The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective
Abstract
1. Introduction
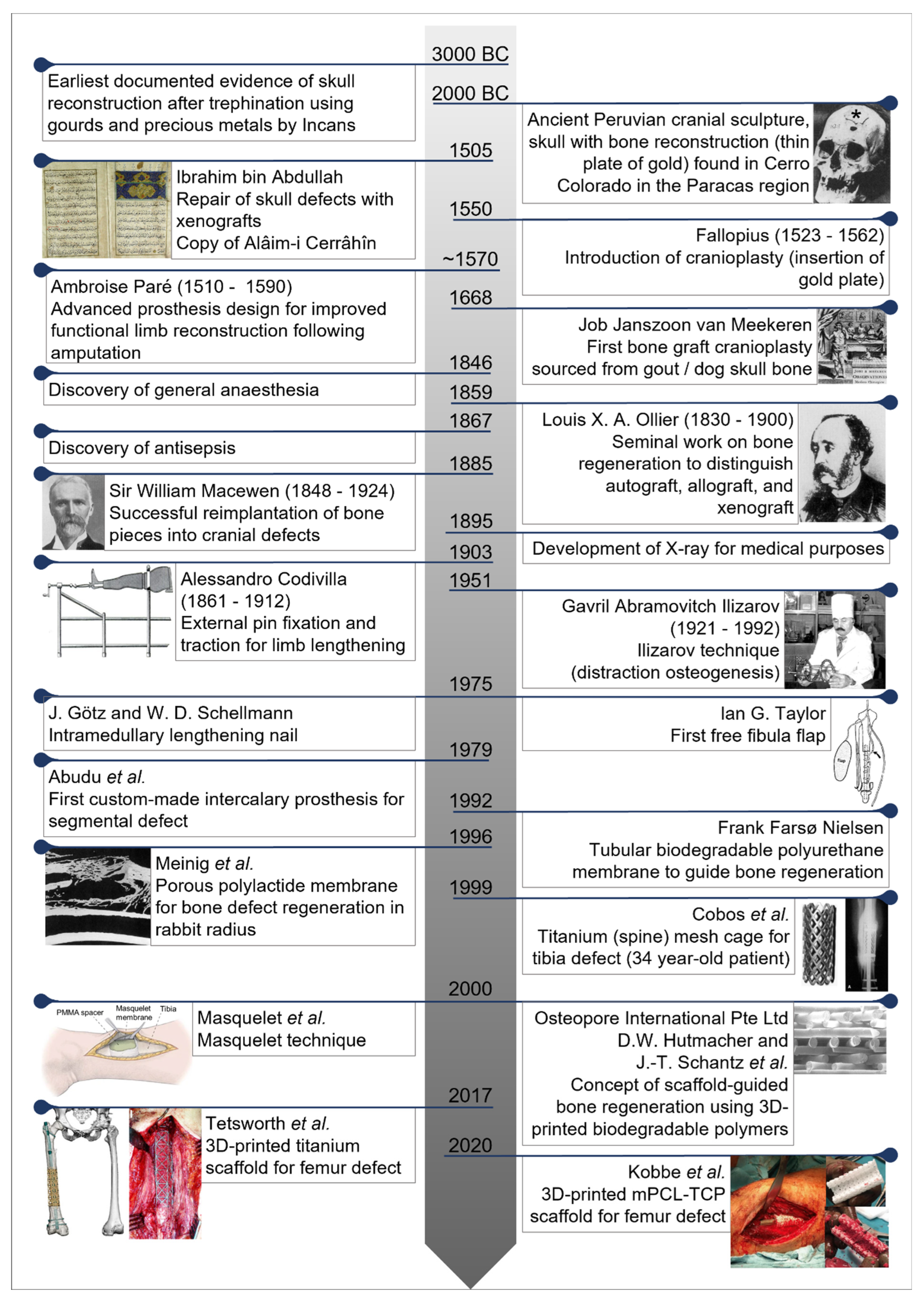
Central Historical Events
2. Bone Defects: Current Treatment Methods
3. An alternative Concept: ‘Guided Tissue Engineering’
3.1. Preclinical Testing (Spinal) Porous Titanium Mesh Cages for Long Bone Defects
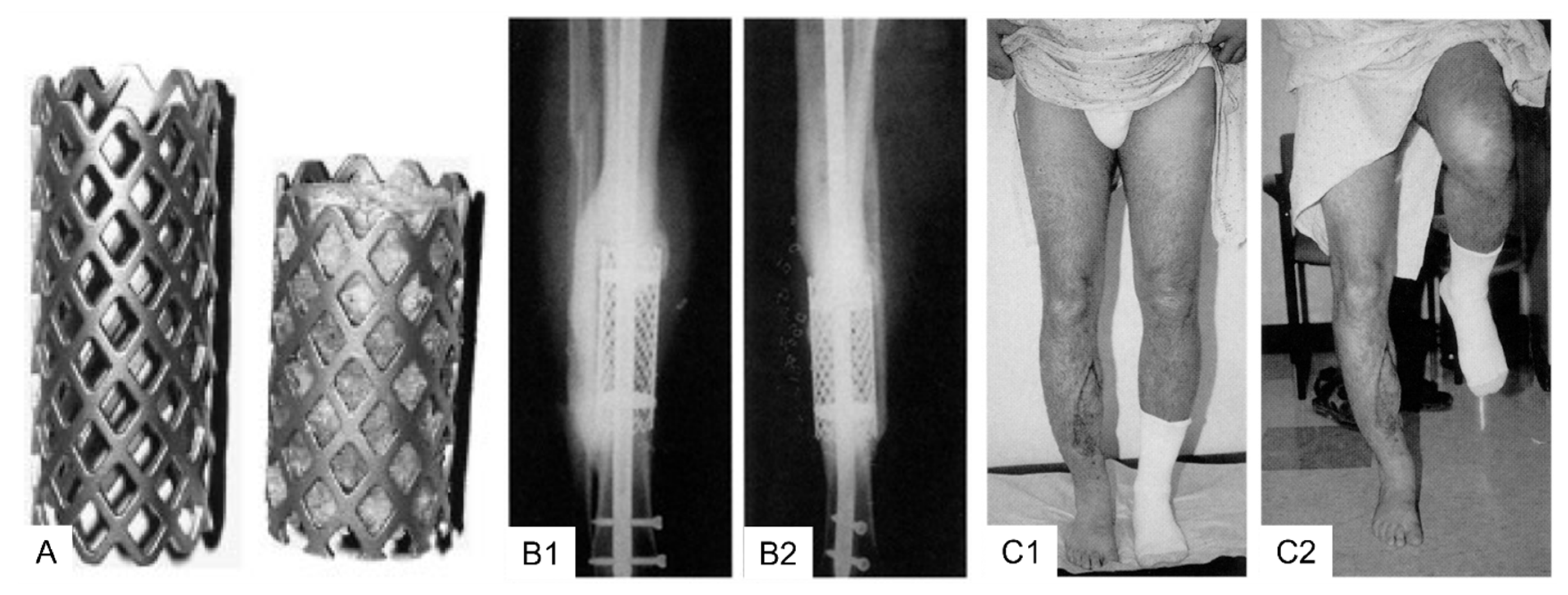
3.2. Clinical Application Cylindrical (Spinal) Titanium Mesh Cages for Long Bone Defects
3.3. Importance of Graft Material Compartmentalization
4. Bone Defect Regeneration in the Era of Advanced 3D-Printing Technology Platforms
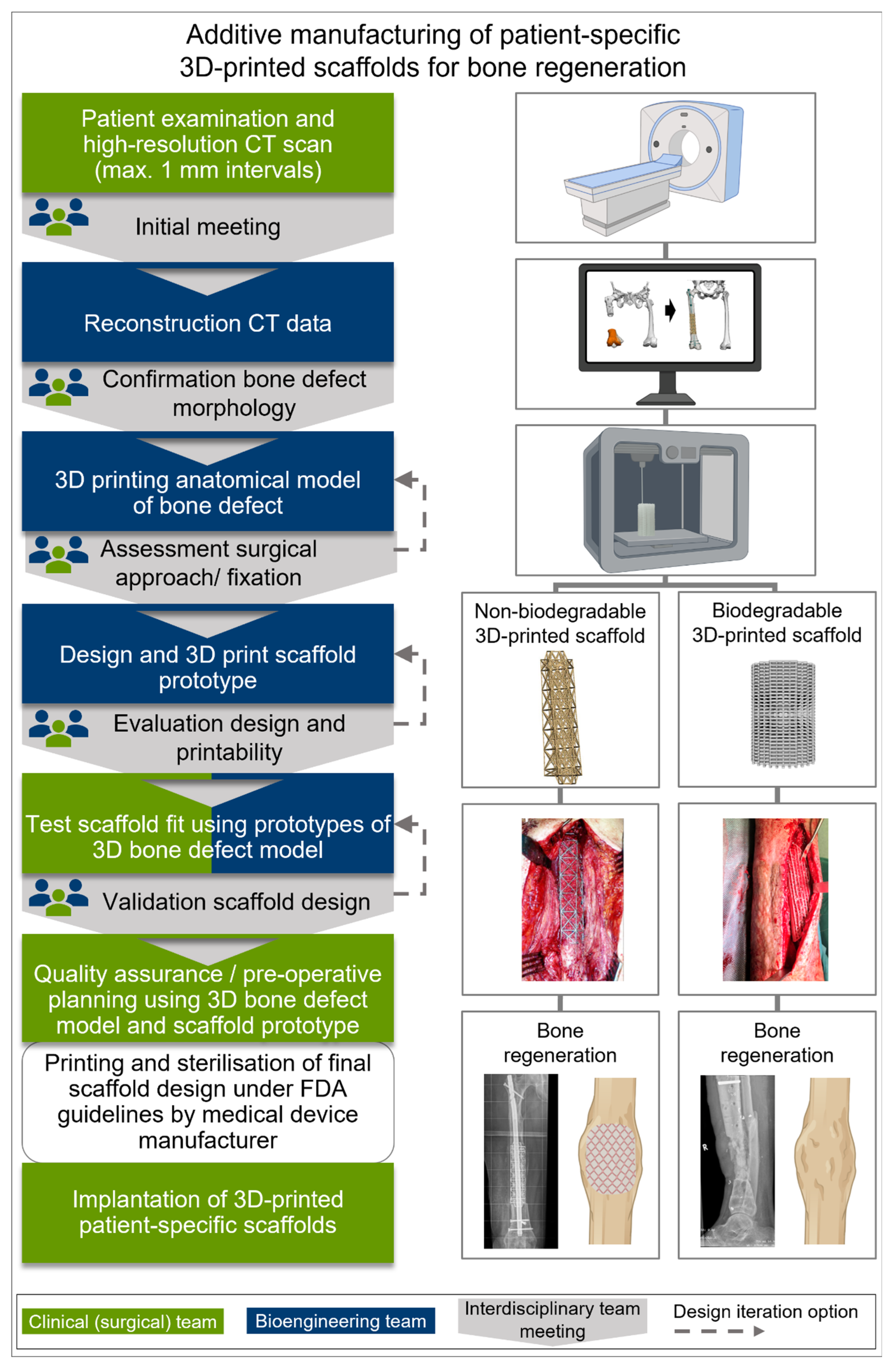
4.1. Additive Manufacturing and Surgical Utilization of Implants for SGBR
4.2. Patient-Specific 3D-Printed Titanium Scaffolds
| Reference (Year) | Number of Patients (Mean Age and Range) | Anatomical Location | Pathology | Defect Size | Masquelet Technique | Implant for Fixation | Bone/Synthetic Graft Substitute | Perioperative Complications | Patient Outcome | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Case studies with patient-specific 3D-printed medical-grade titanium (Ti-6Al-4V powder) scaffold using the ‘electron beam melting (EBM)’ printing technology | ||||||||||
| Tetsworth et al. [140] 2019 | N = 5 (49.0 years; 26–73) | Femur | Post-traumatic defects | Mean length 14.0 cm (10.3–18.4 cm); mean volume 192.4 cc (114–292 cc) | Two-stage Masquelet technique | Intra-medullary nail or lateral locked plate | Anterior iliac crest bone graft/graft material harvested with RIA system/allograft cancellous chips | No deep infections, fractures, nerve injuries, loss of alignment, or non-unions identified during follow-up | All patients achieved union clinically and radiographically. At latest follow-up, all 5 were ambulating, fully weight-bearing, and pain-free, with 1 patient using a cane when ambulating distances. | 21.8 months (range 12–33 months) |
| Gamieldien et al. [156] | N = 9 (36 years; 19–52) | Femur (n = 7); tibia (n = 2) | Chronic osteo-myelitis (n = 3); acute trauma with bone loss (n = 3); infected non-union (n = 2); aseptic bone defect non-union (n = 1) | Mean length 9.6 cm (3–20.5 cm) | 8/9 patients two-stage Masquelet technique | Intra-medullary nail | Graft material harvested with RIA system (n = 8)/Posterior iliac crest bone graft (n = 1) | No peri- or post-operative complications occurred | All cases progressed to functional union at a mean of 3.1 months (range 2–4.6 months). RUST score [185]: 4/9 union at a mean of 4.9 months (range 2.6–7 months). 6/9 features of radiological union with callus enveloping both ends of truss at a mean of 4.9 months (range 2.6–7.4 months) | 11.3 months (range 4.6–29 months) |
| Case report and case studies with patient-specific 3D-printed medical-grade PCL-TCP scaffolds using ‘fused deposition modelling (FDM)’ printing technology | ||||||||||
| Kobbe et al. [23] 2020 | N = 1 (29 years) | Femur | Post-traumatic defects | Circum-ferential bony defect, 6 cm at medial and 11 cm at lateral aspect of femur | No Masquelet technique | Intra-medullary nail | Graft material harvested with RIA system/rhBMP-2 | None reported | Advanced bone fusion at scaffold–host bone interface and bone formation, both inside and outside the fully interconnected scaffold architecture. | 12 months |
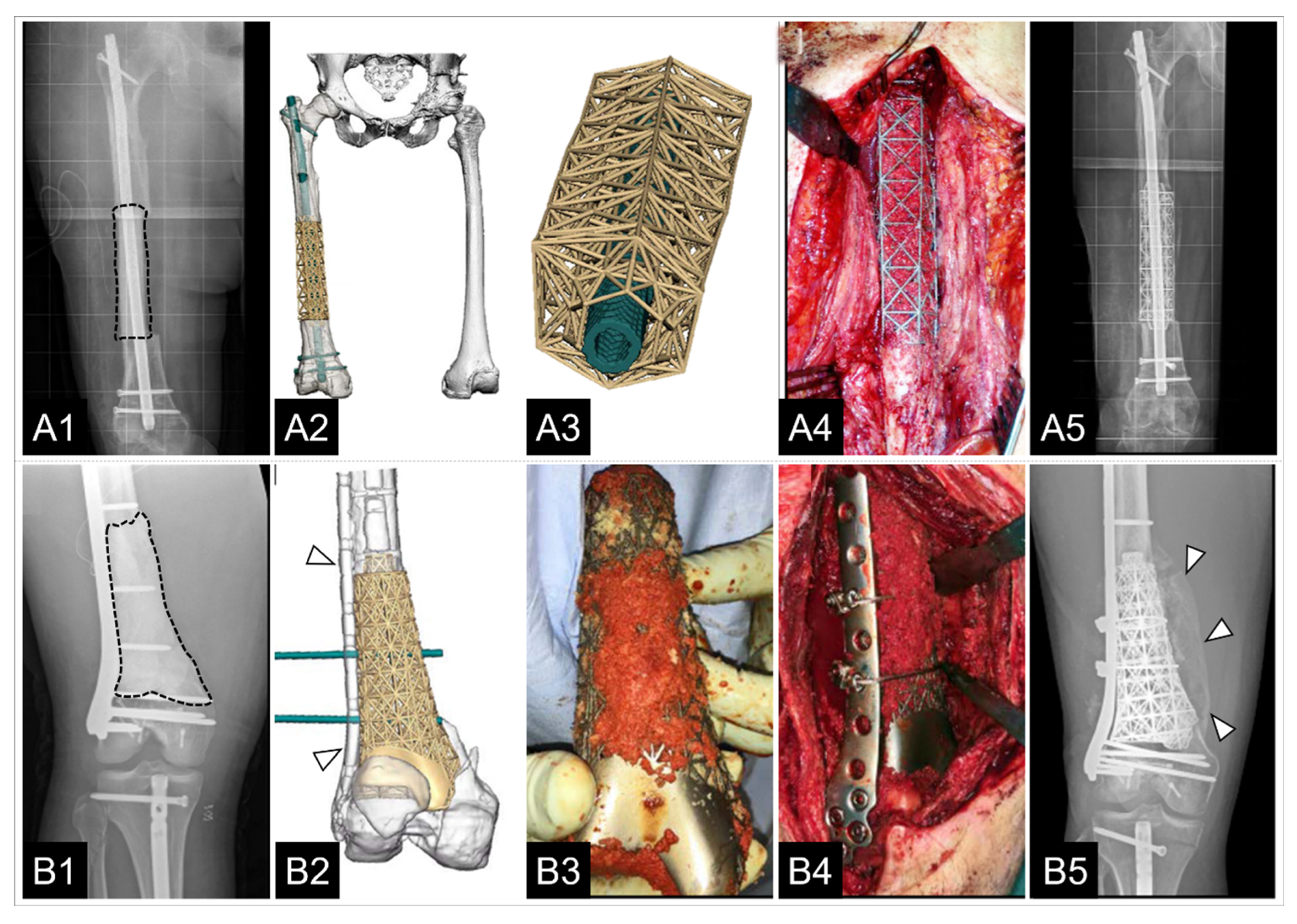
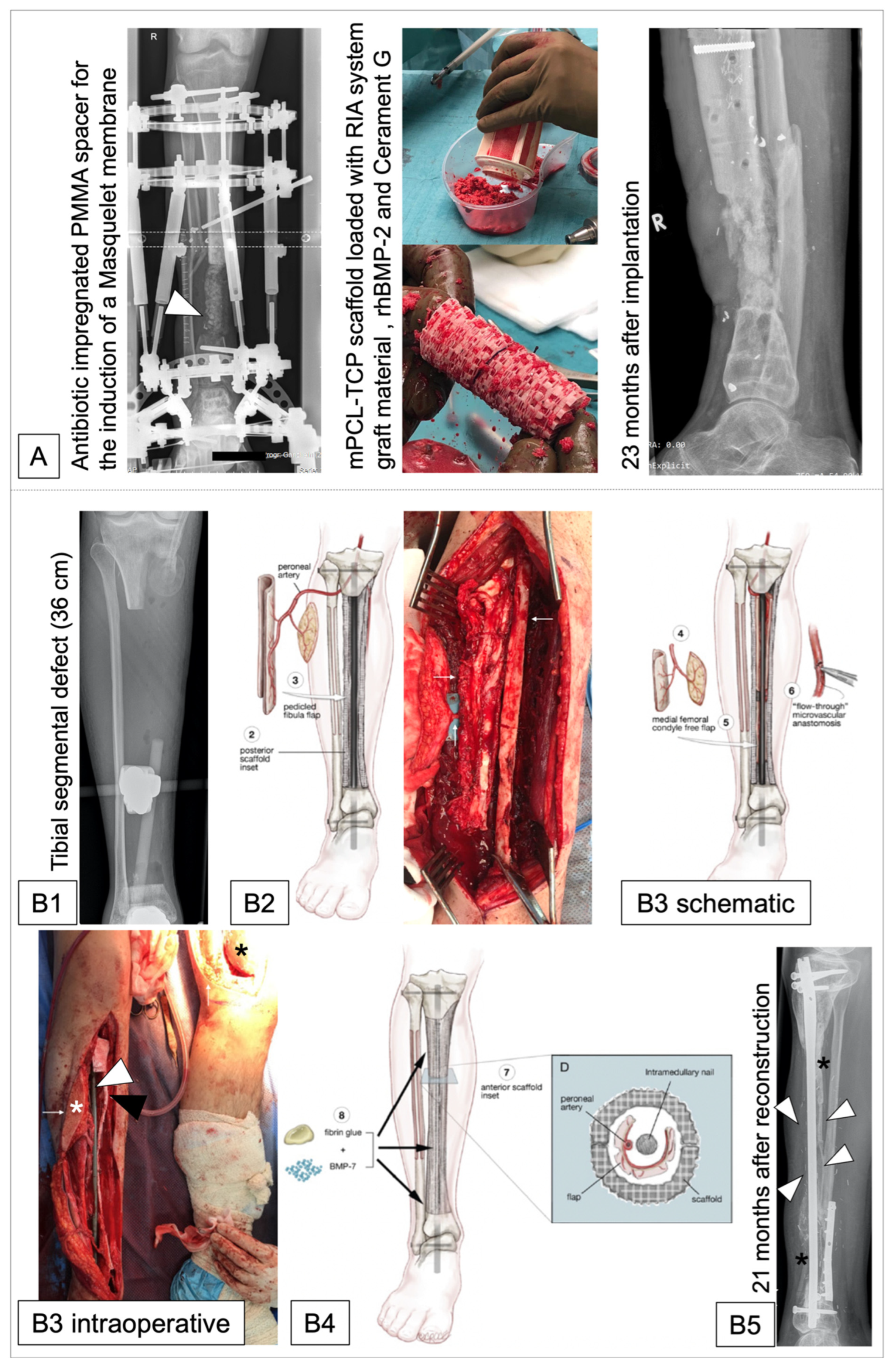
| Laubach et al. [137] 2022 | N = 4 (23–42 years) | Femur (n = 2); tibia (n = 2) | Post-traumatic defects | Volume 29.89 cm3–165.72 cm3 | Two-stage Masquelet technique | Plate or external fixator | Graft material harvested with RIA system/rhBMP-2/Cerament G | No peri-operative adverse events | In all cases, scaffolds matched the actual anatomical defect well; 3/4 cases showed evidence of bone ingrowth into the large honeycomb pores and fully interconnected scaffold architecture with indicated bony bridges 8–9 months after implant placement. In 1/4 cases, extensive bone regeneration and full loading capacity was achieved after 23 months. | 8–23 months |
| Castrisos et al. [157] 2022 | N = 2 (Case 1: 16 years; case 2: 27 years) | Tibia | Case 1: Ewing sarcoma; case 2: Osteo-myelitis | Volume case 1: 64.641 cm2; Volume case 2: 149.285 cm2 (length 36 cm) | No Masquelet technique | Case 1: Load bearing intra-medullary nail; Case 2: Load bearing plate and screws | Vascularized cortico-periosteal-cutaneous flap (CPCF) plus rhBMP-7. Case 1: ipsilateral medial femoral condyle; Case 2: 2x CPCF (1. Ipsilateral fibula; 2. Contralateral medial femoral condyle) | No intraoperative complications; Case 1: post-OP day 2: extensive blistering of the native skin distal to the CPCF skin paddle; Case 2: 19 months post-OP: Revision with IC ABG and 8 mL of rhBMP-7 for 6 mm defect at junction middle-distal third of the reconstruction | Case 1: good volumes of regenerated bone with osteosynthesis to native bone. Case 2: regenerated bone developed throughout the scaffold over 24 months. | Case 1: 24 months; Case 2: 48 months |
4.3. Patient-Specific 3D-Printed mPCL-TCP Scaffolds
5. Synergistic Effect of 3D-Printed Scaffolds with the Masquelet Technique and Bone Transport to Facilitate Bone Regeneration
6. Patient-Specific 3D-Printed Scaffolds to Regenerate Long Bone Defects: Convergence and Clinical Translation
6.1. Torrential Stream following the “Valley of Death”
6.2. Three-Dimensional-Printed Bone Regeneration Scaffolds—Quo Vadis? Consensus via Stakeholder Workshop
7. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Crane, G.M.; Ishaug, S.L.; Mikos, A.G. Bone tissue engineering. Nat. Med. 1995, 1, 1322–1324. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Paprosky, W.G.; Perona, P.G.; Lawrence, J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty: A 6-year follow-up evaluation. J. Arthroplast. 1994, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Krettek, C.; Lowenberg, D.W.; Tosounidis, T.; Borrelli, J., Jr. Fracture Healing Adjuncts–The World’s Perspective on What Works. J. Orthop. Trauma 2018, 32, S43–S47. [Google Scholar] [CrossRef]
- Ferreira, N.; Tanwar, Y.S. Systematic Approach to the Management of Post-traumatic Segmental Diaphyseal Long Bone Defects: Treatment Algorithm and Comprehensive Classification System. Strateg. Trauma Limb Reconstr. 2020, 15, 106–116. [Google Scholar] [CrossRef]
- Mauffrey, C.; Hake, M.E.; Chadayammuri, V.; Masquelet, A.C. Reconstruction of long bone infections using the induced membrane technique: Tips and tricks. J. Orthop. Trauma 2016, 30, e188–e193. [Google Scholar] [CrossRef]
- McClure, P.K.; Alrabai, H.M.; Conway, J.D. Preoperative Evaluation and Optimization for Reconstruction of Segmental Bone Defects of the Tibia. J. Orthop. Trauma 2017, 31, S16–S19. [Google Scholar] [CrossRef]
- Pacha, T.O.; Mommsen, P.; Brauckmann, V.; Aktas, G.; Krempec, M.; Wilhelmi, B.; Clausen, J.D.; März, V.; Krezdorn, N.; Vogt, P.M.; et al. Interdisciplinary extremity board in the treatment of complex injuries. Unfallchirurgie 2023, 126, 175–183. [Google Scholar] [CrossRef]
- Tetsworth, K.; Block, S.; Glatt, V. Putting 3D modelling and 3D printing into practice: Virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT-J. 2017, 3, 16. [Google Scholar] [CrossRef]
- Pollak, A.N.; Ficke, J.R.; Injuries, E.W., III. Extremity War Injuries: Challenges in Definitive Reconstruction. JAAOS-J. Am. Acad. Orthop. Surg. 2008, 16, 628–634. [Google Scholar] [CrossRef]
- DeCoster, T.A.; Gehlert, R.J.; Mikola, E.A.; Pirela-Cruz, M.A. Management of Posttraumatic Segmental Bone Defects. JAAOS-J. Am. Acad. Orthop. Surg. 2004, 12, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, B.; Neugebauer, E. Outcome after polytrauma. Langenbeck’s Arch. Surg. 1998, 383, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Zelle, B.; Lohse, R.; Hildebrand, F.; Krettek, C.; Panzica, M.; Duhme, V.; Sittaro, N.A. Evaluation and outcome of patients after polytrauma—Can patients be recruited for long-term follow-up? Injury 2006, 37, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, I.b. Alâim-i Cerrâhîn [in Ottoman Turkish]; Süleymaniye Library: Istanbul, Turkey, 1505. [Google Scholar]
