Zinc Oxide-Based Nanomaterials for Microbiostatic Activities: A Review
Abstract
1. Introduction
2. Brief History and Properties of ZnO
3. Challenges of Working with Microorganisms
3.1. Culturing and Containment Risks
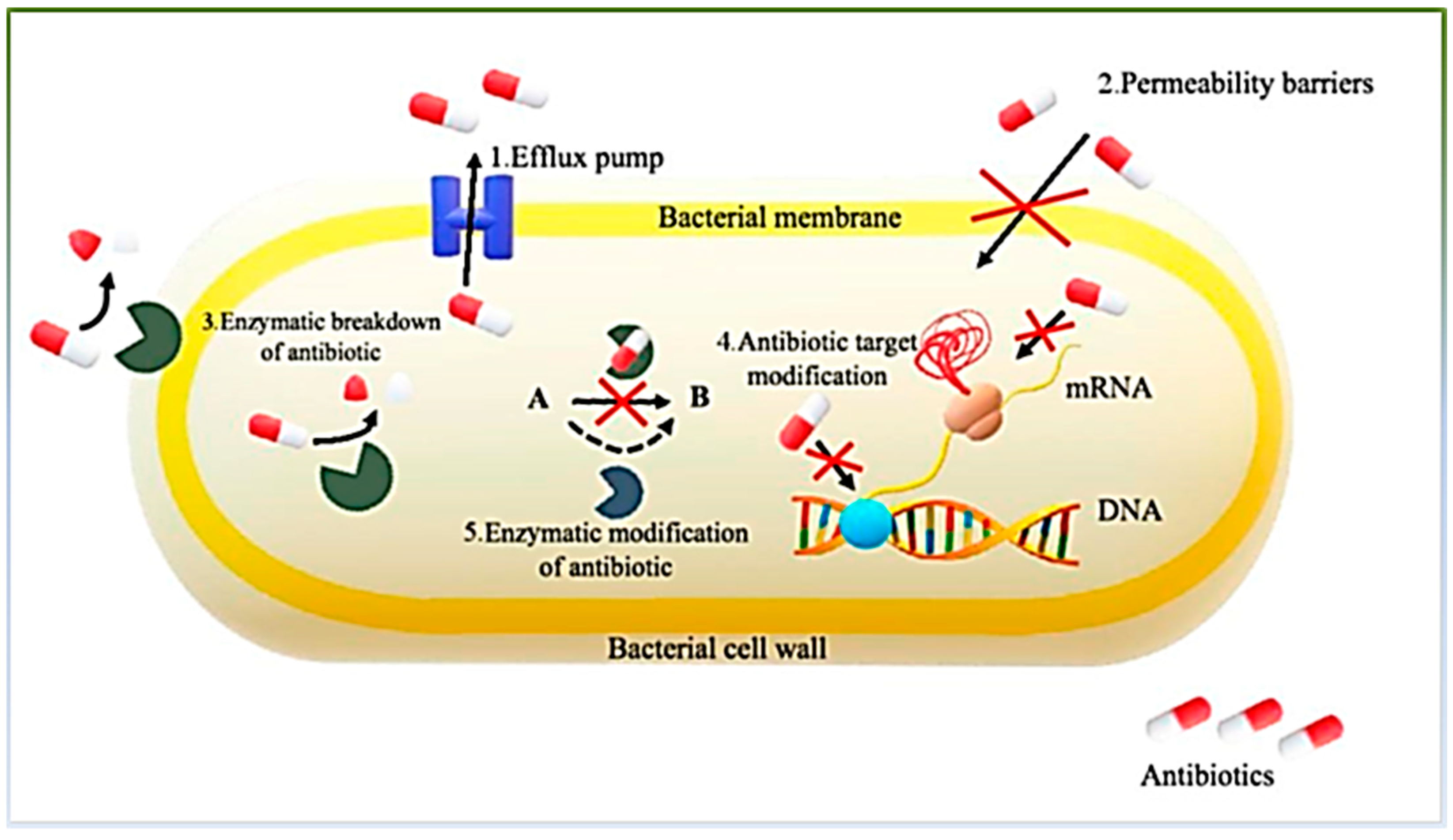
3.2. Development of Resistance
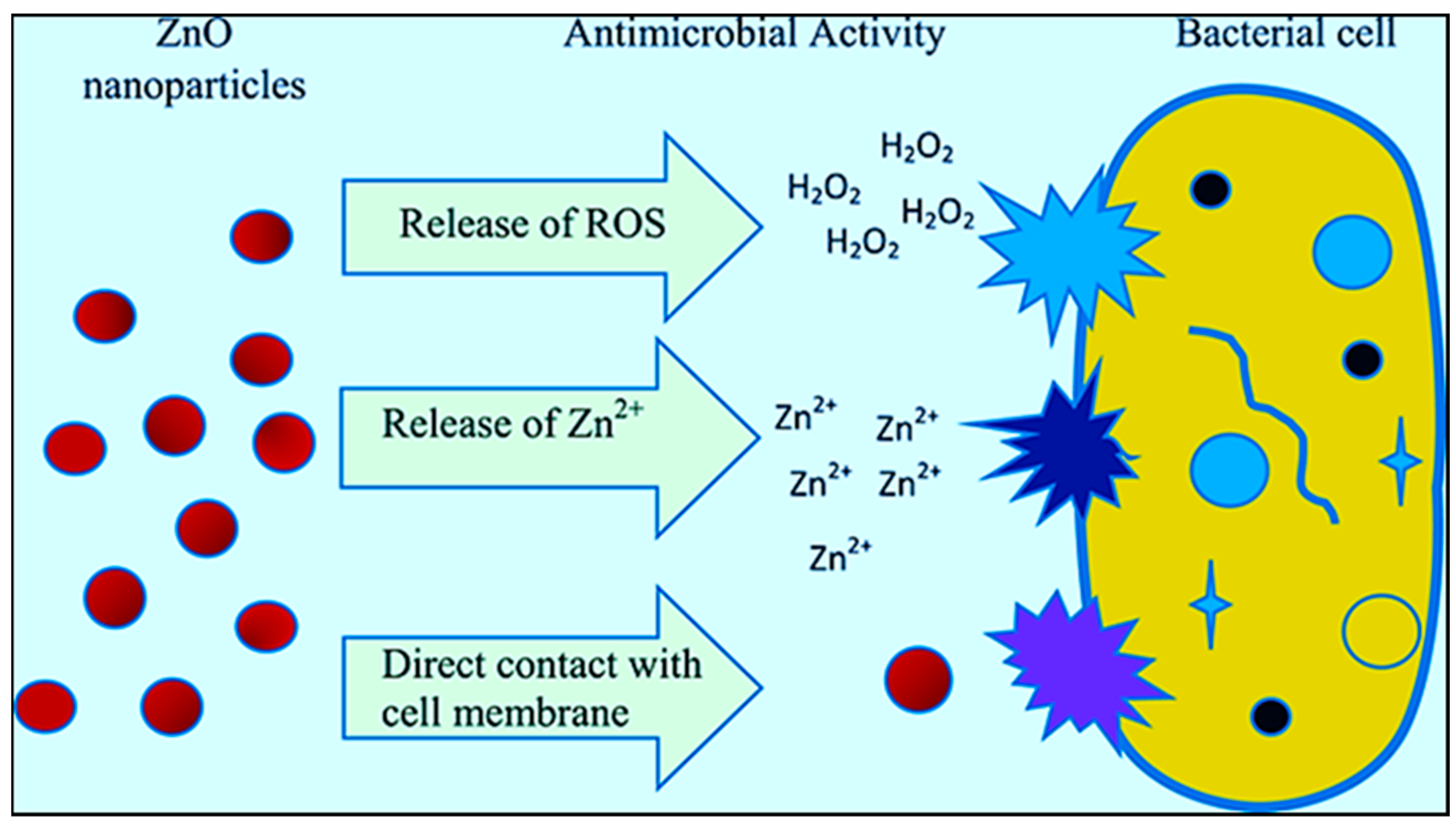
4. Antimicrobial Activity of ZnO
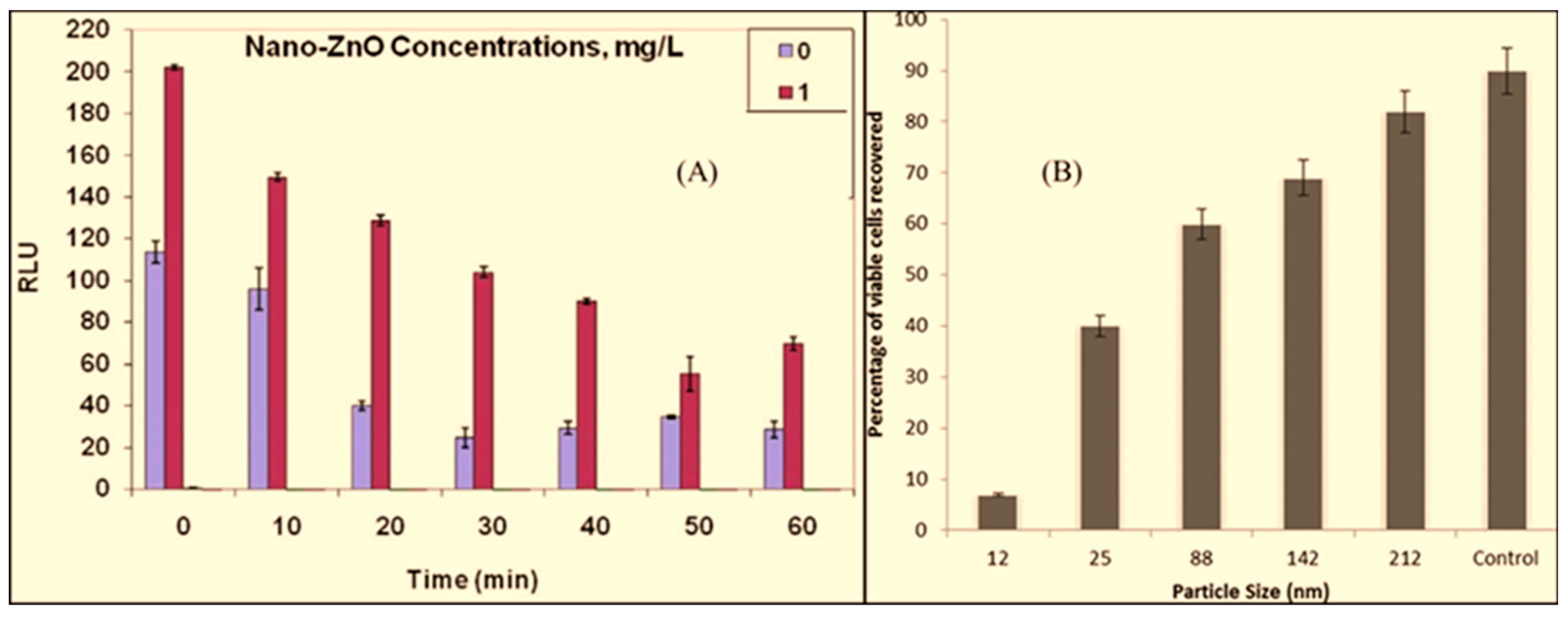
4.1. Time Course of ZnO for Antimicrobial Activity
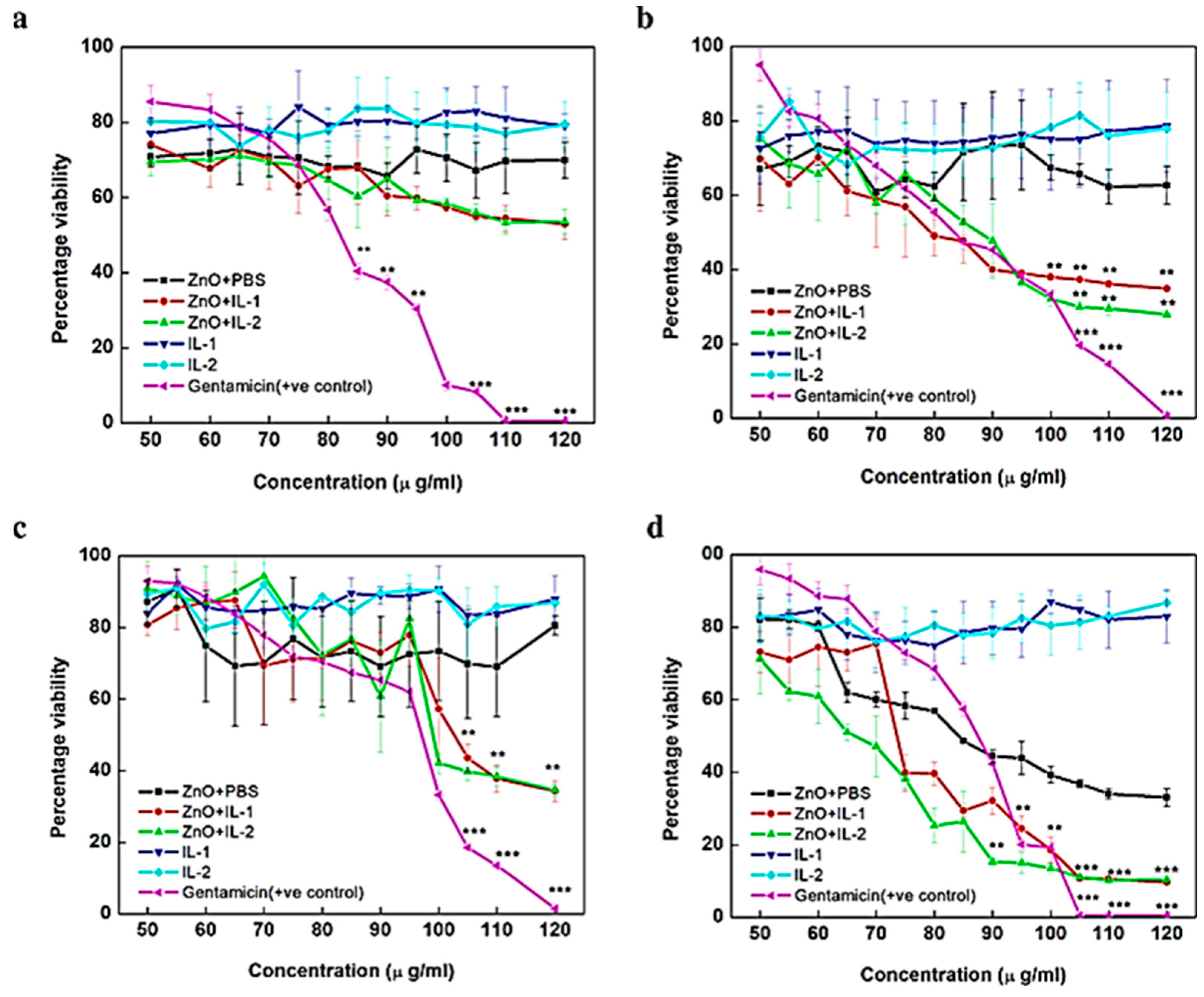
4.2. ZnO NP-Based Materials
| Material | Target Microbe | Time, Temp | Synthesis Method | Note | Ref. |
|---|---|---|---|---|---|
| ZnO NPs | S. mutans | 24 h, 37 °C | Precipitation–diffusion | The inhibition ability was determined using the liquid dilution method. The minimum inhibition concentration (MIC) was found to be 500 ± 306.18 μg/mL. Size reduction in the NPs increases the contact surface and improves the performance. NP size = 125 nm | [132] |
| E. coli, S. aureus P. aeruginosa, E. faecalis, P. aeruginosa, A. baumannii | 24 h, 37 °C | Plant-mediated biosynthesis | Aristolochia indica leaf was served as a source for the NP synthesis. The MIC was determined via Macro-broth dilution. With a size of 22.5 nm and zeta potential of −21.9 ± 1 mV exhibited, the MIC increased from 25 to 200 μg/mL. NP size = 50–70 nm. | [139] | |
| Pseudomonas putida KT2440 | 24 h, 28 °C | N/A | Commercial ZnO NPs were used. Assayed via dilution plating on salt-free Luria Broth. Bulk equivalents of these NPs showed no inhibitory activity, indicating that particle size was determinant in activity. Zn ions and nano-ZnO were effective bacteriostatic agents, unlike the bulk-ZnO in 10 mg Zn/L. NP size < 100 nm. | [133] | |
| S. pyogenes | 24 h, 37 °C | Commercial product | Shape: Spherical with rod mixture. The turbidity method was used to determine the bacteriostatic effect of ZnO NPs. The turbidity of the bacterial suspension treated with 10, 50, and 100 μg/mL of ZnO was reduced by 35.75 ± 5.28, 70.29 ± 6.86, and 81.18 ± 5.70%, respectively, within 24 h. Binding ZnO to bacterial cell wall: Electrostatic force between Zn+ and anionic groups on bacterial cell wall. NP size = 31.4–66.3 nm. | [134] | |
| S. epidermidis, S. pyogenes, E. faecalis, B. cereus, P. vulgaris, S. typhimurium S. flexinari, P. alcaligenes, E. aerogenes | Room temperature and solvothermal | Methicillin resistant and sensitive strains were tested. A 4–7 mM colloidal suspension of ZnO NPs inhibited > 95% of growth for most of the microorganisms, except S. typhimurium, as its growth was inhibited by 50% under ambient lighting conditions. The release of free Zn2+ ions from ZnO had minimal effect on the performance. Bacteriostatic activity of ZnO NPs: through the accumulation of NPs in the cytoplasm or on the outer membranes. NP size = 12–307 nm. | [135] | ||
| M. tuberculosis | 24 h, 37 °C | Chemical precipitation | A Microplate Alamar Blue Assay (MABA) was used to determine the MIC of ZnO; 1 μg/mL of ZnO was the lowest concentration inhibiting the growth of the bacteria. ZnO NPs did not show bactericidal effect against M. tuberculosis. NP size = 9.3 ± 3.9 nm. | [136] | |
| S. epidermidis, S. pyogenes, S. marcescens, K. pneumoniae, P. aeruginosa | 24 h, 37 °C | Facile microplasma | Shape: Nanosheets (40–50 nm size), nanodrums, and nanoneedles. ZnO used: 1 mg/mL. The antibacterial activity of the ZnO nanostructures was determined using the Agar well diffusion method. A maximum inhibition zone of 21 mm was recorded for S. marcescens. Growth inhibition was higher in ZnO dissolved in dimethyl sulfoxide than that of dry ZnO powder. Mechanism: release of Zn2+ ions and a higher surface area-to-volume ratio. | [137] |
| Material | Target Microbe | Time, Temp | Synthesis Method | Note | Ref. |
|---|---|---|---|---|---|
| ZnO-EG a | E. coli and S. aureus | 24 h, 37 °C | Electro-spinning | Antimicrobial activity was performed using the disc diffusion method; 1, 1.5, and 2 wt.% ZnO NPs showed inhibitory diameters of 0.69, 1.30, and 1.61 mm/mg against E. coli and 0.75, 1.17, and 1.33 mm/mg against S. aureus, respectively. Efficiency was enhanced via UV irradiation. Excellent hydrophobicity, water stability, and antibacterial performance. NP size = 30 nm. | [144] |
| ZnO-GPTMS b | E. coli and S. aureus | 24 h, 37 °C | Sol−gel method and surface modification | The preparation of the bacterial inoculum was carried out using the McFarland scale. The reaction time of the ZnO NP synthesis did not make changes in size or antibacterial activity. Antibacterial results with different treatments were better for S. aureus compared to E. coli. Parameters such as dyeing, softening, and number of washes did not affect the efficiency. NP size = 5 nm. | [145] |
| ZnO-L-RMGIC c | Cariogenic | 24 h, 37 °C | Probe sonication | NP size ranged from 10 to 150 nm. Zinc ion was released from the NPs. The highest Zn ion releases over 1, 14, and 28 days were 12.59, 13.5, and 14.1 mg/L, respectively. After 24 h, the highest and the lowest bacterial count were 2.79 × 104 and 1.5 × 103 cfu/mL, respectively. | [146] |
| ZnO-ILs d | E. coli, B. subtilis, K. pneumoniae, and S. epidermidis | 24 h, 37 °C | Precipitation and dispersion | ZnO NPs (60 nm) were dispersed in choline acetate and 1-butyl-3-methylimidazolium chloride to avoid aggregation. The ionic liquids served for dispersion and as an antibacterial agent. ZnO NPs exhibited the highest antibacterial activity in 1-butyl-3-methylimidazolium against S. epidermidis. The production of ROS increases efficiency. | [147] |
| ZnO-NFC e | S. aureus, B. cereus, and K. pneumoniae | 20/24 h, 30/37 °C | Electrostatic assembly | The AATCC 100 standard test method was used to assess the antimicrobials activity. In total, 4 mg of the composite suspension (100 μL of nutrient broth) or 1.5 cm by 1.5 cm specimens of a coated paper sheet (100 μL of a solution of 12.5% diluted nutrient broth) were used. The test was performed in the presence and absence of light. NP size = 40.7 ± 14.5 nm. | [158] |
| ZnO-PVP/PVA/PGA f | E. coli and S. aureus | 24 h, 37 °C | Hydrothermal | ZnO NPs were stabilized using PVP, PVA and PGA polymers; 2.1 × 107 CFU/mL and 4.1 × 107 CFU/mL of S. aureus and E. coli, respectively, were used. Cell reduction activity of ZnO NP was performed using the colony count method in liquid. NP size = 30–100 nm. | [159] |
| ZnO-PDDA/RMGM g | E. coli | 48 h, 37 °C | Hydrothermal | The standard plate counting method was used for the antimicrobial effect. About 107 CFU/mL of E. coli was used. It is reusable with a rate of over 98%. NP size = 16.95 nm. | [160] |
