Peptide-Based Hydrogels: Template Materials for Tissue Engineering
Abstract
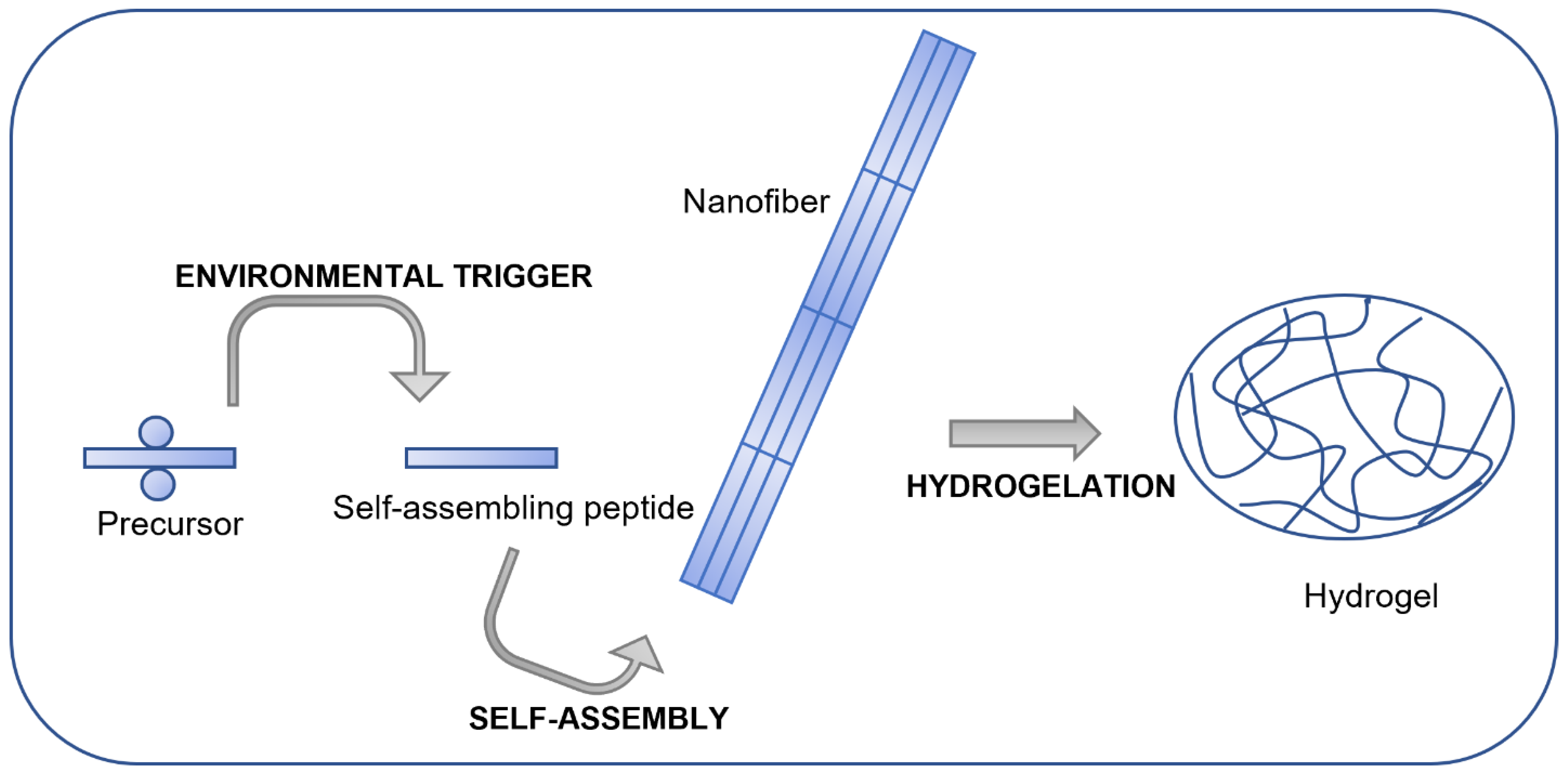
:1. Introduction
2. Main Features of Peptide Hydrogels as 3D Scaffolds
2.1. Mechanical Features
2.2. Biodegradability
2.3. Bioactivity
3. Peptide Hydrogel Scaffolds in Tissue Engineering Applications
3.1. Angiogenesis and Vascularization
3.2. Neural Tissue Engineering
3.2.1. Peptide Hydrogels
3.2.2. Hydrogels Made of Peptides and Organic/Inorganic Components
3.3. Cartilage Regeneration
3.4. Bone Regeneration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative Medicine: Current Therapies and Future Directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic Peptide Hydrogels as 3D Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef]
- Chandra, P.K.; Soker, S.; Atala, A. Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Hoffman, T.; Antovski, P.; Tebon, P.; Xu, C.; Ashammakhi, N.; Ahadian, S.; Morsut, L.; Khademhosseini, A. Synthetic Biology and Tissue Engineering: Toward Fabrication of Complex and Smart Cellular Constructs. Adv. Funct. Mater. 2020, 30, 1909882. [Google Scholar] [CrossRef]
- Fisher, M.B.; Mauck, R.L. Tissue Engineering and Regenerative Medicine: Recent Innovations and the Transition to Translation. Tissue Eng. Part B Rev. 2013, 19, 1–13. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Stevens, M.M. Using Remote Fields for Complex Tissue Engineering. Trends Biotechnol. 2020, 38, 254–263. [Google Scholar] [CrossRef]
- Kengla, C.; Lee, S.J.; Yoo, J.J.; Atala, A. 3-D Bioprinting Technologies for Tissue Engineering Applications. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–288. [Google Scholar]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Li, S.; Roy, K. Induced Pluripotent Stem Cells for Regenerative Medicine. Annu. Rev. Biomed. Eng. 2014, 16, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S. Engineering Complex Synthetic Transcriptional Programs with CRISPR RNA Scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Pulgarin, D.A.V.; Bowlin, G.L.; Nyberg, W.A.; Espinosa, A. CRISPR/CAS Systems in Tissue Engineering: A Succinct Overview of Current Use and Future Opportunities. Curr. Trends Biomed. Eng. Biosci. 2017, 5, 93–96. [Google Scholar]
- Stratakis, E. Novel Biomaterials for Tissue Engineering 2018. Int. J. Mol. Sci. 2018, 19, 3960. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-Dimensional Printing of Multilayered Tissue Engineering Scaffolds. Mater. Today 2018, 21, 861–874. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 19, 17. [Google Scholar] [CrossRef]
- Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Parmeggiani, D.; Barbarisi, A. Smart Materials as Scaffolds for Tissue Engineering. J. Cell. Physiol. 2005, 203, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Del Giudice, A.; Mignardi, S.; Amato, F.; Marrani, A.G.; Sivori, F.; Cavallo, I.; Di Domenico, E.G.; Palocci, C.; Chronopoulou, L. Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels 2022, 8, 700. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Self-assembling Peptide Nanofiber Hydrogels in Tissue Engineering and Regenerative Medicine: Progress, Design Guidelines, and Applications. J. Biomed. Mater. Res. Part A 2016, 104, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, M.; Zhou, Y.; Li, L.; Wang, C.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Chen, Y.; et al. A Self-Assembling Peptide Hydrogel-Based Drug Co-Delivery Platform to Improve Tissue Repair after Ischemia-Reperfusion Injury. Acta Biomater. 2020, 103, 102–114. [Google Scholar] [CrossRef]
- Huang, L.-C.; Wang, H.-C.; Chen, L.-H.; Ho, C.-Y.; Hsieh, P.-H.; Huang, M.-Y.; Wu, H.-C.; Wang, T.-W. Bioinspired Self-Assembling Peptide Hydrogel with Proteoglycan-Assisted Growth Factor Delivery for Therapeutic Angiogenesis. Theranostics 2019, 9, 7072. [Google Scholar] [CrossRef]
- Bruggeman, K.F.; Rodriguez, A.L.; Parish, C.L.; Williams, R.J.; Nisbet, D.R. Temporally Controlled Release of Multiple Growth Factors from a Self-Assembling Peptide Hydrogel. Nanotechnology 2016, 27, 385102. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X. Biomimetic Self-Assembling Peptide Hydrogels for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1064, 297–312. [Google Scholar] [PubMed]
- Sun, L.; Zheng, C.; Webster, T.J. Self-Assembled Peptide Nanomaterials for Biomedical Applications: Promises and Pitfalls. Int. J. Nanomed. 2017, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Gazit, E. The Physical Properties of Supramolecular Peptide Assemblies: From Building Block Association to Technological Applications. Chem. Soc. Rev. 2014, 43, 6881–6893. [Google Scholar] [CrossRef]
- Yan, C.; Altunbas, A.; Yucel, T.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable Solid Hydrogel: Mechanism of Shear-Thinning and Immediate Recovery of Injectable β-Hairpin Peptide Hydrogels. Soft Matter 2010, 6, 5143–5156. [Google Scholar] [CrossRef]
- Bakota, E.L.; Wang, Y.; Danesh, F.R.; Hartgerink, J.D. Injectable Multidomain Peptide Nanofiber Hydrogel as a Delivery Agent for Stem Cell Secretome. Biomacromolecules 2011, 12, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-Matrix Tethering Regulates Stem-Cell Fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Chang, Q.; Darabi, M.A.; Lin, B.; Zhong, W.; Xing, M. Highly Flexible and Resilient Elastin Hybrid Cryogels with Shape Memory, Injectability, Conductivity, and Magnetic Responsive Properties. Adv. Mater. 2016, 28, 7758–7767. [Google Scholar] [CrossRef] [PubMed]