- Tello, J.C. Antiguo Peru: Primera Epoca; Comisión Organizadora del Segundo Congreso Sudamericano de Turismo: Lima, Peru, 1929. [Google Scholar]
- Meekeren, J.J. Observationes Medico-Chirugicae; Ex Officina Henrici & Vidnae Theodori Boom: Amsterdam, The Netherlands, 1682.
- Taylor, G.I.; Miller, G.D.H.; Ham, F.J. THE FREE VASCULARIZED BONE GRAFT: A Clinical Extension of Microvascular Techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Aciduman, A.; Belen, D. The earliest document regarding the history of cranioplasty from the Ottoman era. Surg. Neurol. 2007, 68, 349–352. [Google Scholar] [CrossRef]
- Sanan, A.; Haines, S.J. Repairing Holes in the Head: A History of Cranioplasty. Neurosurgery 1997, 40, 588–603. [Google Scholar]
- Meinig, R.P.; Rahn, B.; Perren, S.M.; Gogolewski, S. Bone Regeneration with Resorbable Polymeric Membranes: Treatment of Diaphyseal Bone Defects in the Rabbit Radius with Poly(L-Lactide) Membrane. A Pilot Study. J. Orthop. Trauma 1996, 10, 178–190. [Google Scholar] [CrossRef]
- Gugala, Z.; Lindsey, R.W.; Gogolewski, S. New Approaches in the Treatment of Critical-Size Segmental Defects in Long Bones. Macromol. Symp. 2007, 253, 147–161. [Google Scholar] [CrossRef]
- Kobbe, P.; Laubach, M.; Hutmacher, D.W.; Alabdulrahman, H.; Sellei, R.M.; Hildebrand, F. Convergence of scaffold-guided bone regeneration and RIA bone grafting for the treatment of a critical-sized bone defect of the femoral shaft. Eur. J. Med. Res. 2020, 25, 70. [Google Scholar] [CrossRef]
- Codivilla, A. On the Means of Lengthening, in the Lower Limbs, the Muscles and Tissues Which Are Shortened through Deformity. JBJS 1905, 2, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Gubin, A.V.; Borzunov, D.Y.; Marchenkova, L.O.; Malkova, T.A.; Smirnova, I.L. Contribution of G.A. Ilizarov to bone reconstruction: Historical achievements and state of the art. Strateg. Trauma Limb Reconstr. 2016, 11, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Krappinger, D.; Lindtner, R.A.; Zegg, M.; Dal Pont, A.; Huber, B. Masquelet technique for the treatment of large dia- and metaphyseal bone defects. Oper. Orthop. Traumatol. 2015, 27, 357–368. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, C.M.; Kumar, A.R.; Gerszten, P.C. The history of military cranioplasty. Neurosurg. Focus FOC 2014, 36, E18. [Google Scholar] [CrossRef]
- Feroze, A.H.; Walmsley, G.G.; Choudhri, O.; Lorenz, H.P.; Grant, G.A.; Edwards, M.S. Evolution of cranioplasty techniques in neurosurgery: Historical review, pediatric considerations, and current trends. J. Neurosurg. 2015, 123, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Burwell, R. History of bone grafting and bone substitutes with special reference to osteogenic induction. In Bone Grafts, Derivatives, and Substitutes; Urist, M.R., O’Connor, B.T., Burwell, R.G., Eds.; Butterworth-Heinemann Medical: Oxford, UK, 1994. [Google Scholar]
- Laubach, M.; Kobbe, P.; Hutmacher, D.W. Biodegradable interbody cages for lumbar spine fusion: Current concepts and future directions. Biomaterials 2022, 288, 121699. [Google Scholar] [CrossRef]
- Rifkinson-Mann, S. Cranial surgery in ancient Peru. Neurosurgery 1988, 23, 411–416. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Aldea, P.A.; Shaw, W.W. The Evolution of the Surgical Management of Severe Lower Extremity Trauma. Clin. Plast. Surg. 1986, 13, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Dreesmann, H. Ueber Knochenplombirung. Dtsch. Med. Wochenschr. 1893, 19, 445–446. [Google Scholar] [CrossRef]
- Peltier, L.F.; Bickel, E.Y.; Lillo, R.; Thein, M.S. The use of plaster of paris to fill defects in bone. Ann. Surg. 1957, 146, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lister, J. On the Antiseptic Principle in the Practice of Surgery. Br. Med. J. 1867, 2, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.H.; Toledo, A.H. Historical development of modern anesthesia. J. Investig. Surg. 2012, 25, 141–149. [Google Scholar] [CrossRef]
- Underwood, E.A. Wilhelm Conrad Röntgen (1845–1923) and the Early Development of Radiology. Proc. R. Soc. Med. 1945, 38, 697–706. [Google Scholar] [CrossRef]
- Bigelow, H.J. Insensibility during surgical operations produced by inhalation. Boston Med. Surg. J. 1846, 35, 309–317. [Google Scholar] [CrossRef]
- Klar, R.M. The Induction of Bone Formation: The Translation Enigma. Front. Bioeng. Biotechnol. 2018, 6, 74. [Google Scholar] [CrossRef]
- Wagels, M.; Rowe, D.; Senewiratne, S.; Theile, D.R. History of lower limb reconstruction after trauma. ANZ J. Surg. 2013, 83, 348–353. [Google Scholar] [CrossRef]
- Simpson, D. Titanium in Cranioplasty. J. Neurosurg. 1965, 22, 292–293. [Google Scholar] [CrossRef]
- Alkhaibary, A.; Alharbi, A.; Alnefaie, N.; Oqalaa Almubarak, A.; Aloraidi, A.; Khairy, S. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects, and Complications. World Neurosurg. 2020, 139, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.J.; Goldstein, R.Y.; McLaurin, T.M.; Grant, A. The evolution of the Ilizarov technique: Part 1: The history of limb lengthening. Bull. Hosp. Jt. Dis. (2013) 2013, 71, 89–95. [Google Scholar] [PubMed]
- Wiedemann, M. Callus distraction: A new method? A historical review of limb lengthening. Clin. Orthop. Relat. Res. 1996, 327, 291–304. [Google Scholar] [CrossRef]
- Brand, R.A. Advances in limb lengthening and reconstruction: Alessandro Codivilla, MD, 1861-1912. Clin. Orthop. Relat. Res. 2008, 466, 2901–2902. [Google Scholar] [CrossRef] [PubMed]
- Codivilla, A. Sulla correzione della deformita de frattura del femore. Bull. Sci. Med. 1903, 3, 246–249. [Google Scholar]
- Cech, O. Prof. Ilizarov and his contribution to the challenge of limb lengthening. Injury 1993, 24 (Suppl. S2), S2–S8. [Google Scholar] [CrossRef]
- Paterson, D. Leg-lengthening procedures. A historical review. Clin. Orthop. Relat. Res. 1990, 250, 27–33. [Google Scholar] [CrossRef]
- Wagner, W. Die temporäre Resektion des Schädeldaches an stelle der Trepanation. Zentralbl. Chir. 1889, 16, 833–838. [Google Scholar]
- Sparks, D.S.; Saleh, D.B.; Rozen, W.M.; Hutmacher, D.W.; Schuetz, M.A.; Wagels, M. Vascularised bone transfer: History, blood supply and contemporary problems. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1–11. [Google Scholar] [CrossRef]
- Götz, J.; Schellmann, W.D. Continuous lengthening of the femur with intramedullary stabilisation (author’s transl). Arch. Orthop. Unfallchir. 1975, 82, 305–310. [Google Scholar] [CrossRef]
- Thaller, P.H.; Frankenberg, F.; Degen, N.; Soo, C.; Wolf, F.; Euler, E.; Fürmetz, J. Complications and Effectiveness of Intramedullary Limb Lengthening: A Matched Pair Analysis of Two Different Lengthening Nails. Strategies Trauma Limb Reconstr. 2020, 15, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Degen, N.; de Almeida Lopes, N.; Wolf, F.; Fürmetz, J.; Euler, E.; Böcker, W.; Thaller, P.H. Pain levels during distraction osteogenesis with lengthening nails in 168 cases. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, C.; Rölfing, J.D.; Langlais, T.; Sales de Gauzy, J.; Accadbled, F. No osteolysis at the telescopic junction of 128 FITBONE lengthening nails. Orthop. Traumatol. Surg. Res. 2023, 109, 103501. [Google Scholar] [CrossRef]
- Glatt, V.; Evans, C.H.; Tetsworth, K. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front. Physiol. 2017, 7, 678. [Google Scholar] [CrossRef]
- Thaller, P.H.; Wolf, F.; Kucukkaya, M. Surgical Techniques for Lengthening and Deformity Correction of the Tibia with Lengthening Nails. Tech. Orthop. 2014, 29, 150–157. [Google Scholar] [CrossRef]