| ZnO, ZnO-PVA h | E. coli and S. aureus | 24 h, 37 °C | Solvothermal | Antimicrobial activity was analyzed using an agarose diffusion assay. The density of bacterial cells in the liquid cultures was measured at a 600 nm wavelength. Cell suspension for antibacterial activity was 1 × 105 colony-forming units (CFUs) mL−1. The MIC was determined using a modified resazurin method. In total, 100 μL of nutrient broth or sterile saline was used on the plates, and a 5 × 106 CFU/mL bacterial suspension was added. ZnO-PVA was used for anti-infection (female mice, 5 × 106 CFU/mL E. coli in 50 μL of sterile phosphate-buffered saline). NP size = 4 nm. | [161] |
| ZnO/SBA i | E. coli and S. aureus | 24–72 h, 37 °C | Co-condensation/impregnation/calcination | In total, 2 mg of ZnO/SBA powder was added to 20 mL of LB agar, and 100 μL of each of S. aureus (105 CFU mL−1) and E. coli (105 CFU mL−1) were used. Photocatalytic antibacterial activity. SBA/ZnO showed a bacteriostatic effect with inhibition rates of 32.61 and 38.33% against E. coli and S. aureus, respectively. NP size = 40 nm | [162] |
| ZnO/Ag-Haw j | E. coli and S. aureus | 24 h, 37 °C | Template-oriented precipitation/sol–gel method | ZnO/Ag-HAw was sintered at 600 C for 10 h before use. ZnO/Ag-HAw showed non-cytotoxicity, and ZnO had an average particle size less than 30 nm. Monkey bone marrow mesenchymal stem cells were used. Antimicrobial activity was investigated using the plate colony-counting method. The measured ZnO in the sample was 9.97 wt.%, which was about 66.5% of the theoretical value. The material had a better antibacterial effect against S. aureus than E. coli. | [140] |
| ZnO-PLGA k | E. coli | 24 h, 37 °C | Laser ablation/low-temperature technology | Rod-like ZnO with an average hydrodynamic NP diameter of 47 nm (90% ZnO and 10% metallic Zn). The number of cells on surface of the composite with 0.001% and 0.01% ZnO decreased by 2 and 10 times, respectively. The PLGA–ZnO NP composite containing 0.1% ZnO NPs had bacteriostatic properties. At ZnO NP concentrations of 0.001%, 0.01%, and 0.1%, the rate of 8-oxoguanine formation in DNA increased 1.5, 2.3, and 2.8 times, respectively. PLGA had no antibacterial effect. | [163] |
| ZnO/PVA/Cel l | C. albicans, E. coli, and S. aureus | 24 h, 30/37 °C | Molding | An antibacterial test was performed using the viable shake-flask method. Colony: 105–106 CFU/mL. Solution shaken at 150 rpm at a certain temperature (bacteria: 37 °C, fungus: 30 °C) for 24 h in a water bath oscillator. The thickness of the film was 63–69 μm. Zn2+ reached a maximum release value of 4.20 mgL−1 after 24 h. | [164] |
| ZnO-PHB m | E. coli and S. aureus | 24 h, 37 °C | Electro-spinning and electrospraying | It has an average porosity of around 85% and is thermally stable, and 3 and 5 wt.% ZnO were used to form the composite. The growth inhibition by ZnO-PHB was about 95–97%. The PHB alone did not inhibit bacterial growth. NP size = 8–20 nm | [165] |
| ZnO-PLA-SiO2 n | S. aureus | 18 h, 37 °C | sol–gel method and coating | When 1.5% ZnO and 1.5% SiO2 were used, the highest growth inhibition was 20%. SiO2 reduced the bacterial inhibition capacity. With an increase in ZnO and SiO2 contents, the bacteriostatic effect was disturbed. Only PLA + 1% ZnO was effective bactericidal (90% bacterial cell growth inhibited); 1% ZnO + 1% SiO2—bacteriostatic property. | [166] |
| ZnO/PAN@NFMs o | E. coli and S. aureus | 24 h, 37 °C | Solution blow-spinning | Antimicrobial activity was evaluated using a plate count method assay. For S. aureus, the bacteriostatic rate can reach 100%. For E. coli, the best antibacterial effect was achieved when the mass of ZnO NPs was 5 wt.%, and the bacteriostatic rate can reach 99.9%. The bacteriostatic rate for E. coli remained 99% after 10 cycles. NP size = 32.8–40.7 nm | [167] |
| ZnO/TiO2 p | E. coli and S. aureus | 24 h, 37 °C | Hydrothermal | Size: 100 nm ≥ diameter of the particles, and the composite displayed a rhomboid shape. Synthesis temperature affects the performance. The maximum bacteriostatic activity reached 99 and 90% against S. aureus and E. coli, respectively. Antibacterial mechanism: through the ROS formation and release of Zn2+ ions. The smaller the size of the ZnO/TiO2 nanoarray, the stronger the piezoelectric and antibacterial activity. | [23] |
| ZnO-SCF/PEEK q | E. coli and S. aureus | 24 h, 37 °C | In situ/hydrothermal | The addition of ZnO improves the binding force between the SCF and PEEK. The composite has good wear resistance too. The composition of ZnO, SCF, and PEEK with 7.5, 15, and 77.5 wt.%, respectively, has the best antimicrobial effect. It produced diameters of 28.9 and 22.2 mm for E. coli and S. aureus, respectively. | [148] |
| Sb-ZnO Mg-ZnO | E. coli, S. aureus, Saccharomyces, and A. niger | 18/24 h, 37 °C | Sol–gel method | The bacteriostatic rate of Sb-doped ZnO was only 12% as the plates were incubated in the dark. Under irradiated incubation, Mg-ZnO showed an improvement in its bacteriostatic rate from 9.8% without irradiating to 83.5%. However, the bactericidal effect was higher than the bacteriostatic effect. | [142] |
| CTS/-ZnO r | E. coli and S. aureus | 24 h, 37 °C | Room temp. and casting | A nano-ZnO solution was prepared with particle sizes of 5 μm, 100 nm, and 50 nm. The smaller the particle size of the ZnO, the greater the bacteriostatic activity observed. The composite material had a better inhibitory effect on S. aureus than on E. coli. The material containing 0.3% of 50 nm nano-ZnO had the best antibacterial effect on both target microbes. | [168] |
| CA/ZnO/Ag NPs s | E. coli and S. aureus | 24/108 h, 37 °C | Electro-spinning | Antibacterial activity was evaluated using the Kirby Bauer disc diffusion assay, performed on an agar plate and in liquid medium. The material effectively inhibited the growth of both the strains up to 108 h; 100% bactericidal effect (0% viable cells) against both strains. NP size = 17.85 nm. | [141] |
| ZnO-carvacrol | C. jejuni | 48 h, 37 °C | N/A | ZnO NPs and carvacrol were tested separately and combined. ZnO NPs: <12.5 μg/mL had little inhibition effect, and bacteriostatic and bactericidal effects with 25 and 50 μg/mL, respectively. Synergistic: carvacrol had a better effect than ZnO NPs. ZnO NP effect: physically induce cell leakage. | [169] |
| ZnO-Mk t | S. aureus, L. fusiformis, P. vulgaris, and Pr. vermicola | 24 h, 37 °C | Co-precipitation | The microbiostatic effect of Mk-ZnO NPs was determined through the MIC, live and dead, and antibiofilm assay. Mk-ZnO NPs inhibit the growth of Gram-positive and Gram-negative bacteria at 40 and 50 μg/mL, respectively. A 90–50% cell viability at concentrations of 10–100 μg/mL. It also exhibited a mosquito larva controlling capacity. NP size = 10–15 nm. | [170] |