- Gyarmati, B.; Mészár, E.Z.; Kiss, L.; Deli, M.A.; László, K.; Szilágyi, A. Supermacroporous Chemically Cross-Linked Poly (Aspartic Acid) Hydrogels. Acta Biomater. 2015, 22, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Chakraborty, P.; Guterman, T.; Adadi, N.; Yadid, M.; Brosh, T.; Adler-Abramovich, L.; Dvir, T.; Gazit, E. A Self-Healing, All-Organic, Conducting, Composite Peptide Hydrogel as Pressure Sensor and Electrogenic Cell Soft Substrate. ACS Nano 2018, 13, 163–175. [Google Scholar] [CrossRef]
- Sieminski, A.L.; Was, A.S.; Kim, G.; Gong, H.; Kamm, R.D. The Stiffness of Three-Dimensional Ionic Self-Assembling Peptide Gels Affects the Extent of Capillary-like Network Formation. Cell Biochem. Biophys. 2007, 49, 73–83. [Google Scholar] [CrossRef]
- Huebsch, N.; Mooney, D.J. Inspiration and Application in the Evolution of Biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y. Chapter 19—Three-Dimensional Scaffolds. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 343–360. ISBN 978-0-12-818422-6. [Google Scholar]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Pochan, D.J. Rheological Properties of Peptide-Based Hydrogels for Biomedical and Other Applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A.J. Interplay of Matrix Stiffness and Protein Tethering in Stem Cell Differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef] [PubMed]
- De Leon Rodriguez, L.M.; Hemar, Y.; Cornish, J.; Brimble, M.A. Structure–Mechanical Property Correlations of Hydrogel Forming β-Sheet Peptides. Chem. Soc. Rev. 2016, 45, 4797–4824. [Google Scholar] [CrossRef]
- Li, Y.; Qin, M.; Cao, Y.; Wang, W. Designing the Mechanical Properties of Peptide-Based Supramolecular Hydrogels for Biomedical Applications. Sci. China Phys. Mech. Astron. 2014, 57, 849–858. [Google Scholar] [CrossRef]
- Jung, J.P.; Jones, J.L.; Cronier, S.A.; Collier, J.H. Modulating the Mechanical Properties of Self-Assembled Peptide Hydrogels via Native Chemical Ligation. Biomaterials 2008, 29, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Park, J.; Jiang, Y.; Woodrow, K.A. Rational Design of Charged Peptides That Self-Assemble into Robust Nanofibers as Immune-Functional Scaffolds. Acta Biomater. 2017, 55, 183–193. [Google Scholar] [CrossRef]
- Clarke, D.E.; Parmenter, C.D.J.; Scherman, O.A. Tunable Pentapeptide Self-Assembled b-Sheet Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 7709–7713. [Google Scholar] [CrossRef]
- Ghosh, A.; Haverick, M.; Stump, K.; Yang, X.; Tweedle, M.F.; Goldberger, J.E. Fine-Tuning the PH Trigger of Self-Assembly. J. Am. Chem. Soc. 2012, 134, 3647–3650. [Google Scholar] [CrossRef]
- Chen, Y.; Gan, H.X.; Tong, Y.W. PH-Controlled Hierarchical Self-Assembly of Peptide Amphiphile. Macromolecules 2015, 48, 2647–2653. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A New Method for Maintaining Homogeneity during Liquid–Hydrogel Transitions Using Low Molecular Weight Hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Raeburn, J.; Pont, G.; Chen, L.; Cesbron, Y.; Lévy, R.; Adams, D.J. Fmoc-Diphenylalanine Hydrogels: Understanding the Variability in Reported Mechanical Properties. Soft Matter 2012, 8, 1168–1174. [Google Scholar] [CrossRef]
- Ramachandran, S.; Taraban, M.B.; Trewhella, J.; Gryczynski, I.; Gryczynski, Z.; Yu, Y.B. Effect of Temperature During Assembly on the Structure and Mechanical Properties of Peptide-Based Materials. Biomacromolecules 2010, 11, 1502–1506. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Qin, M.; Cao, Y.; Wang, W. Photo-Cross-Linking Approach to Engineering Small Tyrosine-Containing Peptide Hydrogels with Enhanced Mechanical Stability. Langmuir 2013, 29, 13299–13306. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hou, X.; Zhang, X.; Zhao, Y.; Sun, Y.; Wang, J. One- and Two-Photon Responsive Injectable Nano-Bundle Biomaterials from Co-Assembled Lipopeptides for Controlling Molecular Diffusion. Soft Matter 2019, 15, 6476–6484. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Fei, J.; Li, Q.; Li, J. Photo-Induced Reversible Structural Transition of Cationic Diphenylalanine Peptide Self-Assembly. Small 2015, 11, 1787–1791. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Pandolfi, D.; Paradossi, G.; Palocci, C. Biofabrication of Genipin-Crosslinked Peptide Hydrogels and Their Use in the Controlled Delivery of Naproxen. New Biotechnol. 2017, 37, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800221. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angew. Chem. Int. Ed. 2012, 51, 7396–7417. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Chwalek, K.; Prokoph, S.; Zieris, A.; Levental, K.R.; Freudenberg, U.; Werner, C. Defined Polymer–Peptide Conjugates to Form Cell-Instructive StarPEG–Heparin Matrices In Situ. Adv. Mater. 2013, 25, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Rachel Ee, P.L.; Ke, C.Y.; Hedrick, J.L.; Yang, Y.Y. Biodegradable Poly(Ethylene Glycol)–Peptide Hydrogels with Well-Defined Structure and Properties for Cell Delivery. Biomaterials 2009, 30, 1453–1461. [Google Scholar] [CrossRef]
- Tzokova, N.; Fernyhough, C.M.; Topham, P.D.; Sandon, N.; Adams, D.J.; Butler, M.F.; Armes, S.P.; Ryan, A.J. Soft Hydrogels from Nanotubes of Poly(Ethylene Oxide)−Tetraphenylalanine Conjugates Prepared by Click Chemistry. Langmuir 2009, 25, 2479–2485. [Google Scholar] [CrossRef]
- Castelletto, V.; Newby, G.E.; Zhu, Z.; Hamley, I.W.; Noirez, L. Self-Assembly of PEGylated Peptide Conjugates Containing a Modified Amyloid β-Peptide Fragment. Langmuir 2010, 26, 9986–9996. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.J.; Romano, N.H.; Wirtz, D.; Yu, S.M. PEG-Based Hydrogels with Collagen Mimetic Peptide-Mediated and Tunable Physical Cross-Links. Biomacromolecules 2010, 11, 2336–2344. [Google Scholar]