- Kazmirchuk, A.; Yarmoliuk, Y.; Lurin, I.; Gybalo, R.; Burianov, O.; Derkach, S.; Karpenko, K. Ukraine’s Experience with Management of Combat Casualties Using NATO’s Four-Tier “Changing as Needed” Healthcare System. World, J. Surg. 2022, 46, 2858–2862. [Google Scholar] [CrossRef]
- Trutyak, I.; Los, D.; Medzyn, V.; Trunkvalter, V.; Zukovsky, V. Treatment of combat surgical trauma of the limbs in the conditions of modern war. Proceeding Shevchenko Sci. Society. Med. Sci. 2022, 69. [Google Scholar] [CrossRef]
- Carbone, E.J.; Jiang, T.; Nelson, C.; Henry, N.; Lo, K.W.H. Small molecule delivery through nanofibrous scaffolds for musculoskeletal regenerative engineering. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1691–1699. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N. Natural Medicinal Compounds in Bone Tissue Engineering. Trends Biotechnol. 2020, 38, 404–417. [Google Scholar] [CrossRef]
- Leanora Anne, M.; Simpson, A.H.R.W. The relative incidence of fracture non-union in the Scottish population (5.17 million): A 5-year epidemiological study. BMJ Open 2013, 3, e002276. [Google Scholar] [CrossRef]
- Cillóniz, C.; Rodríguez-Hurtado, D.; Torres, A. Characteristics and Management of Community-Acquired Pneumonia in the Era of Global Aging. Med. Sci. 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; McKee, M.D.; Einhorn, T.A.; Watson, J.T.; Li, R.; Schemitsch, E.H. Managing bone defects. J. Orthop. Trauma 2011, 25, 462–466. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-graft substitutes: Facts, fictions, and applications. J. Bone Jt. Surg. Am. 2001, 83 Pt 2 (Suppl. S2), 98–103. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Sales de Gauzy, J.; Bauer, T.; Fabre, A.; Fitoussi, F.; Hannouche, D.; Jouve, J.L.; Karger, C.; Le Nen, D.; Mathevon, H.; et al. Reconstruction of post-traumatic diaphyseal bone defects: Preoperative planning, guideline, and future developments. Rev. Chir. Orthopédique Traumatol. 2012, 98, 94–103. [Google Scholar] [CrossRef]
- Madison, R.D.; Nowotarski, P.J. The Reamer-Irrigator-Aspirator in Nonunion Surgery. Orthop. Clin. N. Am. 2019, 50, 297–304. [Google Scholar] [CrossRef]
- Weiland, A.J.; Phillips, T.W.; Randolph, M.A. Bone grafts: A radiologic, histologic, and biomechanical model comparing autografts, allografts, and free vascularized bone grafts. Plast. Reconstr. Surg. 1984, 74, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C. Muscle reconstruction in reconstructive surgery: Soft tissue repair and long bone reconstruction. Langenbeck’s Arch. Surg. 2003, 388, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Cierny, G., III; Mader, J.T.; Penninck, J.J. The Classic: A Clinical Staging System for Adult Osteomyelitis. Clin. Orthop. Relat. Res. 2003, 414, 7–24. [Google Scholar] [CrossRef] [PubMed]
- McPherson, E.J.; Tontz, W.; Patzakis, M.; Woodsome, C.; Holtom, P.; Norris, L.; Shufelt, C. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am. J. Orthop. 1999, 28, 161–165. [Google Scholar] [PubMed]
- McPherson, E.J.; Woodson, C.; Holtom, P.; Roidis, N.; Shufelt, C.; Patzakis, M. Periprosthetic Total Hip Infection: Outcomes Using a Staging System. Clin. Orthop. Relat. Res. 2002, 403, 8–15. [Google Scholar] [CrossRef]
- Friedrich, J.B.; Moran, S.L.; Bishop, A.T.; Shin, A.Y. Free vascularized fibula grafts for salvage of failed oncologic long bone reconstruction and pathologic fractures. Microsurgery 2009, 29, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Pederson, W.C.; Person, D.W. Long bone reconstruction with vascularized bone grafts. Orthop. Clin. 2007, 38, 23–35. [Google Scholar] [CrossRef]
- Ghert, M.; Colterjohn, N.; Manfrini, M. The use of free vascularized fibular grafts in skeletal reconstruction for bone tumors in children. JAAOS-J. Am. Acad. Orthop. Surg. 2007, 15, 577–587. [Google Scholar] [CrossRef]
- Kong, L.-c.; Li, H.A.; Kang, Q.-l.; Li, G. An update to the advances in understanding distraction histogenesis: From biological mechanisms to novel clinical applications. J. Orthop. Transl. 2020, 25, 3–10. [Google Scholar] [CrossRef]
- Dugan, T.R.; Hubert, M.G.; Siska, P.A.; Pape, H.-C.; Tarkin, I.S. Open supracondylar femur fractures with bone loss in the polytraumatized patient–Timing is everything! Injury 2013, 44, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Bosse, M.J.; MacKenzie, E.J.; Kellam, J.F.; Burgess, A.R.; Webb, L.X.; Swiontkowski, M.F.; Sanders, R.W.; Jones, A.L.; McAndrew, M.P.; Patterson, B.M.; et al. An Analysis of Outcomes of Reconstruction or Amputation after Leg-Threatening Injuries. N. Engl. J. Med. 2002, 347, 1924–1931. [Google Scholar] [CrossRef]
- Appleton, P.; Moran, M.; Houshian, S.; Robinson, C.M. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J. Bone Jt. Surg. Br. 2006, 88, 1065–1070. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Mekhail, A.O.; Abraham, E.; Gruber, B.; Gonzalez, M. Bone Transport in the Management of Posttraumatic Bone Defects in the Lower Extremity. J. Trauma Acute Care Surg. 2004, 56, 368–378. [Google Scholar] [CrossRef]
- Petri, M.; Namazian, A.; Wilke, F.; Ettinger, M.; Stübig, T.; Brand, S.; Bengel, F.; Krettek, C.; Berding, G.; Jagodzinski, M. Repair of segmental long-bone defects by stem cell concentrate augmented scaffolds: A clinical and positron emission tomography--computed tomography analysis. Int. Orthop. 2013, 37, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, B.; Gilbert, F.; Prodinger, P.M.; Raab, P.; Knebel, C.; Wille, M.-L.; Rudert, M.; Hutmacher, D.W. 3D-printed, patient-specific scaffolds for regenerative therapy of osseous defects of long bone: Successfully bridging the gap between basic research and clinical application [own translation; conference abstract in German]. In Proceedings of the Deutscher Kongress für Orthopädie und Unfallchirurgie (DKOU 2019), Berlin, Germany, 22–25 October 2019. [Google Scholar]
- Higgins, T.F.; Klatt, J.B.; Beals, T.C. Lower Extremity Assessment Project (LEAP)--the best available evidence on limb-threatening lower extremity trauma. Orthop. Clin. N. Am. 2010, 41, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, R.; Siwach, K.; Devgan, A.; Singh, R.; Wadhwani, J.; Ahmed, N. Outcome of distraction osteogenesis by ring fixator in infected, large bone defects of tibia. J. Clin. Orthop. Trauma 2016, 7, 201–209. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Ren, Y.; Song, H.-J.; Ali, A.A.; Meng, X.-Q.; Xu, L.; Zhang, H.-A.; Fang, J.; Qin, C.-H. One-stage debridement and bone transport versus first-stage debridement and second-stage bone transport for the management of lower limb post-traumatic osteomyelitis. J. Orthop. Transl. 2021, 28, 21–27. [Google Scholar] [CrossRef]
- Clements, J.R.; Carpenter, B.B.; Pourciau, J.K. Treating segmental bone defects: A new technique. J. Foot Ankle Surg. 2008, 47, 350–356. [Google Scholar] [CrossRef]
- Iacobellis, C.; Berizzi, A.; Aldegheri, R. Bone transport using the Ilizarov method: A review of complications in 100 consecutive cases. Strateg. Trauma Limb Reconstr. 2010, 5, 17–22. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Faour, O.; Goff, T.; Kanakaris, N.; Dimitriou, R. Masquelet technique for the treatment of bone defects: Tips-tricks and future directions. Injury 2011, 42, 591–598. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010, 41, 27–37, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.; Kishi, T.; Schneider, L.; Fitoussi, F.; Masquelet, A.C. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 2012, 98, 97–102. [Google Scholar] [CrossRef]
- Pelissier, P.; Martin, D.; Baudet, J.; Lepreux, S.; Masquelet, A.C. Behaviour of cancellous bone graft placed in induced membranes. Br. J. Plast. Surg. 2002, 55, 596–598. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar]