| ZnO@PVA/KGM u | E. coli and B. subtilis | 24 h 37 °C | Electro-spinning and ultra-sonication | The material was treated @140 °C in citric acid to improve water insolubility. The highest antibacterial activities for E. coli and B. subtilis were found in 1.0 and 2.0 wt.% ZnO@PVA/KGM, respectively. When the ZnO content is >1.0 wt.%, the antibacterial activity for E. coli decreased. Reason: as the value of ZnO NPs increased, the particles gathered into clusters randomly. The material has good photocatalytic activity and filtration efficiency. NP size = 30 ± 10 nm. | [171] |
| ZnO-ALG v | E. coli | 48 h, 37 °C | Electro-spinning | Thin and homogeneous nanofiber with a size of 100 ± 30 nm. It exhibited good stability for more than 10 days in physiological conditions. It has similar mechanical properties as human skin. It has 21.0 ± 3.5 MPa and 6.0 ± 1.3% in tensile strength and elongation break, respectively. | [172] |
| ZnO-MO w | P. aeruginosa, A. baumannii, K. pneumoniae, and C. albicans | 3–24 h, 37 °C | Solvo-chemical/reduction | ZnO−Ag2O/Ag, ZnO−CuO, and ZnO−SnO2 composite NPs (<4 nm) were synthesized to gain broad-spectrum activity. The broth dilution method showed the MIC for A. baumannii as the best result. The antibacterial activities of the samples were investigated using the Luria broth (LB) method. Highly effective antibacterial activity was obtained at 12 h of incubation, and the ZnO−AgO2/Ag composite was the best. ZnO−AgO2/Ag showed high antibacterial activity after just 3 h at a 50 μg/mL. | [143] |
4.3. One-Dimensional ZnO Nanostructures and Their Composites
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Fauci, A.S.; Morens, D.M. The Perpetual Challenge of Infectious Diseases. N. Engl. J. Med. 2012, 366, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Gössling, S.; Scott, D.; Hall, C.M.; Gössling, S.; Scott, D.; Pandemics, C.M.H. Pandemics, Tourism and Global Change: A Rapid Assessment of COVID-19. J. Sustain. Tour. 2021, 29, 1–20. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K., Jr.; Bradley, J.; Guidos, R.J.; Jones, R.N. 10 × ’20 Progress—Development of New Drugs Active Against Gram-Negative Bacilli: An Update From the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wu, R.; Xiong, Y.H.; Ren, H.M.; Lei, C.; Zhao, Y.Q.; Zhang, X.Y.; Xu, F.J. Multifunctional Antimicrobial Materials: From Rational Design to Biomedical Applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F. The Resistance Tsunami, Antimicrobial Stewardship, and the Golden Age of Microbiology. Vet. Microbiol. 2014, 171, 273–278. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Walsh, C.T.; Wencewicz, T.A. Prospects for New Antibiotics: A Molecule-Centered Perspective. J. Antibiot. 2014, 67, 7–22. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Anand Kumar, P.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Nelson, R.E.; Hyun, D.; Jezek, A.; Samore, M.H. Mortality, Length of Stay, and Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections among Elderly Hospitalized Patients in the United States. Clin. Infect. Dis. 2022, 74, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial Properties and Toxicity from Metallic Nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial Magnetic Nanoparticles Based-Therapies for Controlling Infectious Diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered Nanomaterials for Antimicrobial Applications: A Review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Visnapuu, M.; Rosenberg, M.; Truska, E.; Nõmmiste, E.; Šutka, A.; Kahru, A.; Rähn, M.; Vija, H.; Orupõld, K.; Kisand, V.; et al. UVA-Induced Antimicrobial Activity of ZnO/Ag Nanocomposite Covered Surfaces. Colloids Surf. B Biointerfaces 2018, 169, 222–232. [Google Scholar] [CrossRef]
- Mandapalli, P.K.; Labala, S.; Chawla, S.; Janupally, R.; Sriram, D.; Venuganti, V.V.K. Polymer–Gold Nanoparticle Composite Films for Topical Application: Evaluation of Physical Properties and Antibacterial Activity. Polym. Compos. 2017, 38, 2829–2840. [Google Scholar] [CrossRef]
- Iyigundogdu, Z. Synergistic Effects of Zinc Borate and Graphene on Enhanced Thermal Stability and Antimicrobial Properties of Poly(Methyl Methacrylate). Polym. Compos. 2023, 44, 3939–3951. [Google Scholar] [CrossRef]
- Doghish, A.S.; Hashem, A.H.; Shehabeldine, A.M.; Sallam, A.A.M.; El-Sayyad, G.S.; Salem, S.S. Nanocomposite Based on Gold Nanoparticles and Carboxymethyl Cellulose: Synthesis, Characterization, Antimicrobial, and Anticancer Activities. J. Drug Deliv. Sci. Technol. 2022, 77, 103874. [Google Scholar] [CrossRef]
- Pang, S.; He, Y.; Zhong, R.; Guo, Z.; He, P.; Zhou, C.; Xue, B.; Wen, X.; Li, H. Multifunctional ZnO/TiO2 Nanoarray Composite Coating with Antibacterial Activity, Cytocompatibility and Piezoelectricity. Ceram. Int. 2019, 45, 12663–12671. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial Metals and Alloys for Potential Biomedical Implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Cun, J.E.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; He, B.; Pu, Y. Copper-Based Metal–Organic Frameworks for Biomedical Applications. Adv. Colloid Interface Sci. 2022, 305, 102686. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Q.; Chen, Q.; Cui, C.; Duan, S.; Kang, Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S.; et al. Biomedical Polymers: Synthesis, Properties, and Applications. Sci. China Chem. 2022, 65, 1010–1075. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, F.; Swain, S.K. Silver Nanoparticles Decorated Polyethylmethacrylate/Graphene Oxide Composite: As Packaging Material. Polym. Compos. 2019, 40, E1199–E1207. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Han, S.S. Dual-Crosslinked Poly (Vinyl Alcohol)/Sodium Alginate/Silver Nanocomposite Beads—A Promising Antimicrobial Material. Food Chem. 2017, 234, 103–110. [Google Scholar] [CrossRef]