- Liyanage, W.; Vats, K.; Rajbhandary, A.; Benoit, D.S.W.; Nilsson, B.L. Multicomponent Dipeptide Hydrogels as Extracellular Matrix-Mimetic Scaffolds for Cell Culture Applications. Chem. Commun. 2015, 51, 11260–11263. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, A.; Bochicchio, B.; Smith, A.; Workman, V.L.; Castillo Diaz, L.A.; Saiani, A.; Pepe, A. Tuning of Hydrogel Stiffness Using a Two-Component Peptide System for Mammalian Cell Culture. J. Biomed. Mater. Res. Part A 2019, 107, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Shi, S.; Wang, B.K.; Li, I.-C.; Jalan, A.A.; Sarkar, B.; Wickremasinghe, N.C.; Hartgerink, J.D. Drug-Triggered and Cross-Linked Self-Assembling Nanofibrous Hydrogels. J. Am. Chem. Soc. 2015, 137, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.C.; Smith, K.H.; Tejeda-Montes, E.; Engel, E.; Reis, R.L.; Azevedo, H.S.; Mata, A. Co-Assembled and Microfabricated Bioactive Membranes. Adv. Funct. Mater. 2013, 23, 430–438. [Google Scholar] [CrossRef]
- Chen, J.; Tao, N.; Fang, S.; Chen, Z.; Liang, L.; Sun, X.; Li, J.; Liu, Y.-N. Incorporation of Fmoc-Y Nanofibers into Ca-Alginate Hydrogels for Improving Their Mechanical Properties and the Controlled Release of Small Molecules. New J. Chem. 2018, 42, 9651–9657. [Google Scholar] [CrossRef]
- Pugliese, R.; Gelain, F. Characterization of Elastic, Thermo-Responsive, Self-Healable Supramolecular Hydrogel Made of Self-Assembly Peptides and Guar Gum. Mater. Des. 2020, 186, 108370. [Google Scholar] [CrossRef]
- Chaudhuri, O. Viscoelastic Hydrogels for 3D Cell Culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Lee, H.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical Confinement Regulates Cartilage Matrix Formation by Chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.N. Hydrogels with Tunable Stress Relaxation Regulate Stem Cell Fate and Activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Giano, M.C.; Pochan, D.J.; Schneider, J.P. Controlled Biodegradation of Self-Assembling β-Hairpin Peptide Hydrogels by Proteolysis with Matrix Metalloproteinase-13. Biomaterials 2011, 32, 6471–6477. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, J.; Rollo, J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Biomaterials and Scaffold Design: Key to Tissue-engineering Cartilage. Biotechnol. Appl. Biochem. 2007, 46, 73–84. [Google Scholar] [PubMed]
- Sokic, S.; Christenson, M.C.; Larson, J.C.; Appel, A.A.; Brey, E.M.; Papavasiliou, G. Evaluation of MMP Substrate Concentration and Specificity for Neovascularization of Hydrogel Scaffolds. Biomater. Sci. 2014, 2, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.; Luo, Y.; Cheung, A.C.Y.; Nagai, Y.; Zhang, S.; Kobler, J.B.; Zeitels, S.M.; Langer, R. Incorporation of a Matrix Metalloproteinase-Sensitive Substrate into Self-Assembling Peptides–a Model for Biofunctional Scaffolds. Biomaterials 2008, 29, 1713–1719. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Jun, H.; Yuwono, V.; Paramonov, S.E.; Hartgerink, J.D. Enzyme-mediated Degradation of Peptide-amphiphile Nanofiber Networks. Adv. Mater. 2005, 17, 2612–2617. [Google Scholar]
- Son, J.; Kalafatovic, D.; Kumar, M.; Yoo, B.; Cornejo, M.A.; Contel, M.; Ulijn, R.V. Customizing Morphology, Size, and Response Kinetics of Matrix Metalloproteinase-Responsive Nanostructures by Systematic Peptide Design. ACS Nano 2019, 13, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ferreira, D.S.; Banerjee, J.; Pickford, A.R.; Azevedo, H.S. Tuning the Matrix Metalloproteinase-1 Degradability of Peptide Amphiphile Nanofibers through Supramolecular Engineering. Biomater. Sci. 2019, 7, 5132–5142. [Google Scholar] [CrossRef] [PubMed]
- Qorri, B.; Kalaydina, R.-V.; Velickovic, A.; Kaplya, Y.; Decarlo, A.; Szewczuk, M.R. Agonist-Biased Signaling via Matrix Metalloproteinase-9 Promotes Extracellular Matrix Remodeling. Cells 2018, 7, 117. [Google Scholar] [CrossRef]
- Daviran, M.; Caram, H.S.; Schultz, K.M. Role of Cell-Mediated Enzymatic Degradation and Cytoskeletal Tension on Dynamic Changes in the Rheology of the Pericellular Region Prior to Human Mesenchymal Stem Cell Motility. ACS Biomater. Sci. Eng. 2018, 4, 468–472. [Google Scholar] [CrossRef]
- Daviran, M.; Schultz, K.M. Characterizing the Dynamic Rheology in the Pericellular Region by Human Mesenchymal Stem Cell Re-Engineering in PEG-Peptide Hydrogel Scaffolds. Rheol. Acta 2019, 58, 421–437. [Google Scholar] [CrossRef]
- Swanekamp, R.J.; Welch, J.J.; Nilsson, B.L. Proteolytic Stability of Amphipathic Peptide Hydrogels Composed of Self-Assembled Pleated β-Sheet or Coassembled Rippled β-Sheet Fibrils. Chem. Commun. 2014, 50, 10133–10136. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, A.; He, B.; Zhao, W.; Chen, X.; Jiang, D. Designer D-Form Self-Assembling Peptide Scaffolds Promote the Proliferation and Migration of Rat Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Med. 2017, 40, 679–688. [Google Scholar] [CrossRef]
- Castelletto, V.; Gouveia, R.J.; Connon, C.J.; Hamley, I.W.; Seitsonen, J.; Ruokolainen, J.; Longo, E.; Siligardi, G. Influence of Elastase on Alanine-Rich Peptide Hydrogels. Biomater. Sci. 2014, 2, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jacobsen, M.T.; Pan, H.; Kopeček, J. Synthesis and Characterization of Enzymatically Degradable PEG-based Peptide-containing Hydrogels. Macromol. Biosci. 2010, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of Polymer Degradation and Erosion. Biomater. Silver Jubil. Compend. 1996, 17, 117–128. [Google Scholar]