- Masquelet, A.; Kanakaris, N.K.; Obert, L.; Stafford, P.; Giannoudis, P.V. Bone Repair Using the Masquelet Technique. J. Bone Jt. Surg.-Am. Vol. 2019, 101, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Stafford, P.R.; Norris, B.L. Reamer-irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: A review of 25 cases. Injury 2010, 41 (Suppl. S2), S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Ashman, O.; Phillips, A.M. Treatment of non-unions with bone defects: Which option and why? Injury 2013, 44 (Suppl. S1), S43–S45. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C. The evolution of the induced membrane technique: Current status and future directions. Tech. Orthop. 2016, 31, 3–8. [Google Scholar] [CrossRef]
- Owens, B.D.; Kragh, J.F., Jr.; Macaitis, J.; Svoboda, S.J.; Wenke, J.C. Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J. Orthop. Trauma 2007, 21, 254–257. [Google Scholar] [CrossRef]
- Christou, C.; Oliver, R.A.; Yu, Y.; Walsh, W.R. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PLoS ONE 2014, 9, e114122. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. S4), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Dalisson, B.; Charbonnier, B.; Aoude, A.; Gilardino, M.; Harvey, E.; Makhoul, N.; Barralet, J. Skeletal regeneration for segmental bone loss: Vascularised grafts, analogues and surrogates. Acta Biomater. 2021, 136, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, A.; Yang, X.; Gong, J.; Yu, M.; Yao, X.; Wang, H.; He, Y. 3D Cell Culture—Can It Be As Popular as 2D Cell Culture? Adv. NanoBiomed Res. 2021, 1, 2000066. [Google Scholar] [CrossRef]
- van der Putten, C.; Buskermolen, A.B.C.; Werner, M.; Brouwer, H.F.M.; Bartels, P.A.A.; Dankers, P.Y.W.; Bouten, C.V.C.; Kurniawan, N.A. Protein Micropatterning in 2.5D: An Approach to Investigate Cellular Responses in Multi-Cue Environments. ACS Appl. Mater. Interfaces 2021, 13, 25589–25598. [Google Scholar] [CrossRef]
- de la Zerda, A.; Kratochvil, M.J.; Suhar, N.A.; Heilshorn, S.C. Review: Bioengineering strategies to probe T cell mechanobiology. APL Bioeng. 2018, 2, 021501. [Google Scholar] [CrossRef]
- Kumar, S.; Azam, D.; Raj, S.; Kolanthai, E.; Vasu, K.S.; Sood, A.K.; Chatterjee, K. 3D scaffold alters cellular response to graphene in a polymer composite for orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 732–749. [Google Scholar] [CrossRef]
- Woodley, J.P.; Lambert, D.W.; Asencio, I.O. Understanding Fibroblast Behavior in 3D Biomaterials. Tissue Eng. Part B Rev. 2022, 28, 569–578. [Google Scholar] [CrossRef]
- Zandrini, T.; Florczak, S.; Levato, R.; Ovsianikov, A. Breaking the resolution limits of 3D bioprinting: Future opportunities and present challenges. Trends Biotechnol. 2022, 41, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.M.; Imperio, R.; Wen, M.; Meyers, M.A.; McKittrick, J. Bioinspired Scaffolds with Varying Pore Architectures and Mechanical Properties. Adv. Funct. Mater. 2014, 24, 1978–1987. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Gogolewski, S.; Galletti, G. Degradable, microporous vascular prosthesis from segmented polyurethane. Colloid Polym. Sci. 1986, 264, 854–858. [Google Scholar] [CrossRef]
- Gogolewski, S. Implantable segmented polyurethanes: Controversies and uncertainties. Life Support Syst. J. Eur. Soc. Artif. Organs 1987, 5, 41–46. [Google Scholar]
- Farso Nielsen, F.; Karring, T.; Gogolewski, S. Biodegradable guide for bone regeneration. Polyurethane membranes tested in rabbit radius defects. Acta Orthop. Scand. 1992, 63, 66–69. [Google Scholar] [CrossRef]
- Gogolewski, S.; Meinig, R.; Perren, S.M. Bone Regeneration Membrane. Patent US-5676699-A, 14 October 1997. [Google Scholar]
- Gugala, Z.; Gogolewski, S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: A preliminary study. J. Orthop. Trauma 1999, 13, 187–195. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.W.; Gugala, Z.; Milne, E.; Sun, M.; Gannon, F.H.; Latta, L.L. The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. J. Orthop. Res. 2006, 24, 1438–1453. [Google Scholar] [CrossRef]
- Cobos, J.A.; Lindsey, R.W.; Gugala, Z. The Cylindrical Titanium Mesh Cage for Treatment of a Long Bone Segmental Defect: Description of a New Technique and Report of Two Cases. J. Orthop. Trauma 2000, 14, 54–59. [Google Scholar] [CrossRef]
- Lindsey, R.W.; Gugala, Z. Cylindrical Titanium Mesh Cage for the Reconstruction of Long Bone Defects. Osteosynth. Trauma Care 2004, 12, 108–115. [Google Scholar] [CrossRef]
- Attias, N.; Lindsey, R.W. CASE REPORTS: Management of Large Segmental Tibial Defects Using a Cylindrical Mesh Cage. Clin. Orthop. Relat. Res. 2006, 450, 259–266. [Google Scholar] [CrossRef]
- Attias, N.; Thabet, A.M.; Prabhakar, G.; Dollahite, J.A.; Gehlert, R.J.; DeCoster, T.A. Management of extra-articular segmental defects in long bone using a titanium mesh cage as an adjunct to other methods of fixation. Bone Jt. J. 2018, 100-B, 646–651. [Google Scholar] [CrossRef]
- Tang, K.; Day, W.; Tarpada, S.; Kahn, M.D. Treatment of an Infected Tibial Shaft Non-Union Using a Novel 3D-Printed Titanium Mesh Cage: A Case Report. Cureus 2023, 15, e34212. [Google Scholar] [CrossRef]
- O’Malley, N.T.; Kates, S.L. Advances on the Masquelet technique using a cage and nail construct. Arch. Orthop. Trauma Surg. 2012, 132, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Pobloth, A.-M.; Checa, S.; Razi, H.; Petersen, A.; Weaver, J.C.; Schmidt-Bleek, K.; Windolf, M.; Tatai, A.Á.; Roth, C.P.; Schaser, K.-D.; et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018, 10, eaam8828. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C., Jr.; Bondoc, C.C.; Jung, W.K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981, 194, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Orgill, D.P.; Burke, J.F. Template for Skin Regeneration. Plast. Reconstr. Surg. 2011, 127, 60S–70S. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Savi, F.M.; Saifzadeh, S.; Schuetz, M.A.; Wagels, M.; Hutmacher, D.W. Convergence of Scaffold-Guided Bone Reconstruction and Surgical Vascularization Strategies-A Quest for Regenerative Matching Axial Vascularization. Front. Bioeng. Biotechnol. 2020, 7, 448. [Google Scholar] [CrossRef]
- Laurent, J.; Blin, G.; Chatelain, F.; Vanneaux, V.; Fuchs, A.; Larghero, J.; Théry, M. Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat. Biomed. Eng. 2017, 1, 939–956. [Google Scholar] [CrossRef]
- Tetsworth, K.D.; Mettyas, T. Overview of Emerging Technology in Orthopedic Surgery: What is the Value in 3D Modeling and Printing? Tech. Orthop. 2016, 31, 143–152. [Google Scholar] [CrossRef]
- Smith, K.E.; Dupont, K.M.; Safranski, D.L.; Blair, J.; Buratti, D.; Zeetser, V.; Callahan, R.; Lin, J.; Gall, K. Use of 3D Printed Bone Plate in Novel Technique to Surgically Correct Hallux Valgus Deformities. Tech. Orthop. 2016, 31, 181–189. [Google Scholar] [CrossRef]
- Green, N.; Glatt, V.; Tetsworth, K.; Wilson, L.J.; Grant, C.A. A Practical Guide to Image Processing in the Creation of 3D Models for Orthopedics. Tech. Orthop. 2016, 31, 153–163. [Google Scholar] [CrossRef]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical translation of a patient-specific scaffold-guided bone regeneration concept in four cases with large long bone defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Pobloth, A.M.; Schell, H.; Petersen, A.; Beierlein, K.; Kleber, C.; Schmidt-Bleek, K.; Duda, G.N. Tubular open-porous beta-tricalcium phosphate polycaprolactone scaffolds as guiding structure for segmental bone defect regeneration in a novel sheep model. J. Tissue Eng. Regen. Med. 2018, 12, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Herath, B.; Suresh, S.; Downing, D.; Cometta, S.; Tino, R.; Castro, N.J.; Leary, M.; Schmutz, B.; Wille, M.-L.; Hutmacher, D.W. Mechanical and geometrical study of 3D printed Voronoi scaffold design for large bone defects. Mater. Des. 2021, 212, 110224. [Google Scholar] [CrossRef]