- Cady, N.C.; Behnke, J.L.; Strickland, A.D. Copper-Based Nanostructured Coatings on Natural Cellulose: Nanocomposites Exhibiting Rapid and Efficient Inhibition of a Multi-Drug Resistant Wound Pathogen, A. Baumannii, and Mammalian Cell Biocompatibility in Vitro. Adv. Funct. Mater. 2011, 21, 2506–2514. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, L.; Huang, X.; Chen, J.; Shi, X.; Yang, W.; Hong, M.; Wang, Y.; Dargusch, M.S.; Chen, Z.G. Dual Ag/ZnO-Decorated Micro-/Nanoporous Sulfonated Polyetheretherketone with Superior Antibacterial Capability and Biocompatibility via Layer-by-Layer Self-Assembly Strategy. Macromol. Biosci. 2018, 18, 1800028. [Google Scholar] [CrossRef] [PubMed]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan-Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Naserian, F.; Mesgar, A.S. Development of Antibacterial and Superabsorbent Wound Composite Sponges Containing Carboxymethyl Cellulose/Gelatin/Cu-Doped ZnO Nanoparticles. Colloids Surf. B Biointerfaces 2022, 218, 112729. [Google Scholar] [CrossRef] [PubMed]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric Wound Dressings, an Insight into Polysaccharide-Based Electrospun Membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial Properties of a Novel Silver-Silica Nanocomposite Material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of Recent Studies Dealing with the Antibacterial Properties of Silver Nanoparticles: Fact and Opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef]
- Reda, A.T.; Weldemhret, T.G.; Park, J.Y.; Lim, S.; Debele, N.T.; Choi, S.S.; Cho, C.; Park, Y.T. Synthesis and Characterization of Zinc Basic Salt–Loaded PVA-PEI Polymeric Composite for Antimicrobial Activity and Triboelectric Nanogenerator Applications. Sens. Actuators A Phys. 2024, 370, 115197. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin. Microbiol. Rev. 2019, 32, 1–28. [Google Scholar] [CrossRef]
- Elguindi, J.; Hao, X.; Lin, Y.; Alwathnani, H.A.; Wei, G.; Rensing, C. Advantages and Challenges of Increased Antimicrobial Copper Use and Copper Mining. Appl. Microbiol. Biotechnol. 2011, 91, 237–249. [Google Scholar] [CrossRef]
- Li, S.; Liang, F.; Bai, D.; Liang, X.; Tao, Y. Enhancement of Polyvinylpyrrolidone on Antimicrobial Activity and Mechanism of Copper(II)-β-Cyclodextrin. J. Drug Deliv. Sci. Technol. 2023, 85, 104517. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Yurtdaş-Kırımlıoğlu, G.; Görgülü, Ş. Surface Modification of PLGA Nanoparticles with Chitosan or Eudragit® RS 100: Characterization, Prolonged Release, Cytotoxicity, and Enhanced Antimicrobial Activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102145. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of Essential Oils as Antimicrobial Agents against Spoilage and Pathogenic Microorganisms in Meat Products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.M.; Badawy, M.E.I.; Taktak, N.E.M. Characterization, Antimicrobial Activity, and Antioxidant Activity of the Nanoemulsions of Lavandula Spica Essential Oil and Its Main Monoterpenes. J. Drug Deliv. Sci. Technol. 2021, 65, 102732. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Castillo-Hernández, D.; Pérez-González, M.; Tomás, S.A.; Jiménez-Pérez, J.L.; Sánchez Ramírez, J.F.; Correa-Pacheco, Z.N. Synthesis of Sol-Gel TiO2 Nanoparticles and Assessment of Their Antifungal Activity for the Eventual Conservation of Historical Documents. Appl. Mater. Today 2023, 35, 101999. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Z.; Gao, Z.; Tammina, S.K.; Yang, Y. Nanozymes Used for Antimicrobials and Their Applications. Colloids Surf. B Biointerfaces 2020, 195, 111252. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, J.; Yang, K.; Zhou, C.; Xu, Y.; Song, J.; Gu, Y.; Chen, Z.; Wang, M.; Shoen, C.; et al. A Polymeric Approach toward Resistance-Resistant Antimicrobial Agent with Dual-Selective Mechanisms of Action. Sci. Adv. 2021, 7, eabc9917. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Cano-Vicent, A.; Sabater i Serra, R.; El-Tanani, M.; Aljabali, A.A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the Microbial Resistant Era: Fabrication, Materials, Properties and Tissue Engineering Applications. Mater. Today Bio. 2022, 16, 100412. [Google Scholar] [CrossRef] [PubMed]
- Van Meurs, S.J.; Gawlitta, D.; Heemstra, K.A.; Poolman, R.W.; Vogely, H.C.; Kruyt, M.C. Selection of an Optimal Antiseptic Solution for Intraoperative Irrigation: An in Vitro Study. J. Bone Jt. Surg. 2014, 96, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Haydel, S.E. Evaluation of the Medicinal Use of Clay Minerals as Antibacterial Agents. Int. Geol. Rev. 2010, 52, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zhu, X.Y.; Wu, F.G. Antibacterial Gas Therapy: Strategies, Advances, and Prospects. Bioact. Mater. 2023, 23, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, I.; Orekhov, P.; Rulev, M.; Kovalev, K.; Astashkin, R.; Bratanov, D.; Ryzhykau, Y.; Balandin, T.; Bukhdruker, S.; Okhrimenko, I.; et al. High-Pressure Crystallography Shows Noble Gas Intervention into Protein-Lipid Interaction and Suggests a Model for Anaesthetic Action. Commun. Biol. 2022, 5, 360. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary Ammonium-Based Biomedical Materials: State-of-the-Art, Toxicological Aspects and Antimicrobial Resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, N.; Tasviri, M.; Rahimi, E.; Gholami, M.R. Nano Sized ZnO Composites: Preparation, Characterization and Application as Photocatalysts for Degradation of AB92 Azo Dye. Mater. Sci. Semicond. Process. 2014, 21, 167–179. [Google Scholar] [CrossRef]
- Chen, H.; Deng, H.; Zhong, X.; Zhou, H.; Zhan, J.; Zhou, X. Highly Dispersed Amorphous ZnO on a Petal-like Porous Silica-Clay Composite with Enhanced Antimicrobial Properties. Colloids Surf. B Biointerfaces 2022, 220, 112978. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Ling, W.; Wang, M.; Qiu, B.; Shi, J. Synthesis of Core–Shell ZnO Nanoparticles and Their Effect on Mechanical and Antibacterial Properties for PLLA/ZnO Nanocomposites. Polym. Compos. 2023, 45, 3448–3459. [Google Scholar] [CrossRef]
- Demirel, R.; Suvacı, E.; Şahin, İ.; Dağ, S.; Kiliç, V. Antimicrobial Activity of Designed Undoped and Doped MicNo-ZnO Particles. J. Drug Deliv. Sci. Technol. 2018, 47, 309–321. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter Jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Lallo da Silva, B.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The Contribution of Zinc Ions to the Antimicrobial Activity of Zinc Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 1–5. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Liu, M.; Zhong, C.; Zhang, Q.; Su, Q.Z.; Tan, Z.L.; Han, P.P.; Jia, S.R. Metabolomic Analysis of Antimicrobial Mechanisms of ε-Poly-L-Lysine on Saccharomyces Cerevisiae. J. Agric. Food Chem. 2014, 62, 4454–4465. [Google Scholar] [CrossRef] [PubMed]
