engineering Strategies toward Precision Medicine. Adv. Healthc. Mater. 2022, 11, e2102530. [Google Scholar] [CrossRef]
- Roh, E.J.; Darai, A.; Kyung, J.; Choi, H.; Kwon, S.; Bhujel, B.; Kim, K.; Han, I. Genetic therapy for intervertebral disc degeneration. Int. J. Mol. Sci. 2021, 22, 1579. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Kos, N.; Gradisnik, L.; Velnar, T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421–424. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Shao, Z. The role of structure and function changes of sensory nervous system in intervertebral disc-related low back pain. Osteoarthr. Cartil. 2021, 29, 17–27. [Google Scholar] [CrossRef]
- Moghaddamjou, A.; Fehlings, M. The Beneficial Effect of Early Surgical Decompression for Acute Spinal Cord Injury: Time Is Spine. Neurospine 2021, 18, 20–22. [Google Scholar] [CrossRef]
- Solumsmoen, S.; Bari, T.J.; Woldu, S.; Zielinski, O.B.; Gehrchen, M.; Dahl, B.; Bech-Azeddine, R. A Comparison of Mortality and Morbidity between Complex and Degenerative Spine Surgery in Prospectively Collected Data from 2280 Procedures. Neurospine 2021, 18, 524–532. [Google Scholar] [CrossRef]
- Hasz, M.W. Diagnostic testing for degenerative disc disease. Adv. Orthop. 2012, 2012, 413913. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef] [Green Version]
- Vo, N.; Couch, B.; Lee, J.; Sowa, G.; Kang, J.; Rebecca, S. Actions of Prostaglandins on Human Nucleus Pulposus Metabolism Inferred by Cyclooxygenase 2 Inhibition of Cytokine Activated Cells. Neurospine 2020, 17, 60–68. [Google Scholar] [CrossRef]
- Ding, Q.; Ren, Y.; Che, H.; Ma, C.; Li, H.; Yu, S.; Zhang, Y.; An, H.; O’Keefe, R.J.; Chen, D.; et al. Cyclooxygenase-2 deficiency causes delayed ossification of lumbar vertebral endplates. Am. J. Transl. Res. 2018, 10, 718–730. [Google Scholar]
- Liu, C.; Liang, G.; Deng, Z.; Tan, J.; Zheng, Q.; Lyu, F.-J. The Upregulation of COX2 in Human Degenerated Nucleus Pulposus: The Association of Inflammation with Intervertebral Disc Degeneration. Mediat. Inflamm. 2021, 2021, 2933199. [Google Scholar] [CrossRef]
- Wahlby, C.; Erlandsson, F.; Bengtsson, E.; Zetterberg, A. Sequential immunofluorescence staining and image analysis for detection of large numbers of antigens in individual cell nuclei. Cytometry 2002, 47, 32–41. [Google Scholar] [CrossRef]
- Guan, P.P.; Yu, X.; Zou, Y.H.; Wang, P. Cyclooxygenase-2 is critical for the propagation of beta-amyloid protein and reducing the glycosylation of tau in Alzheimer’s disease. Cell. Mol. Immunol. 2019, 16, 892–894. [Google Scholar] [CrossRef]
- Kang, X.; Qiu, J.; Li, Q.; Bell, K.A.; Du, Y.; Jung, D.W.; Lee, J.Y.; Hao, J.; Jiang, J. Cyclooxygenase-2 contributes to oxidopamine-mediated neuronal inflammation and injury via the prostaglandin E2 receptor EP2 subtype. Sci. Rep. 2017, 7, 9459. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yu, J.; Lin, X.; Tang, W. Inhibition of cyclooxygenase-2 by NS398 attenuates noise-induced hearing loss in mice. Sci. Rep. 2016, 6, 22573. [Google Scholar] [CrossRef] [Green Version]
- Tu, M.; Yang, M.; Yu, N.; Zhen, G.; Wan, M.; Liu, W.; Ji, B.; Ma, H.; Guo, Q.; Tong, P.; et al. Inhibition of cyclooxygenase-2 activity in subchondral bone modifies a subtype of osteoarthritis. Bone Res. 2019, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wei, J.; Shi, J.; He, Q.; Zhou, X.; Gao, X.; Cheng, L. Follistatin-like 1 attenuation suppresses intervertebral disc degeneration in mice through interacting with TNF-α and Smad signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6640751. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.M.; Momin, A.; Kusurkar, R. New and efficient routes for the synthesis of murrayaquinone A and murrayanine. Tetrahedron 2012, 68, 6420–6426. [Google Scholar] [CrossRef]
- Crosby, G.A.; Demas, J. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Choi, U.Y.; Joshi, H.P.; Payne, S.; Kim, K.T.; Kyung, J.W.; Choi, H.; Cooke, M.J.; Kwon, S.Y.; Roh, E.J.; Sohn, S.; et al. An Injectable Hyaluronan-Methylcellulose (HAMC) Hydrogel Combined with Wharton’s Jelly-Derived Mesenchymal Stromal Cells (WJ-MSCs) Promotes Degenerative Disc Repair. Int. J. Mol. Sci. 2020, 21, 7391. [Google Scholar] [CrossRef]