- Shiba, K. Natural and Artificial Peptide Motifs: Their Origins and the Application of Motif-Programming. Chem. Soc. Rev. 2010, 39, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Garip, I.C.; Gulseren, G.; Tekinay, A.B.; Guler, M.O. Bioactive Supramolecular Peptide Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2014, 3, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, X. Self-Assemble Peptide Biomaterials and Their Biomedical Applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Kopesky, P.W.; Vanderploeg, E.J.; Kisiday, J.D.; Frisbie, D.D.; Sandy, J.D.; Grodzinsky, A.J. Controlled Delivery of Transforming Growth Factor Β1 by Self-Assembling Peptide Hydrogels Induces Chondrogenesis of Bone Marrow Stromal Cells and Modulates Smad2/3 Signaling. Tissue Eng. Part A 2011, 17, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Chen, S.; He, B.; Zhao, W.; Chen, X.; Jiang, D. Controlled Release of TGF-Beta 1 from RADA Self-Assembling Peptide Hydrogel Scaffolds. Drug Des. Devel. Ther. 2016, 10, 3043–3051. [Google Scholar] [CrossRef]
- Luo, H.; Xu, C.; Liu, Z.; Yang, L.; Hong, Y.; Liu, G.; Zhong, H.; Cai, X.; Lin, X.; Chen, X. Neural Differentiation of Bone Marrow Mesenchymal Stem Cells with Human Brain-derived Neurotrophic Factor Gene-modified in Functionalized Self-assembling Peptide Hydrogel in Vitro. J. Cell. Biochem. 2019, 120, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Kobayashi, H.; Tabata, Y. Enhanced Angiogenesis through Controlled Release of Basic Fibroblast Growth Factor from Peptide Amphiphile for Tissue Regeneration. Biomaterials 2006, 27, 5836–5844. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cao, Z.-Z.; Luo, X.-L.; Wang, X.-X.; Wang, S.-H.; Wang, D.-L. Fabrication and Characterization of a PDLSCs/BMP-2-PLGA-NP/RADA Peptide Hydrogel Composite for Bone Repair. J. Biomater. Tissue Eng. 2017, 7, 379–385. [Google Scholar]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone Regeneration Using an Alpha 2 Beta 1 Integrin-Specific Hydrogel as a BMP-2 Delivery Vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef]
- Impellitteri, N.A.; Toepke, M.W.; Levengood, S.K.L.; Murphy, W.L. Specific VEGF Sequestering and Release Using Peptide-Functionalized Hydrogel Microspheres. Biomaterials 2012, 33, 3475–3484. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, M.; Lin, B.; Zhao, X. Controlled Release of Fuctional Proteins IGF-1, AFGF and VEGF through Self-Assembling Peptide Nanofiber Hydrogel. J. Biomed. Eng. Shengwu Yixue Gongchengxue Zazhi 2011, 28, 310–313. [Google Scholar]
- Lu, J.; Yan, X.; Sun, X.; Shen, X.; Yin, H.; Wang, C.; Liu, Y.; Lu, C.; Fu, H.; Yang, S.; et al. Synergistic Effects of Dual-Presenting VEGF- and BDNF-Mimetic Peptide Epitopes from Self-Assembling Peptide Hydrogels on Peripheral Nerve Regeneration. Nanoscale 2019, 11, 19943–19958. [Google Scholar] [CrossRef]
- Li, R.; Pang, Z.; He, H.; Lee, S.; Qin, J.; Wu, J.; Pang, L.; Wang, J.; Yang, V.C. Drug Depot-Anchoring Hydrogel: A Self-Assembling Scaffold for Localized Drug Release and Enhanced Stem Cell Differentiation. J. Control. Release 2017, 261, 234–245. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lin, S.-P.; Nelli, S.R.; Zhan, F.-K.; Cheng, H.; Lai, T.-S.; Yeh, M.-Y.; Lin, H.-C.; Hung, S.-C. Self-Assembled Peptide-Based Hydrogels as Scaffolds for Proliferation and Multi-Differentiation of Mesenchymal Stem Cells. Macromol. Biosci. 2017, 17, 1600192. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. RGD and Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Dos Santos, B.P.; Garbay, B.; Fenelon, M.; Rosselin, M.; Garanger, E.; Lecommandoux, S.; Oliveira, H.; Amédée, J. Development of a Cell-Free and Growth Factor-Free Hydrogel Capable of Inducing Angiogenesis and Innervation after Subcutaneous Implantation. Acta Biomater. 2019, 99, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored Integrin-Extracellular Matrix Interactions to Direct Human Mesenchymal Stem Cell Differentiation. Stem Cells Dev. 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Satchell, S.C.; Wertheim, J.A.; Shah, R.N. Poly(Ethylene Glycol)-Crosslinked Gelatin Hydrogel Substrates with Conjugated Bioactive Peptides Influence Endothelial Cell Behavior. Biomaterials 2019, 201, 99–112. [Google Scholar] [CrossRef]
- Aye, S.-S.S.; Li, R.; Boyd-Moss, M.; Long, B.; Pavuluri, S.; Bruggeman, K.; Wang, Y.; Barrow, C.R.; Nisbet, D.R.; Williams, R.J. Scaffolds Formed via the Non-Equilibrium Supramolecular Assembly of the Synergistic ECM Peptides RGD and PHSRN Demonstrate Improved Cell Attachment in 3D. Polymers 2018, 10, 690. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wang, L.-C.; Su, Y.-T.; Wei, W.-Y.; Tsai, K.-J. Synthetic A5β1 Integrin Ligand PHSRN Is Proangiogenic and Neuroprotective in Cerebral Ischemic Stroke. Biomaterials 2018, 185, 142–154. [Google Scholar] [CrossRef]
- Caprini, A.; Silva, D.; Zanoni, I.; Cunha, C.; Volontè, C.; Vescovi, A.; Gelain, F. A Novel Bioactive Peptide: Assessing Its Activity over Murine Neural Stem Cells and Its Potential for Neural Tissue Engineering. New Biotechnol. 2013, 30, 552–562. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Zhang, S. Long-Term Three-Dimensional Neural Tissue Cultures in Functionalized Self-Assembling Peptide Hydrogels, Matrigel and Collagen I. Acta Biomater. 2013, 9, 5162–5169. [Google Scholar] [CrossRef]
- Li, X.; Cheng, S.; Wu, Y.; Ying, J.; Wang, C.; Wen, T.; Bai, X.; Ji, W.; Wang, D.; Ruan, D. Functional Self-Assembled Peptide Scaffold Inhibits Tumor Necrosis Factor-Alpha-Induced Inflammation and Apoptosis in Nucleus Pulposus Cells by Suppressing Nuclear Factor-ΚB Signaling. J. Biomed. Mater. Res. Part A 2018, 106, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Whitfield, C. KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesisof bacterial capsules. Proc. Natl. Acad. Sci. USA 2013, 110, 20753–20758. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.H.; Kim, S.H.; Jung, Y. Skin Regeneration with Self-Assembled Peptide Hydrogels Conjugated with Substance P in a Diabetic Rat Model. Tissue Eng. Part A 2017, 24, 21–33. [Google Scholar] [CrossRef]