- Agnese, A.M.; Pérez, C.; Cabrera, J.L. Adesmia Aegiceras: Antimicrobial Activity and Chemical Study. Phytomedicine 2001, 8, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Omojate Godstime, C.; Enwa Felix, O.; Jewo Augustina, O.; Eze Christopher, O. Mechanisms of Antimicrobial Actions of Phytochemicals against Enteric Pathogens—A Review. J. Pharm. Chem. Biol. Sci. 2014, 2, 77–85. [Google Scholar]
- Saada, N.S.; Abdel-Maksoud, G.; Abd El-Aziz, M.S.; Youssef, A.M. Green Synthesis of Silver Nanoparticles, Characterization, and Use for Sustainable Preservation of Historical Parchment against Microbial Biodegradation. Biocatal. Agric. Biotechnol. 2021, 32, 101948. [Google Scholar] [CrossRef]
- Nawal, R.R.; Parande, M.; Sehgal, R.; Naik, A.; Rao, N.R. A Comparative Evaluation of Antimicrobial Efficacy and Flow Properties for Epiphany, Guttaflow and AH-Plus Sealer. Int. Endod. J. 2011, 44, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, R.T.; Oswald, I.P.; Hieny, S.; James, S.L. The Microbicidal Activity of Interferon-y-Treated Macrophages Against. Eur. J. Immunol. 1992, 22, 2501–2506. [Google Scholar] [CrossRef]
- Granger, D.L.; Hibbs, J.B.; Perfect, J.R.; Durack, D.T. Metabolic Fate of L-Arginine in Relation to Microbiostatic Capability of Murine Macrophages. J. Clin. Investig. 1990, 85, 264–273. [Google Scholar] [CrossRef]
- Karou, D.; Dicko, M.H.; Simpore, J.; Traore, A.S. Antioxidant and Antibacterial Activities of Polyphenols from Ethnomedicinal Plants of Burkina Faso. Afr. J. Biotechnol. 2005, 4, 823–828. [Google Scholar]
- Dao, K.Q.; Hoang, C.H.; Van Nguyen, T.; Nguyen, D.H.; Mai, H.H. High Microbiostatic and Microbicidal Efficiencies of Bacterial Cellulose-ZnO Nanocomposites for in Vivo Microbial Inhibition and Filtering. Colloid Polym. Sci. 2023, 301, 389–399. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, A.E.; Brewer, M.T.; Carlson, S.A. Adverse Effects of Antimicrobials via Predictable or Idiosyncratic Inhibition of Host Mitochondrial Components. Antimicrob. Agents Chemother. 2012, 56, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Netíková, L.; Bogusch, P.; Heneberg, P. Czech Ethanol-Free Propolis Extract Displays Inhibitory Activity against a Broad Spectrum of Bacterial and Fungal Pathogens. J. Food Sci. 2013, 78, M1421–M1429. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.S.; Oliveira, M.; De Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial Nanostructured Starch Based Films for Packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Hsu, C.Y.; Lai, S.M.; Syu, W.J.; Wang, T.Y.; Lai, P.S. Metal Nanobullets for Multidrug Resistant Bacteria and Biofilms. Adv. Drug Deliv. Rev. 2014, 78, 88–104. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current Status and Perspectives of Zinc-Based Absorbable Alloys for Biomedical Applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hasan, M.R.; Mehto, N.K.; Deepak; Bishoyi, A.; Narang, J. 92 Years of Zinc Oxide: Has Been Studied by the Scientific Community since the 1930s—An Overview. Sens. Int. 2022, 3, 100182. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and Immune Function: The Biological Basis of Altered Resistance to Infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc Oxide Particles: Synthesis, Properties and Applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doǧan, S.; Avrutin, V.; Cho, S.J.; Morko̧, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The Neurobiology of Zinc in Health and Disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Surbhi; Chakraborty, I.; Pandey, A. A Review Article on Application of ZnO-Based Nanocomposite Materials in Environmental Remediation. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc Oxide Protects Cultured Enterocytes from the Damage Induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Singh, S.; Aldawsari, H.M.; Alam, A.; Alqarni, M.H.S.; Ranjan, S.; Kesharwani, P. Synthesis and Antimicrobial Activity of Vancomycin–Conjugated Zinc Coordination Polymer Nanoparticles against Methicillin-Resistant Staphylococcus Aureus. J. Drug Deliv. Sci. Technol. 2022, 70, 103255. [Google Scholar] [CrossRef]
- Petchthanasombat, C.; Tiensing, T.; Sunintaboon, P. Synthesis of Zinc Oxide-Encapsulated Poly(Methyl Methacrylate)-Chitosan Core-Shell Hybrid Particles and Their Electrochemical Property. J. Colloid Interface Sci. 2012, 369, 52–57. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial Cytotoxicity Is Composition, Size, and Cell Type Dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.A.C.; Lunn, G.; Coico, R. Biosafety: Guidelines for Working with Pathogenic and Infectious Microorganisms. Curr. Protoc. Microbiol. 2009, 13, 1–14. [Google Scholar] [CrossRef]
- Bhusnure, O.G.; Dongare, R.B.; Gholve, S.B.; Giram, P.S. Chemical Hazards and Safety Managment in Pharmaceutical Industry. J. Pharm. Res. 2018, 12, 357–369. [Google Scholar]
- Yu, Y.; Bu, F.; Zhou, H.; Wang, Y.; Cui, J.; Wang, X.; Nie, G.; Xiao, H. Biosafety Materials: An Emerging New Research Direction of Materials Science from the COVID-19 Outbreak. Mater. Chem. Front. 2020, 4, 1930–1953. [Google Scholar] [CrossRef]
- Report, M.W. FDA Approval of an Extended Period for Administering VariZIG for Postexposure Prophylaxis of Varicella. Am. J. Transplant. 2012, 12, 2554. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Biosafety Manual, 4th ed.; WHO: Geneva, Switzerland, 2020; ISBN 9789275724170. [Google Scholar]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016; Volume 7.
- Denk-Lobnig, M.; Wood, K.B. Antibiotic Resistance in Bacterial Communities. Curr. Opin. Microbiol. 2023, 74, 102306. [Google Scholar] [CrossRef]
- Wilson, D.N.; Hauryliuk, V.; Atkinson, G.C.; O’Neill, A.J. Target Protection as a Key Antibiotic Resistance Mechanism. Nat. Rev. Microbiol. 2020, 18, 637–648. [Google Scholar] [CrossRef]
- De Pascale, G.; Wright, G.D. Antibiotic Resistance by Enzyme Inactivation: From Mechanisms to Solutions. ChemBioChem 2010, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Pauter, K.; Szultka-Młyńska, M.; Buszewski, B. Determination and identification of antibiotic drugs and bacterial strains in biological samples. Molecules 2020, 25, 2556. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Fang, X.; Jia, H.; Liu, M.; Shi, X.; Xue, C.; Chen, T.; Wei, Z.; Fang, F.; Zhu, H.; et al. Zn or O? An Atomic Level Comparison on Antibacterial Activities of Zinc Oxides. Chem. Eur. J. 2016, 22, 8053–8058. [Google Scholar] [CrossRef] [PubMed]
- Blog, C.; Emerging, O.; Blog, C.T.; Demand, W.O.; Seminars, P. EPA Announces Approval of First-Ever Long-Lasting Antiviral Product for Use against COVID-19. Available online: https://www.jdsupra.com/legalnews/epa-announces-approval-of-first-ever-95999/ (accessed on 27 August 2020).