- Tetsworth, K.; Woloszyk, A.; Glatt, V. 3D printed titanium cages combined with the Masquelet technique for the reconstruction of segmental femoral defects: Preliminary clinical results and molecular analysis of the biological activity of human-induced membranes. OTA Int. 2019, 2, e016. [Google Scholar] [CrossRef]
- Mourino, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Won, J.-E.; Lee, Y.S.; Park, J.-H.; Lee, J.-H.; Shin, Y.S.; Kim, C.-H.; Knowles, J.C.; Kim, H.-W. Hierarchical microchanneled scaffolds modulate multiple tissue-regenerative processes of immune-responses, angiogenesis, and stem cell homing. Biomaterials 2020, 227, 119548. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cheng, Y.; Zhang, K.; Song, C.; Leng, H.; Zheng, Y.; Cai, H.; et al. Tailored Surface Treatment of 3D Printed Porous Ti6Al4V by Microarc Oxidation for Enhanced Osseointegration via Optimized Bone In-Growth Patterns and Interlocked Bone/Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 17964–17975. [Google Scholar] [CrossRef]
- Kopp, A.; Derra, T.; Müther, M.; Jauer, L.; Schleifenbaum, J.H.; Voshage, M.; Jung, O.; Smeets, R.; Kröger, N. Influence of design and postprocessing parameters on the degradation behavior and mechanical properties of additively manufactured magnesium scaffolds. Acta Biomater. 2019, 98, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, F.; Fu, J.; Gao, Q.; Liu, A.; Sun, M.; He, Y. Printing@Clinic: From Medical Models to Organ Implants. ACS Biomater. Sci. Eng. 2017, 3, 3083–3097. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Xu, X.; Zhu, D.; Zheng, Y. Design, printing, and engineering of regenerative biomaterials for personalized bone healthcare. Prog. Mater. Sci. 2023, 134, 101072. [Google Scholar] [CrossRef]
- Perier-Metz, C.; Cipitria, A.; Hutmacher, D.W.; Duda, G.N.; Checa, S. An in silico model predicts the impact of scaffold design in large bone defect regeneration. Acta Biomater. 2022, 145, 329–341. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nature Materials 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; Espinosa, M.d.M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 9656938. [Google Scholar] [CrossRef]
- Derby, B. Printing and Prototyping of Tissues and Scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Sun, J.S.; Wu, S.Y.H.; Lin, F.H. The role of muscle-derived stem cells in bone tissue engineering. Biomaterials 2005, 26, 3953. [Google Scholar] [CrossRef] [PubMed]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef]
- Gamieldien, H.; Ferreira, N.; Birkholtz, F.F.; Hilton, T.; Campbell, N.; Laubscher, M. Filling the gap: A series of 3D-printed titanium truss cages for the management of large, lower limb bone defects in a developing country setting. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 497–505. [Google Scholar] [CrossRef]
- Castrisos, G.; Gonzalez Matheus, I.; Sparks, D.; Lowe, M.; Ward, N.; Sehu, M.; Wille, M.-L.; Phua, Y.; Medeiros Savi, F.; Hutmacher, D.; et al. Regenerative matching axial vascularisation of absorbable 3D-printed scaffold for large bone defects: A first in human series. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 2108–2118. [Google Scholar] [CrossRef]
- Lowenberg, D.W.; Githens, M. Complex Limb Reconstruction with Simultaneous Muscle Transfer and Circular External Fixation. Tech. Orthop. 2015, 30, 156–160. [Google Scholar] [CrossRef]
- Lowenberg, D.W.; Feibel, R.J.; Louie, K.W.; Eshima, I. Combined Muscle Flap and Ilizarov Reconstruction for Bone and Soft Tissue Defects. Clin. Orthop. Relat. Res. 1996, 332, 37–51. [Google Scholar] [CrossRef]
- Coriaty, N.; Pettibone, K.; Todd, N.; Rush, S.; Carter, R.; Zdenek, C. Titanium Scaffolding: An Innovative Modality for Salvage of Failed First Ray Procedures. J. Foot Ankle Surg. 2018, 57, 593–599. [Google Scholar] [CrossRef]
- So, E.; Mandas, V.H.; Hlad, L. Large Osseous Defect Reconstruction Using a Custom Three-Dimensional Printed Titanium Truss Implant. J. Foot Ankle Surg. 2018, 57, 196–204. [Google Scholar] [CrossRef]
- Abar, B.; Kwon, N.; Allen, N.B.; Lau, T.; Johnson, L.G.; Gall, K.; Adams, S.B. Outcomes of Surgical Reconstruction Using Custom 3D-Printed Porous Titanium Implants for Critical-Sized Bone Defects of the Foot and Ankle. Foot Ankle Int. 2022, 43, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.R.; Ellington, J.K. Patient-Specific 3-Dimensional Printed Titanium Truss Cage with Tibiotalocalcaneal Arthrodesis for Salvage of Persistent Distal Tibia Nonunion. Foot Ankle Spec. 2015, 8, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mulhern, J.L.; Protzman, N.M.; White, A.M.; Brigido, S.A. Salvage of Failed Total Ankle Replacement Using a Custom Titanium Truss. J. Foot Ankle Surg. 2016, 55, 868–873. [Google Scholar] [CrossRef]
- Dekker, T.J.; Steele, J.R.; Federer, A.E.; Hamid, K.S.; Adams, S.B., Jr. Use of Patient-Specific 3D-Printed Titanium Implants for Complex Foot and Ankle Limb Salvage, Deformity Correction, and Arthrodesis Procedures. Foot Ankle Int. 2018, 39, 916–921. [Google Scholar] [CrossRef]
- Bejarano-Pineda, L.; Sharma, A.; Adams, S.B.; Parekh, S.G. Three-Dimensional Printed Cage in Patients with Tibiotalocalcaneal Arthrodesis Using a Retrograde Intramedullary Nail: Early Outcomes. Foot Ankle Spec. 2021, 14, 401–409. [Google Scholar] [CrossRef]
- Hamid, K.S.; Parekh, S.G.; Adams, S.B. Salvage of Severe Foot and Ankle Trauma with a 3D Printed Scaffold. Foot Ankle Int. 2016, 37, 433–439. [Google Scholar] [CrossRef]
- Nwankwo, E.C.; Chen, F.; Nettles, D.L.; Adams, S.B. Five-Year Follow-Up of Distal Tibia Bone and Foot and Ankle Trauma Treated with a 3D-Printed Titanium Cage. Case Rep. Orthop. 2019, 2019, 7571013. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E. Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: An overview. J. Mater. Res. Technol. 2020, 9, 1087–1103. [Google Scholar] [CrossRef]
- Murr, L.E. Strategies for creating living, additively manufactured, open-cellular metal and alloy implants by promoting osseointegration, osteoinduction and vascularization: An overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Onal, E.; Frith, J.E.; Jurg, M.; Wu, X.; Molotnikov, A. Mechanical Properties and In Vitro Behavior of Additively Manufactured and Functionally Graded Ti6Al4V Porous Scaffolds. Metals 2018, 8, 200. [Google Scholar] [CrossRef]
- Huzum, B.; Puha, B.; Necoara, R.M.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.D.; Alexa, O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef] [PubMed]
- Ciupik, L.; Kierzkowska, A.; Cecek, J.; Pieniazek, J.; Sterna, J.; Cieslik, M. The Use of Incremental Technology to Produce 3D-Truss Ti6Al4V Implants which Improves the Spinal Treatment Effectiveness. Key Eng. Mater. 2016, 687, 179–184. [Google Scholar] [CrossRef]
- Goriainov, V.; Cook, R.; Latham, J.M.; Dunlop, D.G.; Oreffo, R.O. Bone and metal: An orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014, 10, 4043–4057. [Google Scholar] [CrossRef]
- Feng, J.; Liu, B.; Lin, Z.; Fu, J. Isotropic octet-truss lattice structure design and anisotropy control strategies for implant application. Mater. Des. 2021, 203, 109595. [Google Scholar] [CrossRef]
- Bagheri, A.; Buj-Corral, I.; Ferrer, M.; Pastor, M.M.; Roure, F. Determination of the Elasticity Modulus of 3D-Printed Octet-Truss Structures for Use in Porous Prosthesis Implants. Materials 2018, 11, 2420. [Google Scholar] [CrossRef]
- Litrenta, J.; Tornetta, P., III; Mehta, S.; Jones, C.; O’Toole, R.V.; Bhandari, M.; Kottmeier, S.; Ostrum, R.; Egol, K.; Ricci, W.; et al. Determination of Radiographic Healing: An Assessment of Consistency Using RUST and Modified RUST in Metadiaphyseal Fractures. J. Orthop. Trauma 2015, 29, 516–520. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Lange, C.; Reichert, J.; Berner, A.; Chen, F.; Fratzl, P.; Schantz, J.-T.; Hutmacher, D.W. Bone tissue engineering: From bench to bedside. Mater. Today 2012, 15, 430–435. [Google Scholar] [CrossRef]