- Ma, K.; Wu, Y.; Wang, B.; Yang, S.; Wei, Y.; Shao, Z. Effect of a Synthetic Link N Peptide Nanofiber Scaffold on the Matrix Deposition of Aggrecan and Type II Collagen in Rabbit Notochordal Cells. J. Mater. Sci. Mater. Med. 2013, 24, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, L.; Yu, Y.; Chen, Z.; Zhou, R.; Yuan, Z. Peptide REDV-Modified Polysaccharide Hydrogel with Endothelial Cell Selectivity for the Promotion of Angiogenesis. J. Biomed. Mater. Res. Part A 2015, 103, 1703–1712. [Google Scholar] [CrossRef]
- Liu, X.; Huai, J.; Endle, H.; Schlüter, L.; Fan, W.; Li, Y.; Richers, S.; Yurugi, H.; Rajalingam, K.; Ji, H.; et al. PRG-1 Regulates Synaptic Plasticity via Intracellular PP2A/Β1-Integrin Signaling. Dev. Cell 2016, 38, 275–290. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Zhang, K.; Zhang, C. Self-Assembling Peptides with HBMP7 Biological Activity Promote the Differentiation of ADSCs into Nucleus Pulposus-like Cells. J. Orthop. Surg. Res. 2022, 17, 197. [Google Scholar] [CrossRef]
- Green, P.M.; Ludbrook, S.B.; Miller, D.D.; Horgan, C.M.T.; Barry, S.T. Structural Elements of the Osteopontin SVVYGLR Motif Important for the Interaction with A4 Integrins. FEBS Lett. 2001, 503, 75–79. [Google Scholar] [CrossRef]
- Tanaka, S.; Yasuda, T.; Hamada, Y.; Kawaguchi, N.; Fujishita, Y.; Mori, S.; Yokoyama, Y.; Yamamoto, H.; Kogo, M. Synthetic Peptide SVVYGLR Upregulates Cell Motility and Facilitates Oral Mucosal Wound Healing. Peptides 2020, 134, 170405. [Google Scholar] [CrossRef] [PubMed]
- Qin, E.C.; Ahmed, S.T.; Sehgal, P.; Vu, V.H.; Kong, H.; Leckband, D.E. Comparative Effects of N-Cadherin Protein and Peptide Fragments on Mesenchymal Stem Cell Mechanotransduction and Paracrine Function. Biomaterials 2020, 239, 119846. [Google Scholar] [CrossRef]
- Castillo-Díaz, L.A.; Ruiz-Pacheco, J.A.; Elsawy, M.A.; Reyes-Martínez, J.E.; Enríquez-Rodríguez, A.I. Self-Assembling Peptides as an Emerging Platform for the Treatment of Metabolic Syndrome. Int. J. Nanomed. 2020, 15, 10349–10370. [Google Scholar] [CrossRef]
- Loo, Y.; Zhang, S.; Hauser, C.A.E. From Short Peptides to Nanofibers to Macromolecular Assemblies in Biomedicine. Biotechnol. Adv. 2012, 30, 593–603. [Google Scholar] [CrossRef]
- Pugliese, R.; Gelain, F. Peptidic Biomaterials: From Self-Assembling to Regenerative Medicine. Trends Biotechnol. 2017, 35, 145–158. [Google Scholar] [CrossRef]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer Self-Assembling Peptide Nanofiber Scaffolds for Adult Mouse Neural Stem Cell 3-Dimensional Cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef] [PubMed]
- Noh, I. Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1064, ISBN 9811304459. [Google Scholar]
- Zhao, Y.; Yokoi, H.; Tanaka, M.; Kinoshita, T.; Tan, T. Self-Assembled PH-Responsive Hydrogels Composed of the RATEA16 Peptide. Biomacromolecules 2008, 9, 1511–1518. [Google Scholar] [CrossRef]
- Arosio, P.; Owczarz, M.; Wu, H.; Butté, A.; Morbidelli, M. End-to-End Self-Assembly of RADA 16-I Nanofibrils in Aqueous Solutions. Biophys. J. 2012, 102, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Firipis, K.; Boyd-Moss, M.; Long, B.; Dekiwadia, C.; Hoskin, W.; Pirogova, E.; Nisbet, D.R.; Kapsa, R.M.I.; Quigley, A.F.; Williams, R.J. Tuneable Hybrid Hydrogels via Complementary Self-Assembly of a Bioactive Peptide with a Robust Polysaccharide. ACS Biomater. Sci. Eng. 2021, 7, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, Q. Experimental Study on Self-Assembly of KLD-12 Peptide Hydrogel and 3-D Culture of MSC Encapsulated within Hydrogel in Vitro. J. Huazhong Univ. Sci. Technol. Medical Sci. 2009, 29, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Verbraeken, B.; Lammens, M.; Van Rompaey, V.; Ahmed, M.; Szewczyk, K.; Hermans, C.; Menovsky, T. Efficacy and Histopathological Effects of Self-Assembling Peptides RADA16 and IEIK13 in Neurosurgical Hemostasis. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102485. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, S.; Miller, A.F.; Saiani, A. From Fibres to Networks Using Self-Assembling Peptides. Faraday Discuss. 2013, 166, 195–207. [Google Scholar] [CrossRef]
- Eilken, H.M.; Adams, R.H. Dynamics of Endothelial Cell Behavior in Sprouting Angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Lamm, M.S.; Haines-Butterick, L.A.; Pochan, D.J.; Schneider, J.P. Tuning the PH Responsiveness of β-Hairpin Peptide Folding, Self-Assembly, and Hydrogel Material Formation. Biomacromolecules 2009, 10, 2619–2625. [Google Scholar] [CrossRef]
- Li, W.W.; Talcott, K.E.; Zhai, A.W.; Kruger, E.A.; Li, V.W. The Role of Therapeutic Angiogenesis in Tissue Repair and Regeneration. Adv. Skin Wound Care 2005, 18, 491–492. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in Tissue Engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in Hydrogel-Based Vascularized Tissues for Tissue Repair and Drug Screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Yi, X.; Sun, N. Application of Mesenchymal Stem Cells Combined with Nano-Polypeptide Hydrogel in Tissue Engineering Blood Vessel. Regen. Ther. 2022, 21, 277–281. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Qi, Y.; Zhao, Y.; Nie, G.; Wang, X.; Zheng, S. Self-Assembled Peptide Hydrogel Scaffolds with VEGF and BMP-2 Enhanced in Vitro Angiogenesis and Osteogenesis. Oral Dis. 2022, 28, 723–733. [Google Scholar] [CrossRef]
- Yang, S.; Graham, J.; Kahn, J.W.; Schwartz, E.A.; Gerritsen, M.E. Functional Roles for PECAM-1 (CD31) and VE-Cadherin (CD144. in Tube Assembly and Lumen Formation in Three-Dimensional Collagen Gels. Am. J. Pathol. 1999, 155, 887–895. [Google Scholar] [CrossRef]