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Wu, H.; Meng, Y.; Yu, M.; Yang, H. Modulating the Antibacterial Activity of ZnO/Talc by Balancing the Monodispersity of ZnO Nanoparticles. Appl. Clay Sci. 2023, 242, 107024. [Google Scholar] [CrossRef]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial Activity of ZnO Nanoparticle on Gram-Positive and Gram-Negative Bacteria. Afr. J. Microbiol. Res. 2012, 5, 1368–1373. [Google Scholar] [CrossRef]
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial Power of Cu/Zn Mixed Oxide Nanoparticles to Escherichia coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102. [Google Scholar] [CrossRef]
- Sevinç, B.A.; Hanley, L. Antibacterial Activity of Dental Composites Containing Zinc Oxide Nanoparticles. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef]
- Piedade, A.P.; Pinho, A.C.; Branco, R.; Morais, P.V. Evaluation of Antimicrobial Activity of ZnO Based Nanocomposites for the Coating of Non-Critical Equipment in Medical-Care Facilities. Appl. Surf. Sci. 2020, 513, 145818. [Google Scholar] [CrossRef]
- Dhyani, A.; Repetto, T.; Bartikofsky, D.; Mirabelli, C.; Gao, Z.; Snyder, S.A.; Snyder, C.; Mehta, G.; Wobus, C.E.; VanEpps, J.S.; et al. Surfaces with Instant and Persistent Antimicrobial Efficacy against Bacteria and SARS-CoV-2. Matter 2022, 5, 4076–4091. [Google Scholar] [CrossRef] [PubMed]
- Kaniewska, K.; Karbarz, M.; Katz, E. Nanocomposite Hydrogel Films and Coatings—Features and Applications. Appl. Mater. Today 2020, 20, 100776. [Google Scholar] [CrossRef]
- Wentao, W.; Tao, Z.; Bulei, S.; Tongchang, Z.; Qicheng, Z.; Fan, W.; Ninglin, Z.; Jian, S.; Ming, Z.; Yi, S. Functionalization of Polyvinyl Alcohol Composite Film Wrapped in Am-ZnO@CuO@Au Nanoparticles for Antibacterial Application and Wound Healing. Appl. Mater. Today 2019, 17, 36–44. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc Oxide Nanoparticles for Water Disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.S. A Review on ZnO Nanostructured Materials: Energy, Environmental and Biological Applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Cruz Pena, D.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; de Jesús Pozos Guillén, A.; Tapia-Pérez, H.; Martínez Castañón, G. The Antimicrobial Sensitivity of Streptococcus Mutans to Nanoparticles of Silver, Zinc Oxide, and Gold. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Gajjar, P.; Pettee, B.; Britt, D.W.; Huang, W.; Johnson, W.P.; Anderson, A.J. Antimicrobial Activities of Commercial Nanoparticles against an Environmental Soil Microbe, Pseudomonas Putida KT2440. J. Biol. Eng. 2009, 3, 9. [Google Scholar] [CrossRef]
- Liang, S.X.T.; Wong, L.S.; Lim, Y.M.; Lee, P.F.; Djearamane, S. Effects of Zinc Oxide Nanoparticles on Streptococcus Pyogenes. South Afr. J. Chem. Eng. 2020, 34, 63–71. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Bostanabad, S.Z.; Amini, S.M.; Jafari, A.; Nobar, M.G.; Ghodousi, A.; Kamalzadeh, M.; Darban-Sarokhalil, D. The Anti-Mycobacterial Activity of Ag, Zno, and Ag-Zno Nanoparticles against Mdr-and Xdr-Mycobacterium Tuberculosis. Infect. Drug Resist. 2019, 12, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, T.; Aziz, A.; Khan, M.A.; Andleeb, S.; Mahmood, H.; Khan, A.A.; Khan, R.; Shafique, M.; Zaka-ul-Islam, M. Surfactant Assisted Synthesis of ZnO Nanostructures Using Atmospheric Pressure Microplasma Electrochemical Process with Antibacterial Applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 228, 153–159. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective Toxicity of Zinc Oxide Nanoparticles to Prokaryotic and Eukaryotic Systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- Steffy, K.; Shanthi, G.; Maroky, A.S.; Selvakumar, S. Enhanced Antibacterial Effects of Green Synthesized ZnO NPs Using Aristolochia Indica against Multi-Drug Resistant Bacterial Pathogens from Diabetic Foot Ulcer. J. Infect. Public Health 2018, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Jiang, Z.; Li, P.; Chen, Q.; Zhou, J.; Cui, X.; Wang, Q. Novel Hydroxyapatite Whiskers Modified by Silver Ion and Nano Zinc Oxide Used for Bone Defect Repairment. Coatings 2021, 11, 957. [Google Scholar] [CrossRef]
- Jatoi, A.W.; Kim, I.S.; Ogasawara, H.; Ni, Q.Q. Characterizations and Application of CA/ZnO/AgNP Composite Nanofibers for Sustained Antibacterial Properties. Mater. Sci. Eng. C 2019, 105, 110077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, R.; Liu, P.; Fu, L.; Lan, X.; Gong, G. Improvement of the Antibacterial Activity of Nanocrystalline Zinc Oxide by Doping Mg (II) or Sb (III). Int. J. Appl. Ceram. Technol. 2011, 8, 1087–1098. [Google Scholar] [CrossRef]