- Makino, H.; Seki, S.; Yahara, Y.; Shiozawa, S.; Aikawa, Y.; Motomura, H.; Nogami, M.; Watanabe, K.; Sainoh, T.; Ito, H.; et al. A selective inhibition of c-Fos/activator protein-1 as a potential therapeutic target for intervertebral disc degeneration and associated pain. Sci. Rep. 2017, 7, 16983. [Google Scholar] [CrossRef] [Green Version]
- Piazza, M.; Peck, S.H.; Gullbrand, S.; Bendigo, J.R.; Arginteanu, T.; Zhang, Y.; Smith, H.E.; Malhotra, N.R.; Smith, L.J. Quantitative MRI correlates with histological grade in a percutaneous needle injury mouse model of disc degeneration. J. Orthop. Res. 2018, 36, 2771–2779. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Wang, J.; Zhang, S.; Dou, B.; Peng, X. An off–on Cox-2-specific fluorescent probe: Targeting the golgi apparatus of cancer cells. J. Am. Chem. Soc. 2013, 135, 11663–11669. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Wang, K.; Li, J.; Wang, C.; Nie, Y.; Jiang, T.; Mu, H.; Peng, X.; Jiang, K. Highly sensitive naphthalene-based two-photon fluorescent probe for in situ real-time bioimaging of ultratrace cyclooxygenase-2 in living biosystems. Anal. Chem. 2014, 86, 9131–9138. [Google Scholar] [CrossRef]
- Wang, B.; Fan, J.; Wang, X.; Zhu, H.; Wang, J.; Mu, H.; Peng, X. A Nile blue based infrared fluorescent probe: Imaging tumors that over-express cyclooxygenase-2. Chem. Commun. 2015, 51, 792–795. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, T.; Ren, W.X.; Lim, J.-Y.; Won, M.; Heo, J.S.; Lee, S.G.; Kim, J.S. COX-2 targeting indomethacin conjugated fluorescent probe. Dye. Pigment. 2018, 150, 261–266. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, M.K.; Lee, D.J.; Song, D.H.; Lim, C.S.; Noh, C.-K.; Choi, K.S.; Shin, S.J.; Kim, H.M. Development of two-photon fluorescence probe for detecting cyclooxygenase-2 level in human colorectal cancer tissue. Sens. Actuators B Chem. 2021, 330, 129329. [Google Scholar] [CrossRef]
- Uddin, M.J.; Lo, J.H.; Oltman, C.G.; Crews, B.C.; Huda, T.; Liu, J.; Kingsley, P.J.; Lin, S.; Milad, M.; Aleem, A.M.; et al. Discovery of a redox-activatable chemical probe for detection of cyclooxygenase-2 in cells and animals. ACS Chem. Biol. 2022, 17, 1714–1722. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Crews, B.C.; Rowlinson, S.W.; Marnett, A.B.; Kozak, K.R.; Remmel, R.P.; Marnett, L.J. Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: Facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc. Natl. Acad. Sci. USA 2000, 97, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Blobaum, A.L.; Uddin, J.; Felts, A.S.; Crews, B.C.; Rouzer, C.A.; Marnett, L.J. The 2′-trifluoromethyl analogue of indomethacin is a potent and selective COX-2 inhibitor. ACS Med. Chem. Lett. 2013, 4, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.R.; Pelly, V.S.; Moeini, A.; Chiang, S.C.; Flanagan, E.; Bromley, C.P.; Clark, C.; Earnshaw, C.H.; Koufaki, M.A.; Bonavita, E.; et al. Chemotherapy-induced COX-2 upregulation by cancer cells defines their inflammatory properties and limits the efficacy of chemoimmunotherapy combinations. Nat. Commun. 2022, 13, 2063. [Google Scholar] [CrossRef]
- Cunha, C.; Silva, A.J.; Pereira, P.; Vaz, R.; Gonçalves, R.M.; Barbosa, M.A. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res. Ther. 2018, 20, 251. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, C.H.; Roh, E.J.; Kim, J.; Choi, H.; Jang, H.Y.; Lee, G.; Lim, C.S.; Han, I. Development of a COX-2-Selective Fluorescent Probe for the Observation of Early Intervertebral Disc Degeneration. J. Funct. Biomater. 2023, 14, 192. https://doi.org/10.3390/jfb14040192
Heo CH, Roh EJ, Kim J, Choi H, Jang HY, Lee G, Lim CS, Han I. Development of a COX-2-Selective Fluorescent Probe for the Observation of Early Intervertebral Disc Degeneration. Journal of Functional Biomaterials. 2023; 14(4):192. https://doi.org/10.3390/jfb14040192
Chicago/Turabian StyleHeo, Cheol Ho, Eun Ji Roh, Jaehee Kim, Hyemin Choi, Ho Yeon Jang, Giseong Lee, Chang Su Lim, and Inbo Han. 2023. "Development of a COX-2-Selective Fluorescent Probe for the Observation of Early Intervertebral Disc Degeneration" Journal of Functional Biomaterials 14, no. 4: 192. https://doi.org/10.3390/jfb14040192
APA StyleHeo, C. H., Roh, E. J., Kim, J., Choi, H., Jang, H. Y., Lee, G., Lim, C. S., & Han, I. (2023). Development of a COX-2-Selective Fluorescent Probe for the Observation of Early Intervertebral Disc Degeneration. Journal of Functional Biomaterials, 14(4), 192. https://doi.org/10.3390/jfb14040192