- Jian, W.-H.; Wang, H.-C.; Kuan, C.-H.; Chen, M.-H.; Wu, H.-C.; Sun, J.-S.; Wang, T.-W. Glycosaminoglycan-Based Hybrid Hydrogel Encapsulated with Polyelectrolyte Complex Nanoparticles for Endogenous Stem Cell Regulation in Central Nervous System Regeneration. Biomaterials 2018, 174, 17–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Zhao, M.; Wang, C.; Li, L.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Zhang, J.; et al. Injectable Extracellular Vesicle-Released Self-Assembling Peptide Nanofiber Hydrogel as an Enhanced Cell-Free Therapy for Tissue Regeneration. J. Control. Release 2019, 316, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Horii, A.; Wang, X.; Qiao, L.; Zhang, S.; Cui, F.-Z. In Vivo Studies on Angiogenic Activity of Two Designer Self-Assembling Peptide Scaffold Hydrogels in the Chicken Embryo Chorioallantoic Membrane. Nanoscale 2012, 4, 2720–2727. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.-Y.; Wang, X.-M.; Gao, K.; Gao, Y.-Z.; Cao, J.; Zhang, Z.-L.; Lei, J.; Jin, Z.-Y.; Wang, Y.-N. Image-Guided Stem Cells with Functionalized Self-Assembling Peptide Nanofibers for Treatment of Acute Myocardial Infarction in a Mouse Model. Am. J. Transl. Res. 2017, 9, 3723–3731. [Google Scholar]
- Webber, M.J.; Tongers, J.; Newcomb, C.J.; Marquardt, K.-T.; Bauersachs, J.; Losordo, D.W.; Stupp, S.I. Supramolecular Nanostructures That Mimic VEGF as a Strategy for Ischemic Tissue Repair. Proc. Natl. Acad. Sci. USA 2011, 108, 13438–13443. [Google Scholar] [CrossRef]
- Wang, X.; Horii, A.; Zhang, S. Designer Functionalized Self-Assembling Peptide Nanofiber Scaffolds for Growth, Migration, and Tubulogenesis of Human Umbilical Vein Endothelial Cells. Soft Matter 2008, 4, 2388–2395. [Google Scholar] [CrossRef]
- Muylaert, D.E.P.; Fledderus, J.O.; Bouten, C.V.C.; Dankers, P.Y.W.; Verhaar, M.C. Combining Tissue Repair and Tissue Engineering; Bioactivating Implantable Cell-Free Vascular Scaffolds. Heart 2014, 100, 1825–1830. [Google Scholar] [CrossRef]
- Ai, J.; Kiasat-Dolatabadi, A.; Ebrahimi-Barough, S.; Ai, A.; Lotfibakhshaiesh, N.; Norouzi-Javidan, A.; Saberi, H.; Arjmand, B.; Aghayan, H.R. Polymeric Scaffolds in Neural Tissue Engineering: A Review. Arch. Neurosci. 2014, 1, 15–20. [Google Scholar] [CrossRef]
- He, B.; Yuan, X.; Jiang, D. Molecular Self-Assembly Guides the Fabrication of Peptide Nanofiber Scaffolds for Nerve Repair. RSC Adv. 2014, 4, 23610–23621. [Google Scholar] [CrossRef]
- Koss, K.M.; Unsworth, L.D. Neural Tissue Engineering: Bioresponsive Nanoscaffolds Using Engineered Self-Assembling Peptides. Acta Biomater. 2016, 44, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Long, Y.; Dong, X.; Liu, K.; Wei, W.; Chen, Y.; Qiu, T.; Dai, H. Improved Functional Recovery of Rat Transected Spinal Cord by Peptide-Grafted PNIPAM Based Hydrogel. Colloids Surf. B Biointerfaces 2022, 210, 112220. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, T.M.; Baron-Heeris, D.; Houwers, I.G.J.; Keenan, R.; Williams, R.J.; Nisbet, D.R.; Harvey, A.R.; Hodgetts, S.I. Peptide Hydrogel Scaffold for Mesenchymal Precursor Cells Implanted to Injured Adult Rat Spinal Cord. Tissue Eng. Part A 2020, 27, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Hivare, P.; Gangrade, A.; Swarup, G.; Bhavsar, K.; Singh, A.; Gupta, R.; Thareja, P.; Gupta, S.; Bhatia, D. Peptide Functionalized DNA Hydrogel Enhances Neuroblastoma Cell Growth and Differentiation. Nanoscale 2022, 14, 8611–8620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chai, Y.; Zhao, H.; Yang, S.; Liu, W.; Yang, Z.; Ye, W.; Wang, C.; Gao, X.; Kong, X.; et al. Crosstalk between PC12 Cells and Endothelial Cells in an Artificial Neurovascular Niche Constructed by a Dual-Functionalized Self-Assembling Peptide Nanofiber Hydrogel. Nano Res. 2022, 15, 1433–1445. [Google Scholar] [CrossRef]
- Yaguchi, A.; Oshikawa, M.; Watanabe, G.; Hiramatsu, H.; Uchida, N.; Hara, C.; Kaneko, N.; Sawamoto, K.; Muraoka, T.; Ajioka, I. Efficient Protein Incorporation and Release by a Jigsaw-Shaped Self-Assembling Peptide Hydrogel for Injured Brain Regeneration. Nat. Commun. 2021, 12, 6623. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W.; Wu, X.; Zhang, N.; Zhang, Y.; Ouyang, S.; Song, X.; Fang, X.; Seeram, R.; Xue, W.; et al. Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration. ACS Appl. Mater. Interfaces 2016, 8, 2348–2359. [Google Scholar] [CrossRef]
- Berns, E.J.; Sur, S.; Pan, L.; Goldberger, J.E.; Suresh, S.; Zhang, S.; Kessler, J.A.; Stupp, S.I. Aligned Neurite Outgrowth and Directed Cell Migration in Self-Assembled Monodomain Gels. Biomaterials 2014, 35, 185–195. [Google Scholar] [CrossRef]
- Li, A.; Hokugo, A.; Yalom, A.; Berns, E.J.; Stephanopoulos, N.; McClendon, M.T.; Segovia, L.A.; Spigelman, I.; Stupp, S.I.; Jarrahy, R. A Bioengineered Peripheral Nerve Construct Using Aligned Peptide Amphiphile Nanofibers. Biomaterials 2014, 35, 8780–8790. [Google Scholar] [CrossRef]
- Wang, T.-W.; Chang, K.-C.; Chen, L.-H.; Liao, S.-Y.; Yeh, C.-W.; Chuang, Y.-J. Effects of an Injectable Functionalized Self-Assembling Nanopeptide Hydrogel on Angiogenesis and Neurogenesis for Regeneration of the Central Nervous System. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Williams, D.F. Neural Tissue Engineering Options for Peripheral Nerve Regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Zhan, X.; Gao, M.; Jiang, Y.; Zhang, W.; Wong, W.M.; Yuan, Q.; Su, H.; Kang, X.; Dai, X.; Zhang, W. Nanofiber Scaffolds Facilitate Functional Regeneration of Peripheral Nerve Injury. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, H.-K.; Kim, N.H.; Park, J.K.; Kang, E.S.; Kim, Y.-T.; Heo, C.; Lee, O.-S.; Kim, S.-G.; Yun, W.S. Supramolecular Peptide Hydrogel-Based Soft Neural Interface Augments Brain Signals through a Three-Dimensional Electrical Network. ACS Nano 2020, 14, 664–675. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Minas, T. The Quality of Healing: Articular Cartilage. Wound Repair Regen. 