- Pandey, M.; Singh, M.; Wasnik, K.; Gupta, S.; Patra, S.; Gupta, P.S.; Pareek, D.; Chaitanya, N.S.N.; Maity, S.; Reddy, A.B.M.; et al. Targeted and Enhanced Antimicrobial Inhibition of Mesoporous ZnO-Ag2O/Ag, ZnO-CuO, and ZnO-SnO2Composite Nanoparticles. ACS Omega 2021, 6, 31615–31631. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Deng, L.; Zou, L.; Feng, F.; Zhang, H. Hydrophobic Ethylcellulose/Gelatin Nanofibers Containing Zinc Oxide Nanoparticles for Antimicrobial Packaging. J. Agric. Food Chem. 2018, 66, 9498–9506. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.M.; da Silva, B.L.; Sorrechia, R.; Pietro, R.C.L.R.; Chiavacci, L.A. Sustainable Antibacterial Activity of Polyamide Fabrics Containing ZnO Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Muhammad, N.; Kaleem, M.; Nayyar, M.; Qazi, A.S.; Butt, D.Q.; Safi, S.Z.; Khan, A.S. Anticariogenic and Mechanical Characteristics of Resin-Modified Glass Ionomer Cement Containing Lignin-Decorated Zinc Oxide Nanoparticles. ACS Appl. Bio Mater. 2023, 6, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Aditya, A.; Chattopadhyay, S.; Jha, D.; Gautam, H.K.; Maiti, S.; Ganguli, M. Zinc Oxide Nanoparticles Dispersed in Ionic Liquids Show High Antimicrobial Efficacy to Skin-Specific Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 15401–15411. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Cen, J.; Wu, T.; Hou, T.; Chen, K.; Li, X.; Zhang, D. Preparation and Properties of the Poly(Ether Ether Ketone) (PEEK)/Nano-Zinc Oxide (ZnO)-Short Carbon Fiber (SCF) Artificial Joint Composites. ACS Appl. Polym. Mater. 2022, 4, 8869–8877. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-Date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial Polymeric Nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-Based Antimicrobials and Delivery Systems for Biofilm-Infection Control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Lammel, T.; Connolly, M.; Conde, E.; Barrado, A.I.; Derick, S.; Perez, Y.; Fernandez, M.; Furger, C.; Navas, J.M. Comparative Cytotoxicity Induced by Bulk and Nanoparticulated ZnO in the Fish and Human Hepatoma Cell Lines PLHC-1 and Hep G2. Nanotoxicology 2013, 7, 935–952. [Google Scholar] [CrossRef]
- Mihai, C.; Chrisler, W.B.; Xie, Y.; Hu, D.; Szymanski, C.J.; Tolic, A.; Klein, J.A.; Smith, J.N.; Tarasevich, B.J.; Orr, G. Intracellular Accumulation Dynamics and Fate of Zinc Ions in Alveolar Epithelial Cells Exposed to Airborne ZnO Nanoparticles at the Air-Liquid Interface. Nanotoxicology 2015, 9, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Munawar, T.; Yasmeen, S.; Mukhtar, F.; Nadeem, M.S.; Mahmood, K.; Saqib Saif, M.; Hasan, M.; Ali, A.; Hussain, F.; Iqbal, F. Zn0.9Ce0.05M0.05O (M = Er, Y, V) Nanocrystals: Structural and Energy Bandgap Engineering of ZnO for Enhancing Photocatalytic and Antibacterial Activity. Ceram. Int. 2020, 46, 14369–14383. [Google Scholar] [CrossRef]
- Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics 2023, 15, 970. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.C.T.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Antibacterial Paper Based on Composite Coatings of Nanofibrillated Cellulose and ZnO. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 111–119. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of Size Scale and Morphology on Antibacterial Properties of ZnO Powders Hydrothemally Synthesized Using Different Surface Stabilizing Agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Q.; Su, Y.; Wang, D.; Xing, Z.; Fang, L. High-Efficiency Bacteriostatic Material Modified by Nano Zinc Oxide and Polyelectrolyte Diallyl Dimethylammonium Chloride Based on Red Mud. Colloids Surf. B Biointerfaces 2019, 177, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, L.; Liu, H.; Tan, L.; Liu, T.; Meng, X. Solvothermal Synthesis of ZnO Nanoparticles and Anti-Infection Application in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, H.; Shen, Z.; Hao, L.; Chen, H.; Zhou, X. Synthesis, Characterization, and Comparison of Antibacterial Effects and Elucidating the Mechanism of ZnO, CuO and CuZnO Nanoparticles Supported on Mesoporous Silica SBA-3. RSC Adv. 2020, 10, 2767–2785. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Simakin, A.V.; Smirnova, V.V.; Uvarov, O.V.; Ivashkin, P.I.; Kucherov, R.N.; Ivanov, V.E.; Bruskov, V.I.; Sevostyanov, M.A.; Baikin, A.S.; et al. Bacteriostatic and Cytotoxic Properties of Composite Material Based on Zno Nanoparticles in Plga Obtained by Low Temperature Method. Polymers 2022, 14, 49. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, Y.; Cai, P. Cellulose-Based Films Reinforced by in-Situ Generated ZnO for Antimicrobial Packaging. Cellulose 2022, 29, 9375–9391. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Ledezma, A.; Romero, J.; Saldívar, R.; Langlois, V.; Renard, E.; Grande, D. Electrospinning and Electrospraying Techniques for Designing Novel Antibacterial Poly(3-Hydroxybutyrate)/Zinc Oxide Nanofibrous Composites. J. Mater. Sci. 2016, 51, 8593–8609. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Karakaplan, M.B.; Erdoğan, N. Bacteriostatic Polylactic Acid Coatings Enriched with Zinc Oxide and Silica Nanoparticles for Titanium Pedicle Screws. JOM 2021, 73, 4410–4418. [Google Scholar] [CrossRef]
- Du, Z.; Chen, Y.; Jensen, M.; Wang, N.; Li, X.; Zhang, X. Preparation of 3D Crimped ZnO/PAN Hybrid Nanofiber Mats with Photocatalytic Activity and Antibacterial Properties by Blow-Spinning. J. Appl. Polym. Sci. 2021, 138, 49908. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wu, W.; Yang, J.; Yang, Q. Preparation and Characterization of Chitosan/Nano-ZnO Composite Film with Antimicrobial Activity. Bioprocess Biosyst. Eng. 2021, 44, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Windiasti, G.; Feng, J.; Ma, L.; Hu, Y.; Hakeem, M.J.; Amoako, K.; Delaquis, P.; Lu, X. Investigating the Synergistic Antimicrobial Effect of Carvacrol and Zinc Oxide Nanoparticles against Campylobacter Jejuni. Food Control 2019, 96, 39–46. [Google Scholar] [CrossRef]