- Karimi, M.; Asefnejad, A.; Aflaki, D.; Surendar, A.; Baharifar, H.; Saber-Samandari, S.; Khandan, A.; Khan, A.; Toghraie, D. Fabrication of shapeless scaffolds reinforced with baghdadite-magnetite nanoparticles using a 3D printer and freeze-drying technique. J. Mater. Res. Technol. 2021, 14, 3070–3079. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Lu, T.-P.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. Part B Eng. 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.C.; Pérez-Madrigal, M.M.; Arno, M.C.; Dove, A.P. 3D Printing for the Clinic: Examining Contemporary Polymeric Biomaterials and Their Clinical Utility. Biomacromolecules 2020, 21, 1037–1059. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Henson, A.; Popat, K.C. Biodegradable poly(ɛ-caprolactone) nanowires for bone tissue engineering applications. Biomaterials 2009, 30, 780–788. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Yang, M.; Ng, H.J.H.; Nga, V.D.W.; Chou, N.; Yeo, T.T. Cranial reconstruction using a polycaprolactone implant after burr hole trephination. J. 3D Print. Med. 2020, 4, 9–16. [Google Scholar] [CrossRef]
- Sparks, D.S.; Saifzadeh, S.; Savi, F.M.; Dlaska, C.E.; Berner, A.; Henkel, J.; Reichert, J.C.; Wullschleger, M.; Ren, J.; Cipitria, A.; et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020, 15, 877–924. [Google Scholar] [CrossRef]
- Reichert, J.; Cipitria, A.; Epari, D.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.; Schell, H.; Mehta, M.; Schuetz, M.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra193. [Google Scholar] [CrossRef]
- Reichert, J.; Epari, D.; Wullschleger, M.; Saifzadeh, S.; Steck, R.; Lienau, J.; Sommerville, S.; Dickinson, I.; Schütz, M.; Duda, G.; et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng. Part B Rev. 2010, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schutz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Epari, D.R.; Wullschleger, M.E.; Berner, A.; Saifzadeh, S.; Noth, U.; Dickinson, I.C.; Schuetz, M.A.; Hutmacher, D.W. Bone tissue engineering. Reconstruction of critical sized segmental bone defects in the ovine tibia. Orthopade 2012, 41, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Medeiros Savi, F.; Saifzadeh, S.; Wille, M.-L.; Wagels, M.; Hutmacher, D.W. Bone Regeneration Exploiting Corticoperiosteal Tissue Transfer for Scaffold-Guided Bone Regeneration. Tissue Eng. Part C Methods 2022, 28, 202–213. [Google Scholar] [CrossRef]
- Henkel, J.; Medeiros Savi, F.; Berner, A.; Fountain, S.; Saifzadeh, S.; Steck, R.; Epari, D.R.; Woodruff, M.A.; Knackstedt, M.; Schuetz, M.A.; et al. Scaffold-guided bone regeneration in large volume tibial segmental defects. Bone 2021, 153, 116163. [Google Scholar] [CrossRef]
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2018, 58, 164–207. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Cacopardo, L. Chapter 18-Biomaterials and biocompatibility. In Human Orthopaedic Biomechanics; Innocenti, B., Galbusera, F., Eds.; Academic Press: Vienna, Austria, 2022; pp. 341–359. [Google Scholar] [CrossRef]
- Berner, A.; Henkel, J.; Woodruff, M.A.; Saifzadeh, S.; Kirby, G.; Zaiss, S.; Gohlke, J.; Reichert, J.C.; Nerlich, M.; Schuetz, M.A.; et al. Scaffold-cell bone engineering in a validated preclinical animal model: Precursors vs differentiated cell source. J. Tissue Eng. Regen. Med. 2017, 11, 2081–2089. [Google Scholar] [CrossRef]
- Berner, A.; Reichert, J.C.; Woodruff, M.A.; Saifzadeh, S.; Morris, A.J.; Epari, D.R.; Nerlich, M.; Schuetz, M.A.; Hutmacher, D.W. Autologous vs. allogenic mesenchymal progenitor cells for the reconstruction of critical sized segmental tibial bone defects in aged sheep. Acta Biomater. 2013, 9, 7874–7884. [Google Scholar] [CrossRef]
- Cipitria, A.; Lange, C.; Schell, H.; Wagermaier, W.; Reichert, J.C.; Hutmacher, D.W.; Fratzl, P.; Duda, G.N. Porous scaffold architecture guides tissue formation. J. Bone Miner Res. 2012, 27, 1275–1288. [Google Scholar] [CrossRef]
- Cipitria, A.; Reichert, J.C.; Epari, D.R.; Saifzadeh, S.; Berner, A.; Schell, H.; Mehta, M.; Schuetz, M.A.; Duda, G.N.; Hutmacher, D.W. Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 2013, 34, 9960–9968. [Google Scholar] [CrossRef]
- Cipitria, A.; Wagermaier, W.; Zaslansky, P.; Schell, H.; Reichert, J.C.; Fratzl, P.; Hutmacher, D.W.; Duda, G.N. BMP delivery complements the guiding effect of scaffold architecture without altering bone microstructure in critical-sized long bone defects: A multiscale analysis. Acta Biomater. 2015, 23, 282–294. [Google Scholar] [CrossRef]
- Berner, A.; Henkel, J.; Woodruff, M.A.; Steck, R.; Nerlich, M.; Schuetz, M.A.; Hutmacher, D.W. Delayed minimally invasive injection of allogenic bone marrow stromal cell sheets regenerates large bone defects in an ovine preclinical animal model. Stem. Cells Transl. Med. 2015, 4, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.S.; Savi, F.M.; Dlaska, C.E.; Saifzadeh, S.; Brierly, G.; Ren, E.; Cipitria, A.; Reichert, J.C.; Wille, M.L.; Schuetz, M.A.; et al. Convergence of scaffold-guided bone regeneration principles and microvascular tissue transfer surgery. Sci. Adv. 2023, 9, eadd6071. [Google Scholar] [CrossRef]
- Capanna, R.; Bufalini, C.; Campanacci, M. A new technique for reconstructions of large metadiaphyseal bone defects. Orthop. Traumatol. 1993, 2, 159–177. [Google Scholar] [CrossRef]
- Rabitsch, K.; Maurer-Ertl, W.; Pirker-Frühauf, U.; Wibmer, C.; Leithner, A. Intercalary reconstructions with vascularised fibula and allograft after tumour resection in the lower limb. Sarcoma 2013, 2013, 160295. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Wu, P.; Zhou, Z.; Pan, D.; Tang, J.Y. Complication of osteo reconstruction by utilizing free vascularized fibular bone graft. BMC Surg. 2020, 20, 216. [Google Scholar] [CrossRef]
- Beris, A.E.; Lykissas, M.G.; Korompilias, A.V.; Vekris, M.D.; Mitsionis, G.I.; Malizos, K.N.; Soucacos, P.N. Vascularized fibula transfer for lower limb reconstruction. Microsurgery 2011, 31, 205–211. [Google Scholar] [CrossRef]
- Moghaddam, A.; Zietzschmann, S.; Bruckner, T.; Schmidmaier, G. Treatment of atrophic tibia non-unions according to ‘diamond concept’: Results of one- and two-step treatment. Injury 2015, 46 (Suppl. S4), S39–S50. [Google Scholar] [CrossRef]
- Morris, R.; Hossain, M.; Evans, A.; Pallister, I. Induced membrane technique for treating tibial defects gives mixed results. Bone Jt. J. 2017, 99-B, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Pesciallo, C.A.; Garabano, G.; Dainotto, T.; Ernst, G. Masquelet technique in post-traumatic infected femoral and tibial segmental bone defects. Union and reoperation rates with high proportions (up to 64%) of allograft in the second stage. Injury 2021, 52, 3471–3477. [Google Scholar] [CrossRef] [PubMed]
- Grün, W.; Hansen, E.J.J.; Andreassen, G.S.; Clarke-Jenssen, J.; Madsen, J.E. Functional outcomes and health-related quality of life after reconstruction of segmental bone loss in femur and tibia using the induced membrane technique. Arch. Orthop. Trauma Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.T.; Landy, D.C.; Sneed, C.R.; Liu, B.; Kavolus, M.; Pectol, R.W.; Gitajn, I.L.; Oh, J.-K.; Aneja, A. Masquelet Technique for the Tibia: A Systematic Review and Meta-Analysis of Contemporary Outcomes. J. Orthop. Trauma 2023, 37, e36–e44. [Google Scholar] [CrossRef]
- Rohilla, R.; Sharma, P.K.; Wadhwani, J.; Das, J.; Singh, R.; Beniwal, D. Prospective randomized comparison of bone transport versus Masquelet technique in infected gap nonunion of tibia. Arch. Orthop. Trauma Surg. 2022, 142, 1923–1932. [Google Scholar] [CrossRef]