2014, 22, 30–38. [Google Scholar] [CrossRef]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Elsevier: Amsterdam, The Netherlands, 1965; pp. 97–166. [Google Scholar]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced Hydrogels for the Repair of Cartilage Defects and Regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Yang, Z.; Cao, F.; Li, H.; Zhao, T.; Zhang, H.; Zhang, Z.; Yang, S.; Zhu, J.; Liu, Z.; et al. Articular Cartilage Reconstruction with TGF-Β1-Simulating Self-Assembling Peptide Hydrogel-Based Composite Scaffold. Acta Biomater. 2022, 146, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, G.M.; Liesbeny, P.; Barrett, M.; Zlotnick, H.; Frank, E.; Grodzinsky, A.J.; Frisbie, D.D. Microfracture Augmentation With Trypsin Pretreatment and Growth Factor–Functionalized Self-Assembling Peptide Hydrogel Scaffold in an Equine Model. Am. J. Sports Med. 2021, 49, 2498–2508. [Google Scholar] [CrossRef]
- Thomas, J.; Gupta, N.; Joseph, J.P.; Chopra, V.; Pal, A.; Ghosh, D. Mechanical Integrity in a Dynamic Interpenetrating Hydrogel Network of Supramolecular Peptide–Polysaccharide Supports Enhanced Chondrogenesis. ACS Biomater. Sci. Eng. 2021, 7, 5798–5809. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Yang, H.; Zhao, C.; Pan, J. Research Progress of Self-Assembling Peptide Hydrogels in Repairing Cartilage Defects. Front. Mater. 2022, 9, 611. [Google Scholar] [CrossRef]
- Huang, B.; Li, P.; Chen, M.; Peng, L.; Luo, X.; Tian, G.; Wang, H.; Wu, L.; Tian, Q.; Li, H.; et al. Hydrogel Composite Scaffolds Achieve Recruitment and Chondrogenesis in Cartilage Tissue Engineering Applications. J. Nanobiotechnol. 2022, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.; Lafont, J.E.; Buffier, M.; Verset, M.; Cohendet, A.; Contamin, H.; Confais, J.; Sankar, S.; Rioult, M.; Perrier-Groult, E.; et al. Repair of Full-Thickness Articular Cartilage Defects Using IEIK13 Self-Assembling Peptide Hydrogel in a Non-Human Primate Model. Sci. Rep. 2021, 11, 4560. [Google Scholar] [CrossRef]
- Kisiday, J.D.; Jin, M.; DiMicco, M.A.; Kurz, B.; Grodzinsky, A.J. Effects of Dynamic Compressive Loading on Chondrocyte Biosynthesis in Self-Assembling Peptide Scaffolds. J. Biomech. 2004, 37, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-Assembled N-Cadherin Mimetic Peptide Hydrogels Promote the Chondrogenesis of Mesenchymal Stem Cells through Inhibition of Canonical Wnt/β-Catenin Signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Florine, E.M.; Miller, R.E.; Liebesny, P.H.; Mroszczyk, K.A.; Lee, R.T.; Patwari, P.; Grodzinsky, A.J. Delivering Heparin-Binding Insulin-like Growth Factor 1 with Self-Assembling Peptide Hydrogels. Tissue Eng. Part A 2015, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Liebesny, P.H.; Mroszczyk, K.; Zlotnick, H.; Hung, H.-H.; Frank, E.; Kurz, B.; Zanotto, G.; Frisbie, D.; Grodzinsky, A.J. Enzyme Pretreatment plus Locally Delivered HB-IGF-1 Stimulate Integrative Cartilage Repair in Vitro. Tissue Eng. Part A 2019, 25, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.-Y.; Yin, W.-N.; Fan, J.-X.; Zhuo, R.-X.; Zhang, X.-Z. A Novel Function of BMHP1 and CBMHP1 Peptides to Induce the Osteogenic Differentiation of Mesenchymal Stem Cells. Biomater. Sci. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Nowakowski, G.S.; Dooner, M.S.; Valinski, H.M.; Mihaliak, A.M.; Quesenberry, P.J.; Becker, P.S. A Specific Heptapeptide from a Phage Display Peptide Library Homes to Bone Marrow and Binds to Primitive Hematopoietic Stem Cells. Stem Cells 2004, 22, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shen, X.; Sun, X.; Yin, H.; Yang, S.; Lu, C.; Wang, Y.; Liu, Y.; Huang, Y.; Yang, Z. Increased Recruitment of Endogenous Stem Cells and Chondrogenic Differentiation by a Composite Scaffold Containing Bone Marrow Homing Peptide for Cartilage Regeneration. Theranostics 2018, 8, 5039. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous Bone Graft: Properties and Techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Li, R.; Zhou, C.; Chen, J.; Luo, H.; Li, R.; Chen, D.; Zou, X.; Wang, W. Synergistic Osteogenic and Angiogenic Effects of KP and QK Peptides Incorporated with an Injectable and Self-Healing Hydrogel for Efficient Bone Regeneration. Bioact. Mater. 2022, 18, 267–283. [Google Scholar] [CrossRef]
- Stüdle, C.; Vallmajó-Martín, Q.; Haumer, A.; Guerrero, J.; Centola, M.; Mehrkens, A.; Schaefer, D.J.; Ehrbar, M.; Barbero, A.; Martin, I. Spatially Confined Induction of Endochondral Ossification by Functionalized Hydrogels for Ectopic Engineering of Osteochondral Tissues. Biomaterials 2018, 171, 219–229. [Google Scholar] [CrossRef]
- Ji, W.; Álvarez, Z.; Edelbrock, A.N.; Sato, K.; Stupp, S.I. Bioactive Nanofibers Induce Neural Transdifferentiation of Human Bone Marrow Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 41046–41055. [Google Scholar] [CrossRef]
- Panek, M.; Antunović, M.; Pribolšan, L.; Ivković, A.; Gotić, M.; Vukasović, A.; Caput Mihalić, K.; Pušić, M.; Jurkin, T.; Marijanović, I. Bone Tissue Engineering in a Perfusion Bioreactor Using Dexamethasone-Loaded Peptide Hydrogel. Materials 2019, 12, 919. [Google Scholar] [CrossRef]
- Misawa, H.; Kobayashi, N.; Soto-Gutierrez, A.; Chen, Y.; Yoshida, A.; Rivas-Carrillo, J.D.; Navarro-Alvarez, N.; Tanaka, K.; Miki, A.; Takei, J. PuraMatrixTM Facilitates Bone Regeneration in Bone Defects of Calvaria in Mice. Cell Transplant. 2006, 15, 903–910. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ou, Y.; Chen, S.; Zhao, W.; Zhou, A.; Zhao, J.; Li, H.; Jiang, D.; Zhu, Y. Designer BFGF-Incorporated d-Form Self-Assembly Peptide Nanofiber Scaffolds to Promote Bone Repair. Mater. Sci. Eng. C 2017, 74, 451–458. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ou, Y.; Zhou, A.; Chen, S.; Zhao, W.; Zhao, J.; Li, H.; Zhu, Y.; Zhao, Z.; Jiang, D. Functionalized D-Form Self-Assembling Peptide Hydrogels for Bone Regeneration. Drug Des. Devel. Ther. 2016, 10, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, L.R.; Hofmeister, A.M.; Hruska, K.A. Differential Growth Factor Control of Bone Formation through Osteoprogenitor Differentiation. Bone 2004, 34, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Luong, L.N.; Ramaswamy, J.; Kohn, D.H. Effects of Osteogenic Growth Factors on Bone Marrow Stromal Cell Differentiation in a Mineral-Based Delivery System. Biomaterials 2012, 33, 283–294. [Google Scholar] [CrossRef]