- Yazhiniprabha, M.; Vaseeharan, B.; Sonawane, A.; Behera, A. In Vitro and In Vivo Toxicity Assessment of Phytofabricated ZnO Nanoparticles Showing Bacteriostatic Effect and Larvicidal Efficacy against Culex Quinquefasciatus. J. Photochem. Photobiol. B Biol. 2019, 192, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Scarfi, S.; Pozzolini, M.; Vicini, S.; Alloisio, M.; Castellano, M. Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. ACS Appl. Mater. Interfaces 2020, 12, 3371–3381. [Google Scholar] [CrossRef]
- Bojarska, M.; Nowak, B.; Skowroński, J.; Piątkiewicz, W.; Gradoń, L. Growth of ZnO Nanowires on Polypropylene Membrane Surface—Characterization and Reactivity. Appl. Surf. Sci. 2017, 391, 457–467. [Google Scholar] [CrossRef]
- Jain, A.; Bhargava, R.; Poddar, P. Probing Interaction of Gram-Positive and Gram-Negative Bacterial Cells with ZnO Nanorods. Mater. Sci. Eng. C 2013, 33, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, H.; Zhang, F.; Zhao, S.; Liu, Y.; Xu, Y.; Wu, R.; Li, D.; Yang, Y.; Liao, L.; et al. Antibacterial Properties of Bilayer Biomimetic Nano-ZnO for Dental Implants. ACS Biomater. Sci. Eng. 2020, 6, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Ann, L.C.; Mahmud, S.; Bakhori, S.K.M.; Sirelkhatim, A.; Mohamad, D.; Hasan, H.; Seeni, A.; Rahman, R.A. Effect of Surface Modification and UVA Photoactivation on Antibacterial Bioactivity of Zinc Oxide Powder. Appl. Surf. Sci. 2014, 292, 405–412. [Google Scholar] [CrossRef]
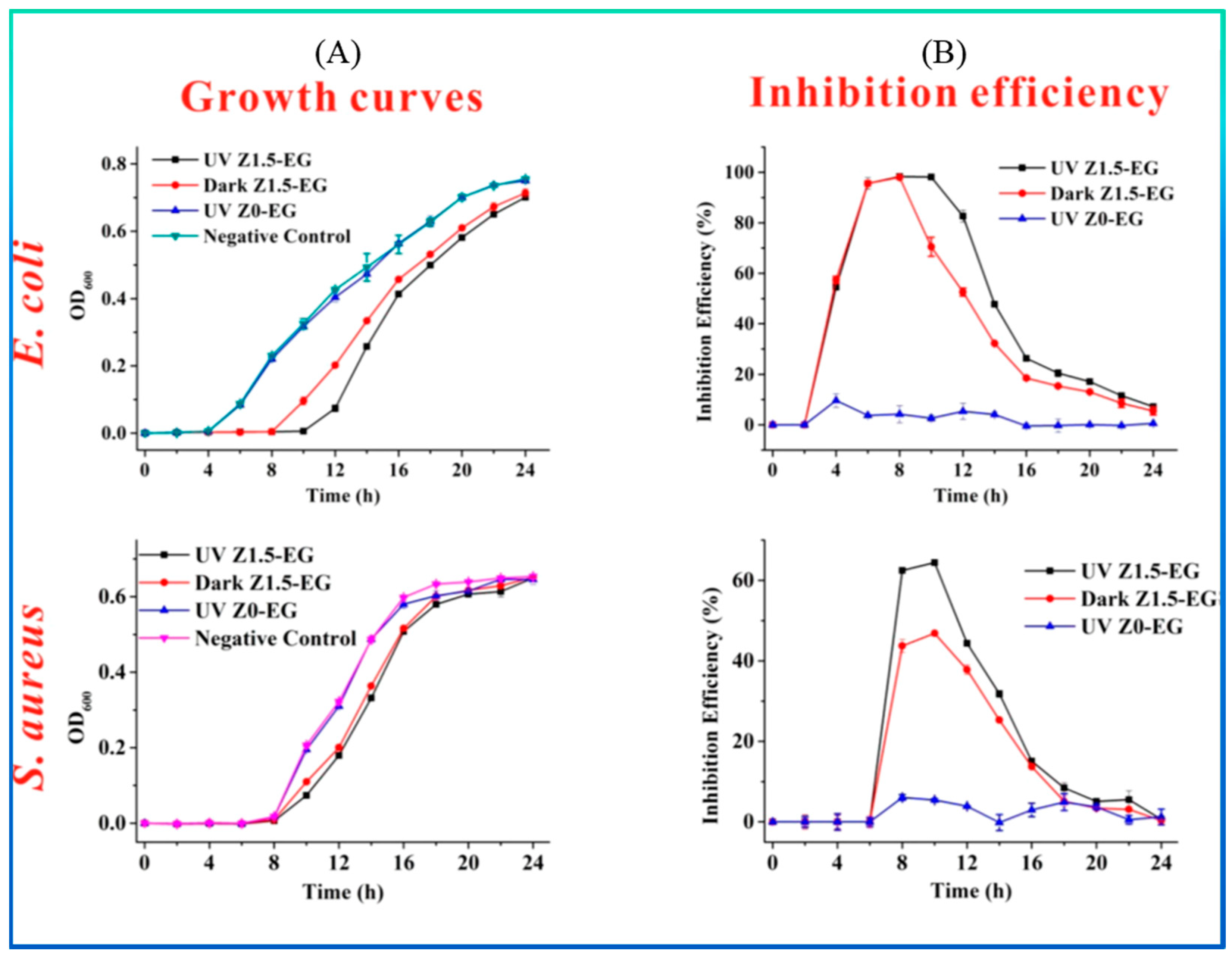
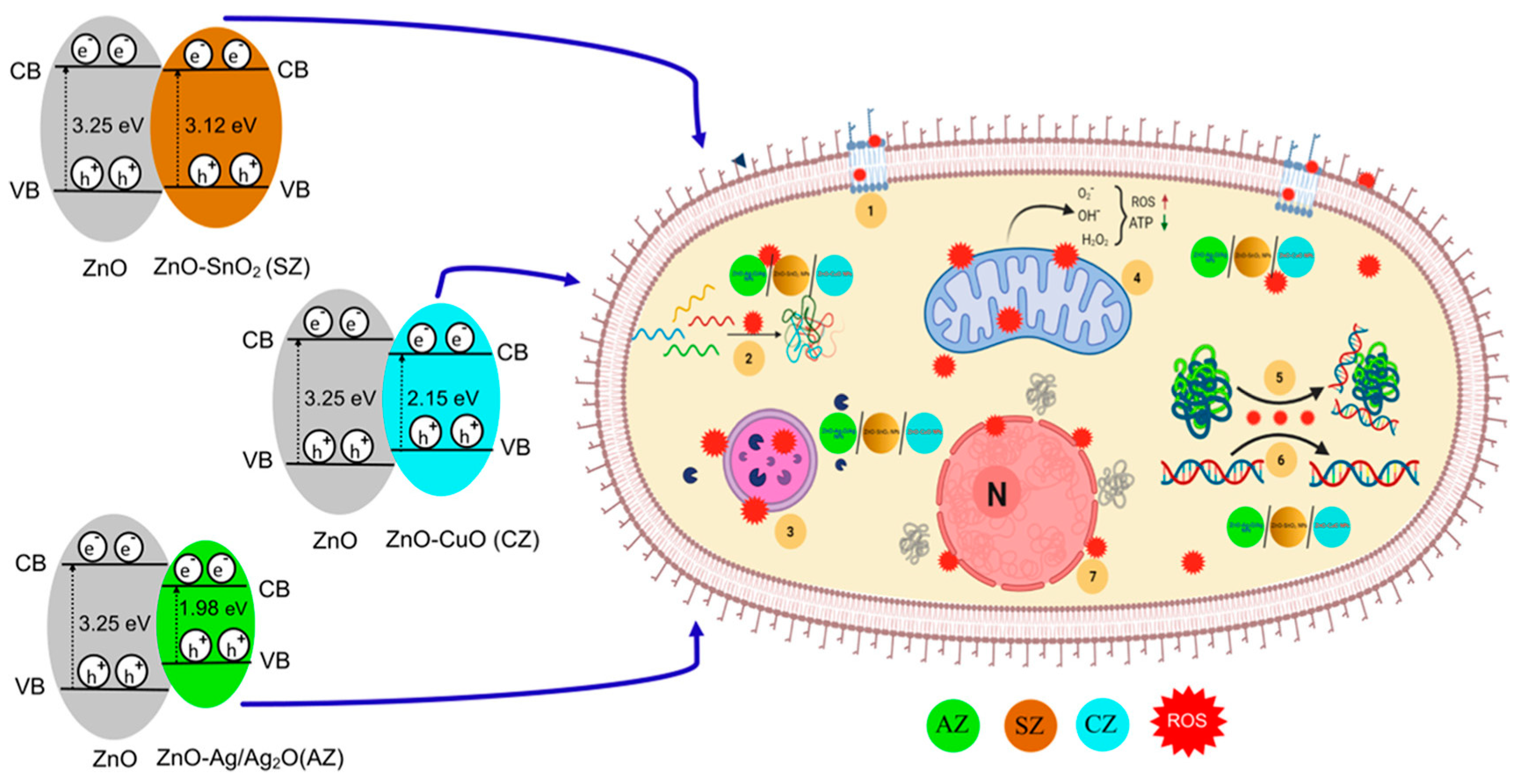
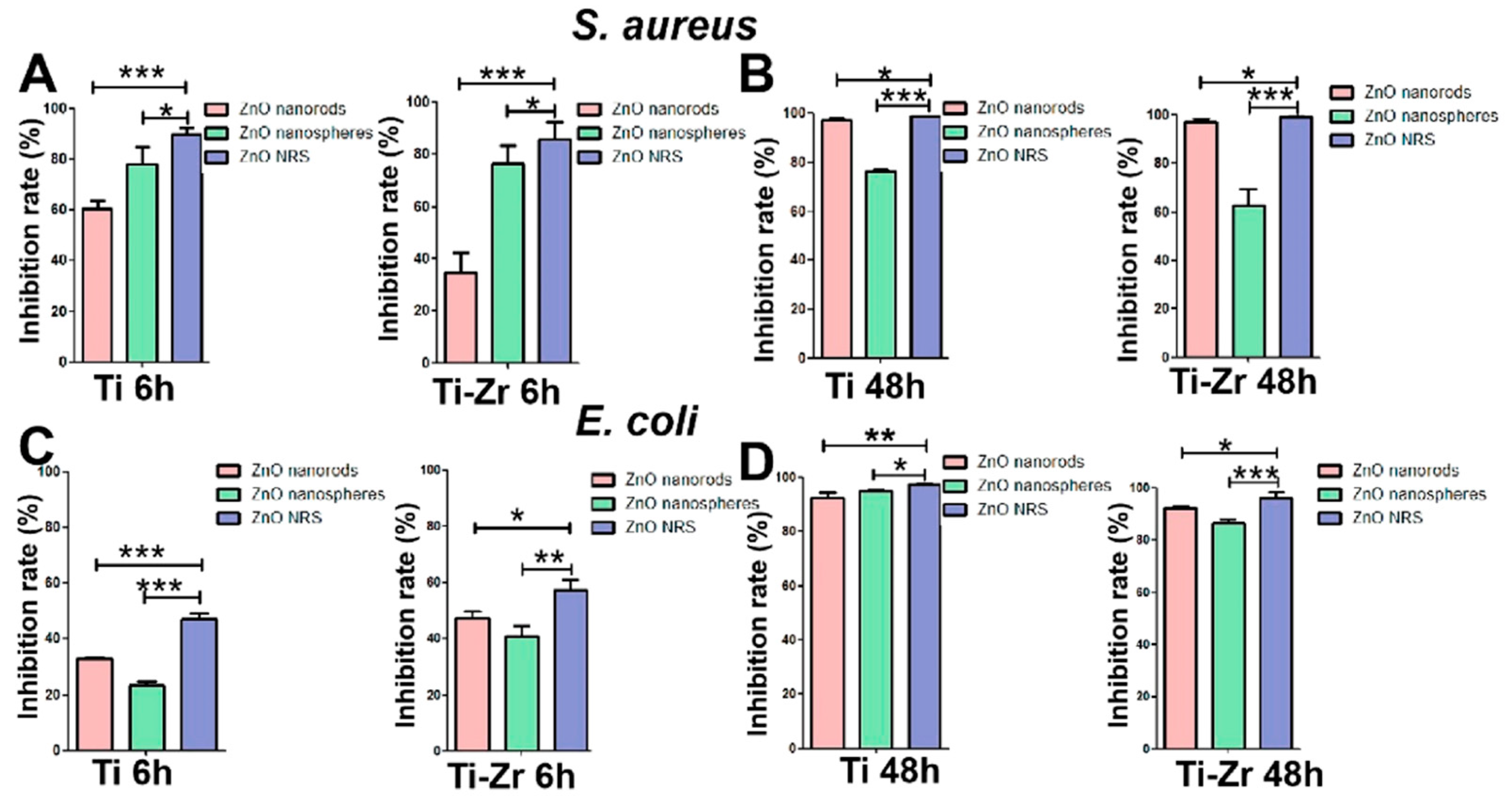
| Material Type | Pros. | Cons. | Ref. |
|---|---|---|---|
| Silver-based materials |
|
| [36,37] |
| Copper-based materials |
|
| [39,40] |
| Quaternary ammonium compounds (QACs) |
|
| [60,61] |
| Chitosan-based materials |
|
| [42,43] |
| Essential oils |
|
| [45,46,62] |
| Nano-materials |
|
| [16,48] |
| Enzymes |
|
| [50,51,52] |
| Polyhexanide-based materials |
|
| [53,54,55] |
| Clay minerals |
|
| [56,57] |
| Noble Gases |
|
| [58,59] |
| Zinc-based materials |
|
| [12], This work |
| Method | Pros. | Cons. |
|---|---|---|
| Microbicidal |
|
|
| Microbiostatic |
|
|
| Activity | Time Course | Note | Ref. |
|---|---|---|---|
| Immediate contact and interaction | Initial minutes |
| [12,120] |
| Early disruption of microbial membrane | Minutes–hours |
| [12,121] |
| Zn2+ ion release and intracellular influence | Hours–days |
| [122,123] |
| Microbial growth inhibition | Days |
| [124] |
| Continuous antimicrobial activity | Days–weeks |
| [125] |
| Long-term residual effects | >Weeks |
| [126] |
| Adaptation and resistance dynamics | Weeks–months |
| [126] |
| Material | Target Microbe | Time Temp | Synthesis Method | Note | Ref. |
|---|---|---|---|---|---|
| ZnO NW@PP a | E. coli B. subtilis | 24 h, 37 °C | Chemical bath deposition | An antimicrobial test was performed with the presence of fluorescent light. Clear growth inhibition was observed for B. subtilis, but almost not for E. coli. A plasma treatment was used before the chemical bath deposition. | [173] |
| ZnO NR b | S. aureus B. subtilis E. coli A. aerogenes | Hydrothermal | The rods have an average diameter and length of 45 and 250 nm, respectively. In the presence of different concentrations of ZnO NR, S. aureus and B. subtilis did not show any growth even at a lower concentration of 100 μg/mL. For E. coli and A. aerogenes, a 500 μg/mL concentration was enough for inhibition observation. | [174] | |
| Ti-ZnO NRS c | E. coli S. aureus | 24 h, 37 °C | Hydrothermal | Different ZnOs produced good long-term antibacterial effects and a poor short-term antibacterial effect with E. coli, due to the weak bacteriostatic property of ZnO against E. coli. Antibacterial effect: due to the rapid release of ZnO nanospheres. | [175] |
| ZnO-rod ZnO-plate | E. coli S. aureus | 24 h, 37 °C | Combustion, O2 annealing | Nano-sized/one-dimensional rod and nano-sized/one-dimensional plate ZnO were prepared from commercial ZnO (bulk). Nano-sized/one-dimensional rod: diameter = 30 to 180 nm and length = 100 to 300 nm. Nano-sized/one-dimensional plate: width = 40 to 250 nm and length = 80 to 350 nm. One-dimensional ZnO size = 30 nm to 300 nm. Oxygen-annealed ZnO showed slightly higher antimicrobial activity than the unannealed ZnO against the target strains. After UV irradiation, the antimicrobial activities of the oxygen-treated materials increased by around 19%. | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reda, A.T.; Park, J.Y.; Park, Y.T. Zinc Oxide-Based Nanomaterials for Microbiostatic Activities: A Review. J. Funct. Biomater. 2024, 15, 103. https://doi.org/10.3390/jfb15040103
Reda AT, Park JY, Park YT. Zinc Oxide-Based Nanomaterials for Microbiostatic Activities: A Review. Journal of Functional Biomaterials. 2024; 15(4):103. https://doi.org/10.3390/jfb15040103
Chicago/Turabian StyleReda, Alemtsehay Tesfay, Jae Yeon Park, and Yong Tae Park. 2024. "Zinc Oxide-Based Nanomaterials for Microbiostatic Activities: A Review" Journal of Functional Biomaterials 15, no. 4: 103. https://doi.org/10.3390/jfb15040103
APA StyleReda, A. T., Park, J. Y., & Park, Y. T. (2024). Zinc Oxide-Based Nanomaterials for Microbiostatic Activities: A Review. Journal of Functional Biomaterials, 15(4), 103. https://doi.org/10.3390/jfb15040103