- Mühlhäusser, J.; Winkler, J.; Babst, R.; Beeres, F.J.P. Infected tibia defect fractures treated with the Masquelet technique. Medicine 2017, 96, e6948. [Google Scholar] [CrossRef]
- Gruber, H.E.; Gettys, F.K.; Montijo, H.E.; Starman, J.S.; Bayoumi, E.; Nelson, K.J.; Hoelscher, G.L.; Ramp, W.K.; Zinchenko, N.; Ingram, J.A.; et al. Genomewide molecular and biologic characterization of biomembrane formation adjacent to a methacrylate spacer in the rat femoral segmental defect model. J. Orthop. Trauma 2013, 27, 290–297. [Google Scholar] [CrossRef]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Viateau, V.; Guillemin, G.; Calando, Y.; Logeart, D.; Oudina, K.; Sedel, L.; Hannouche, D.; Bousson, V.; Petite, H. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: An ovine model. Vet. Surg. 2006, 35, 445–452. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Wu, Y.; Ye, D.; Wang, S.; Zou, D.; Zhang, X.; Kaplan, D.L.; Jiang, X. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cell Mater 2014, 27, 1–12. [Google Scholar] [CrossRef]
- Aronson, J.; Harrison, B.H.; Stewart, C.L.; Harp, J.H., Jr. The histology of distraction osteogenesis using different external fixators. Clin. Orthop. Relat. Res. 1989, 241, 106–116. [Google Scholar] [CrossRef]
- Garcia, F.L.; Picado, C.H.; Garcia, S.B. Histology of the regenerate and docking site in bone transport. Arch. Orthop. Trauma Surg. 2009, 129, 549–558. [Google Scholar] [CrossRef]
- Papakostidis, C.; Bhandari, M.; Giannoudis, P.V. Distraction osteogenesis in the treatment of long bone defects of the lower limbs. Bone Jt. J. 2013, 95-B, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J. Bone Jt. Surg. Am. 1997, 79, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Y.; Jordan, C.J.; McLaurin, T.M.; Grant, A. The evolution of the Ilizarov technique: Part 2: The principles of distraction osteosynthesis. Bull. Hosp. Jt. Dis. (2013) 2013, 71, 96–103. [Google Scholar]
- Tsuchiya, H.; Tomita, K. Distraction osteogenesis for treatment of bone loss in the lower extremity. J. Orthop. Sci. 2003, 8, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Benady, A.; Meyer, S.J.; Golden, E.; Dadia, S.; Katarivas Levy, G. Patient-specific Ti-6Al-4V lattice implants for critical-sized load-bearing bone defects reconstruction. Mater. Des. 2023, 226, 111605. [Google Scholar] [CrossRef]
- Hassan, M.N.; Yassin, M.A.; Suliman, S.; Lie, S.A.; Gjengedal, H.; Mustafa, K. The bone regeneration capacity of 3D-printed templates in calvarial defect models: A systematic review and meta-analysis. Acta Biomater. 2019, 91, 1–23. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Hollister, S.J. Scaffold engineering: A bridge to where? Biofabrication 2009, 1, 012001. [Google Scholar] [CrossRef]
- Hollister, S.J.; Murphy, W.L. Scaffold translation: Barriers between concept and clinic. Tissue Eng. Part B Rev. 2011, 17, 459–474. [Google Scholar] [CrossRef] [PubMed]
- BMBF (German Federal Ministry of Education and Research). Preclinical Studies and Reviews (Fördermaßnahme Präklinische Studien und Reviews). 2018. Available online: https://www.gesundheitsforschung-bmbf.de (accessed on 8 January 2023).
- Vogel, A.L.; Knebel, A.R.; Faupel-Badger, J.M.; Portilla, L.M.; Simeonov, A. A systems approach to enable effective team science from the internal research program of the National Center for Advancing Translational Sciences. J. Clin. Transl. Sci. 2021, 5, e163. [Google Scholar] [CrossRef] [PubMed]
- BMBF (German Federal Ministry of Education and Research). Early Clinical Studies (Fördermaßnahme Frühe Klinische Studien). 2018. Available online: https://www.gesundheitsforschung-bmbf.de (accessed on 8 January 2023).
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Grand View Research. Bone Grafts and Substitutes Market Size, Share & Trends Analysis Report by Material Type (Allograft, Synthetic), by Application (Spinal Fusion, Foot & Ankle, Joint Reconstruction), By Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/bone-grafts-substitutes-market (accessed on 2 January 2023).
- Markides, C.C.; Geroski, P.A. Fast Second: How Smart Companies Bypass Radical Innovation to Enter and Dominate New Markets; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Sparks, D.S.; Wiper, J.; Lloyd, T.; Wille, M.L.; Sehu, M.; Savi, F.M.; Ward, N.; Hutmacher, D.W.; Wagels, M. Protocol for the BONE-RECON trial: A single-arm feasibility trial for critical sized lower limb BONE defect RECONstruction using the mPCL-TCP scaffold system with autologous vascularised corticoperiosteal tissue transfer. BMJ Open 2023, 13, e056440. [Google Scholar] [CrossRef] [PubMed]
- Liodakis, E.; Pacha, T.O.; Aktas, G.; Sehmisch, S.; Mommsen, P. Biological reconstruction of large bone defects: Masquelet technique and new procedures. Die Unfallchirurgie 2023, 126, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Callanan, A. Recent Methods for Modifying Mechanical Properties of Tissue-Engineered Scaffolds for Clinical Applications. Biomimetics 2023, 8, 205. [Google Scholar] [CrossRef]
- Gawande, A. Two Hundred Years of Surgery. N. Engl. J. Med. 2012, 366, 1716–1723. [Google Scholar] [CrossRef]
- Tsang, S.-T.J.; Ferreira, N.; Simpson, A.H.R.W. The reconstruction of critical bone loss. Bone Jt. Res. 2022, 11, 409–412. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S. Lost in Translation: The Gap in Scientific Advancements and Clinical Application. Front. Bioeng. Biotechnol. 2016, 4, 43. [Google Scholar] [CrossRef]
- Young, N.S.; Ioannidis, J.P.A.; Al-Ubaydli, O. Why Current Publication Practices May Distort Science. PLoS Med. 2008, 5, e201. [Google Scholar] [CrossRef] [PubMed]
- Smaldino, P.E.; McElreath, R. The natural selection of bad science. R. Soc. Open Sci. 2016, 3, 160384. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.A.; Roy, S. Academic Research in the 21st Century: Maintaining Scientific Integrity in a Climate of Perverse Incentives and Hypercompetition. Environ. Eng. Sci. 2016, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Naudet, F.; Cristea, I.A.; Miedema, F.; Ioannidis, J.P.A.; Goodman, S.N. Assessing scientists for hiring, promotion, and tenure. PLoS Biol. 2018, 16, e2004089. [Google Scholar] [CrossRef] [PubMed]
- Schömig, F.; Palmowski, Y.; Schitz, F.; Winkler, T.; Perka, C.; Pumberger, M. Scientific Productivity of University Orthopaedics and Trauma Surgery in Germany, Austria, and Switzerland. Z. Orthop. Unfall. 2022. [Google Scholar] [CrossRef]
- Kools, F.R.W.; Mirali, S.; Holst-Bernal, S.; Nijhof, S.L.; Cavalli, G.; Grandner, M.A. Publications Are Not the Finish Line: Focusing on Societal Rather Than Publication Impact. Front. Med. 2018, 5, 314. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N. Expanding Options for Scientific Publication. Circulation 2013, 127, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Drude, N.; Martinez-Gamboa, L.; Haven, T.; Holman, C.; Holst, M.; Kniffert, S.; McCann, S.; Rackoll, T.; Schulz, R.; Weschke, S. Finding the best fit for improving reproducibility: Reflections from the QUEST Center for Responsible Research. BMC Res. Notes 2022, 15, 270. [Google Scholar] [CrossRef]
- Drude, N.I.; Martinez-Gamboa, L.; Danziger, M.; Collazo, A.; Kniffert, S.; Wiebach, J.; Nilsonne, G.; Konietschke, F.; Piper, S.K.; Pawel, S.; et al. Planning preclinical confirmatory multicenter trials to strengthen translation from basic to clinical research—A multi-stakeholder workshop report. Transl. Med. Commun. 2022, 7, 24. [Google Scholar] [CrossRef]
- Guo, A.X.Y.; Cheng, L.; Zhan, S.; Zhang, S.; Xiong, W.; Wang, Z.; Wang, G.; Cao, S.C. Biomedical applications of the powder-based 3D printed titanium alloys: A review. J. Mater. Sci. Technol. 2022, 125, 252–264. [Google Scholar] [CrossRef]
- Elbadawi, M.; McCoubrey, L.E.; Gavins, F.K.H.; Ong, J.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Disrupting 3D printing of medicines with machine learning. Trends Pharmacol. Sci. 2021, 42, 745–757. [Google Scholar] [CrossRef]