- Castillo Diaz, L.A.; Elsawy, M.; Saiani, A.; Gough, J.E.; Miller, A.F. Osteogenic Differentiation of Human Mesenchymal Stem Cells Promotes Mineralization within a Biodegradable Peptide Hydrogel. J. Tissue Eng. 2016, 7, 2041731416649789. [Google Scholar] [CrossRef]
- Ghosh, M.; Halperin-Sternfeld, M.; Grinberg, I.; Adler-Abramovich, L. Injectable Alginate-Peptide Composite Hydrogel as a Scaffold for Bone Tissue Regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.P.; Gonzalez-Fernandez, T.; Lin, J.B.; Campbell, T.; Lee, Y.B.; Panitch, A.; Alsberg, E.; Leach, J.K. Multi-Peptide Presentation and Hydrogel Mechanics Jointly Enhance Therapeutic Duo-Potential of Entrapped Stromal Cells. Biomaterials 2020, 245, 119973. [Google Scholar] [CrossRef] [PubMed]



| Peptide Motif | Bioactivities | Origin | Integrin(s), Cell/Proteins Binded | Ref. |
|---|---|---|---|---|
| RGD | Cell adhesion enhancement | ECM proteins (Fibronectin, collagen, vitronectin) | α5β1, α8β1, αvβ1, αvβ3, αvβ3, αvβ5, αvβ6, αvβ8, αIIbβ3 | [105,106] |
| IKVAV | Cell growth enhancing along with neural differentiation promoting and nerve regeneration | Laminin (α1 chain) | α3β1, α4β1, α6β1 | [107,108] |
| YIGSR | Enhancement of cell adhesion and migration | Laminin (β1 chain) | α3β1, α4β1, α6β1 | [109] |
| PHSRN | Cell adhesion enhancement | ECM proteins (same as RGD) | α5β1 | [110,111] |
| KLPGWSG | Neuronal differentiation enhancement | Stem cells proteins | Neural stem cells (NSCs) | [112] |
| PFSSTKT | Neural cell proliferation and differentiation; human adipose stem cell homing promotion | Bone marrow homing | Nerve and spinal cord | [113] |
| KPSS | Promotion of accumulation of ECM; induction of bone marrow MSCs migration | Morphogenic proteins derived from bone | β-Kdo-transferases | [114,115] |
| Substance P (RPKPQQFFGL) | Cartilage regeneration improvement; wound healing promotion | Neuropeptides (endogenous type) | β2 | [116] |
| Link N (DHLSDNYTLDHDRAIH) | Stabilization of proteoglycan aggregates | Derived from link protein exists in disk tissues | N/A | [117] |
| REDV | Induction of angiogenesis; enhancement of endothelial cell adhesion | Fibronectin | α4β1 | [118] |
| KLT | Acts as an analog of VEGF. | VEGF mimetic peptide | VEGF receptors | [103] |
| PRG | Possesses homology to the lipid phosphate phosphatases (LPPs) in nervous system | Integral membrane protein | β1 (Protein phosphatase 2A, PP2A) | [119] |
| SNVI | Displaying bone morphogenic peptide-7 (BMP-7) bioactivity | Bioactive sequence of BMP-7 | N/A | [120] |
| SVVYGLR | Angiogenesis, production of collagen III, and fibroblast differentiation into myofibroblasts | Osteopontin protein | α4β1, α9β1, α4β7 | [121,122] |
| HAVDI | Cell adhesion | N-Cadherin (calcium-dependent cell-cell adhesion) protein | Extracellular domain 1 (ECD1) of N-cadherin protein | [123] |
| QLK | Covalent binding to transglutaminase to protect GFs from proteolytic | N/A | N/A | [124] |
| LRK | Joining angiogenic inducers (HGF, and VEGF) | N/A | Kinases in plants | [124] |
| Self-Assembling Peptides | Abbreviation | Self-Assembly Mechanism | Higher-Order Structure | Application and Features |
|---|---|---|---|---|
| CH3CO-RATARAEARATARAEA-CONH2 | RATEA16 | Hydrophobic interactions, intermolecular hydrogen bonds, electrostatic interactions | β-sheet nanofibers | Use in controlled release of therapeutics through pH-response and in diffusion release [129] |
| CH3CO-(RADA)4-CONH2 | RADA16 | Hydrophobic interactions, intermolecular hydrogen bonds, electrostatic interactions | Antiparallel β-sheet structure | Stable fibril units with high water content for making three-dimensional scaffolds for cell culture [130] |
| Fmoc-DIKVAV | - | π-π, and electrostatic interactions, hydrogen bonds | β-sheet structure | Petide-based biomaterial combined with polysaccharides to afford a wide range of achievable physico-chemical properties [131] |
| CH3CO-KLDLKLDLKLDL-CONH2 | KLD-12 | Electrostatic interactions, hydrogen bonds | β-sheet structure | Protein-based nanostructured templates with enhanced versatility for tissue engineering of bones and teeth [132] |
| CH3CO-IEIKIEIKIEIKI-CONH2 | IEIK-13 | Hydrophobic and electrostatic interactions, hydrogen bonds | β-sheet structure | Hemostatic potential and safety of RADA16 and IEIK13 for hemostasis in the rat brain [133] |
| FEFEFKFK | - | π-π, and electrostatic interactions, hydrogen bonds | β-sheet structure | The self-assembly and gelation properties of FEFEFKFK depend on pH media [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binaymotlagh, R.; Chronopoulou, L.; Palocci, C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. J. Funct. Biomater. 2023, 14, 233. https://doi.org/10.3390/jfb14040233
Binaymotlagh R, Chronopoulou L, Palocci C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. Journal of Functional Biomaterials. 2023; 14(4):233. https://doi.org/10.3390/jfb14040233
Chicago/Turabian StyleBinaymotlagh, Roya, Laura Chronopoulou, and Cleofe Palocci. 2023. "Peptide-Based Hydrogels: Template Materials for Tissue Engineering" Journal of Functional Biomaterials 14, no. 4: 233. https://doi.org/10.3390/jfb14040233