- Kimmelman, J.; Mogil, J.S.; Dirnagl, U. Distinguishing between Exploratory and Confirmatory Preclinical Research Will Improve Translation. PLoS Biol. 2014, 12, e1001863. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Matheus, I.; Hutmacher, D.W.; Olson, S.; Redmond, M.; Sutherland, A.; Wagels, M. A Medical-Grade Polycaprolactone and Tricalcium Phosphate Scaffold System with Corticoperiosteal Tissue Transfer for the Reconstruction of Acquired Calvarial Defects in Adults: Protocol for a Single-Arm Feasibility Trial. JMIR Res. Protoc. 2022, 11, e36111. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-hay, A.; Barhoum, A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. In Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Reumann, M.K.; Braun, B.J.; Menger, M.M.; Springer, F.; Jazewitsch, J.; Schwarz, T.; Nüssler, A.; Histing, T.; Rollmann, M.F.R. Artificial intelligence and novel approaches for treatment of non-union in bone: From established standard methods in medicine up to novel fields of research. Unfallchirurgie (Heidelberg) 2022, 125, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, K.; Nizak, R.; Noordmans, H.J.; Castelein, R.M.; Weinans, H.; Kruyt, M.C. Challenges in the design and regulatory approval of 3D-printed surgical implants: A two-case series. Lancet Digit. Health 2019, 1, e163–e171. [Google Scholar] [CrossRef]
- Re, F.; Borsani, E.; Rezzani, R.; Sartore, L.; Russo, D. Bone Regeneration Using Mesenchymal Stromal Cells and Biocompatible Scaffolds: A Concise Review of the Current Clinical Trials. Gels 2023, 9, 389. [Google Scholar] [CrossRef]
- Papakostidis, C.; Giannoudis, P.V. Reconstruction of infected long bone defects: Issues and Challenges. Injury 2023, 54, 807–810. [Google Scholar] [CrossRef]
- Bernstein, M.; Fragomen, A.; Rozbruch, S.R. Tibial Bone Transport Over an Intramedullary Nail Using Cable and Pulleys. JBJS Essent. Surg. Tech. 2018, 8, e9. [Google Scholar] [CrossRef]
- Rosteius, T.; Pätzholz, S.; Rausch, V.; Lotzien, S.; Behr, B.; Lehnhardt, M.; Schildhauer, T.A.; Seybold, D.; Geßmann, J. Ilizarov bone transport using an intramedullary cable transportation system in the treatment of tibial bone defects. Injury 2021, 52, 1606–1613. [Google Scholar] [CrossRef]
- Barinaga, G.; Beason, A.M.; Gardner, M.P. Novel Surgical Approach to Segmental Bone Transport Using a Magnetic Intramedullary Limb Lengthening System. J. Am. Acad. Orthop. Surg. 2018, 26, e477–e482. [Google Scholar] [CrossRef] [PubMed]
- Kähler Olesen, U. „Plate-assisted segmental bone transport“ mit Verlängerungsnagel und Platte. Unfallchirurg 2018, 121, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Nolte, P.-C.; Kemmerer, M.; Spranger, N.; Hackl, S.; von Recum, J.; Grützner, P.A.; Reiter, G. Plate-assisted bone segment transport“ bei Knochendefekten an der unteren Extremität. Die Unfallchirurgie 2023, 126, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Borzunov, D.Y.; Kolchin, S.N.; Mokhovikov, D.S.; Malkova, T.A. Ilizarov bone transport combined with the Masquelet technique for bone defects of various etiologies (preliminary results). World, J. Orthop. 2022, 13, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Hamiti, Y.; Yushan, M.; Yalikun, A.; Lu, C.; Yusufu, A. Matched comparative study of trifocal bone transport versus induced membrane followed by trifocal bone transport in the treatment of segmental tibial defects caused by posttraumatic osteomyelitis. BMC Musculoskelet. Disord. 2022, 23, 572. [Google Scholar] [CrossRef]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef]

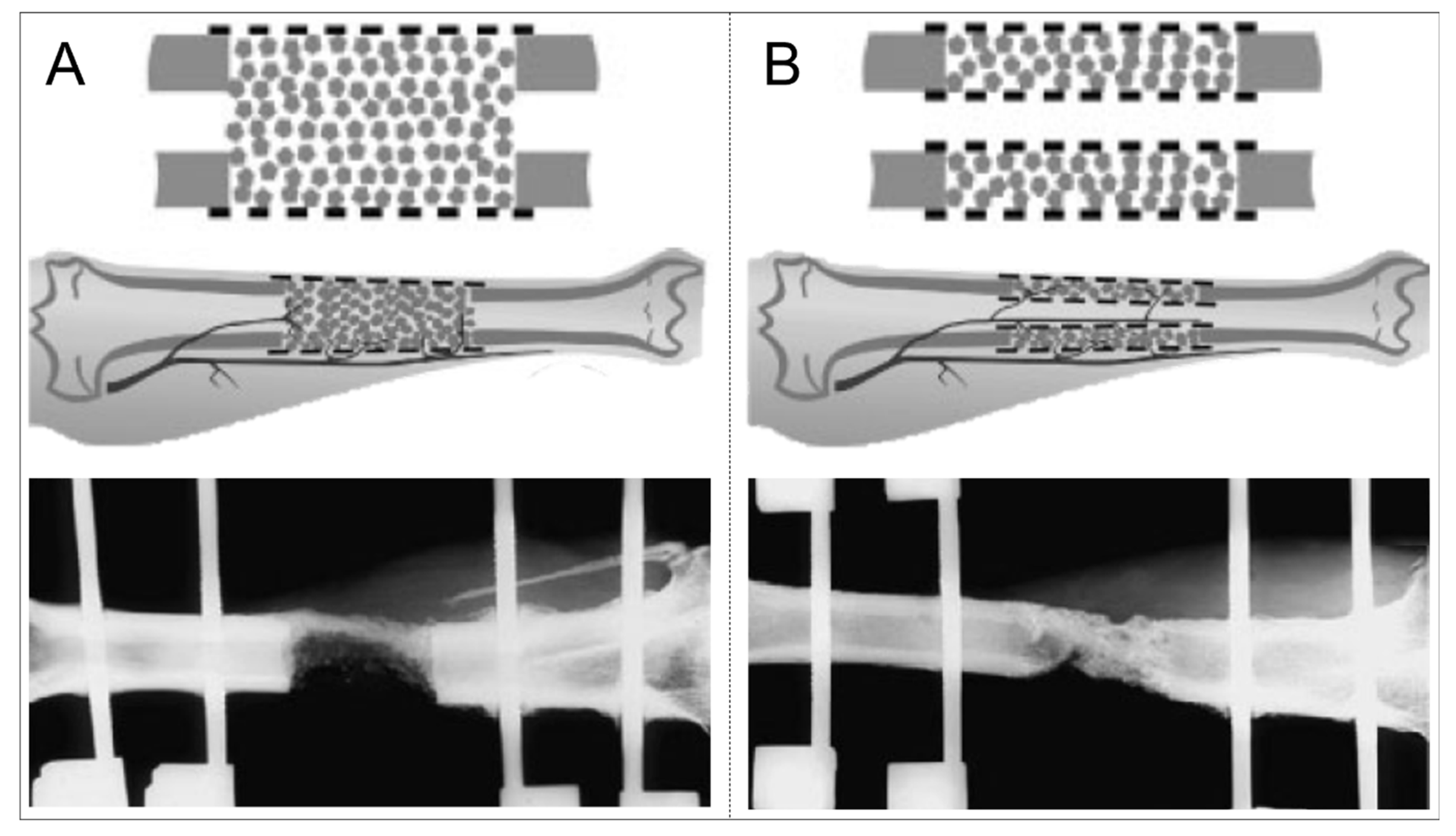

| Bone Fracture Repair | Segmental Bone Defect regeneration | |
|---|---|---|
| Definition 1 |
|
|
| 2.5D Implant | 3D Implant | |
| Definition 2 |
|
|
| Challenge | Interdisciplinary (Stakeholder Workshop) Approach |
|---|---|
| Design of patient-specific 3D-printed scaffolds | The optimization of scaffold design using machine learning [183,261,262] and finite element analysis [139,234,247] methods to avoid complications such as implant failure has been deemed required (Figure S6), especially when using titanium-based scaffolds, but is not yet fully integrated into current workflows [234]. |
| Preclinical and clinical large-scale multicentre studies | Preclinical studies to increase research reproducibility [260,263] and for clinical studies in general in the field of large bone defect treatment using 3D-printed scaffolds can only be performed in purposefully planned and well-powered multicentre large-scale collaborative research projects to have the possibility to correct confounding factors related to host and soft tissue while investigating the respective implant for different defect sizes or for different surgical indications [157,264,265]. Furthermore, as bone defects are rare but very complex diseases with a dramatic socio-economic impact on the healthcare system, there are many open questions that may be better understood in the future through the use of artificial intelligence methods—from predictive models and cost analyses to personalised treatment strategies [266]; however, these methods first need to be validated in preclinical and clinical studies. |
| Regulatory process | Exemptions have been created for patient-specific 3D-printed scaffolds, allowing clinicians to quickly commission the manufacture of custom implants without having to undertake and comply with the complex and time-consuming regulatory process for each individual product; however, the need for strict reporting obligations and manufacturing transparency remains [267], which requires in-depth elaboration from medico-legal experts to reduce barriers and increase standardized use of 3D-printed scaffolds for bone regeneration [247]. |
| Reimbursement strategies | The health economic impact of custom 3D-printed titanium and mPCL-TCP scaffolds for the treatment of large segmental bone defects remains to be defined. Only very limited data are available on costs for 3D-printed implants. An average cost of USD 2329 per 3D-printed titanium implant was reported by Gamieldien et al. [156]. Further, based our experience, approximate costs of mPCL-TCP scaffolds amount to USD 2700. It is important to point out that one of the main factors affecting the cost of 3D-printed scaffolds is the design time for each individual scaffold, and that this time is expected to reduce with increasing experience and new automated methods [149,261,262]. Therefore, in interdisciplinary (stakeholder workshop) meetings, it is a conditio sine qua non for the planning of future studies to include the variables of assessing the direct and indirect costs of different materials and treatment methods for 3D-printed scaffolds for long bone regeneration [268]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J. Funct. Biomater. 2023, 14, 341. https://doi.org/10.3390/jfb14070341
Laubach M, Hildebrand F, Suresh S, Wagels M, Kobbe P, Gilbert F, Kneser U, Holzapfel BM, Hutmacher DW. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. Journal of Functional Biomaterials. 2023; 14(7):341. https://doi.org/10.3390/jfb14070341
Chicago/Turabian StyleLaubach, Markus, Frank Hildebrand, Sinduja Suresh, Michael Wagels, Philipp Kobbe, Fabian Gilbert, Ulrich Kneser, Boris M. Holzapfel, and Dietmar W. Hutmacher. 2023. "The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective" Journal of Functional Biomaterials 14, no. 7: 341. https://doi.org/10.3390/jfb14070341
APA StyleLaubach, M., Hildebrand, F., Suresh, S., Wagels, M., Kobbe, P., Gilbert, F., Kneser, U., Holzapfel, B. M., & Hutmacher, D. W. (2023). The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. Journal of Functional Biomaterials, 14(7), 341. https://doi.org/10.3390/jfb14070341










