Neuroprotective Effect of Platinum Nanoparticles Is Not Associated with Their Accumulation in the Brain of Rats
Abstract
:1. Introduction
2. Materials and Methods
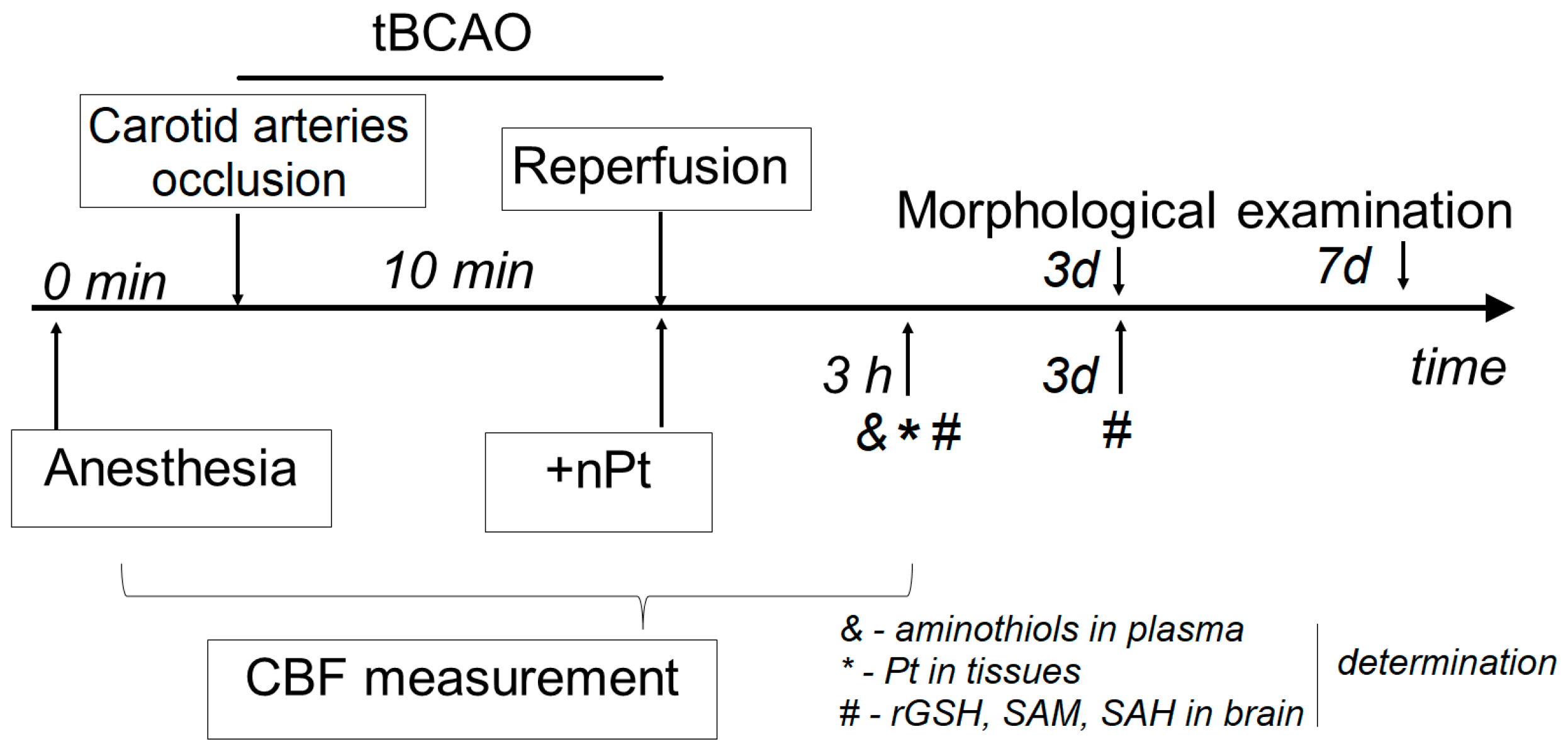
2.1. Animals and Experimental Design
2.2. Surgical Technique (tBCAO) and CBF Determination
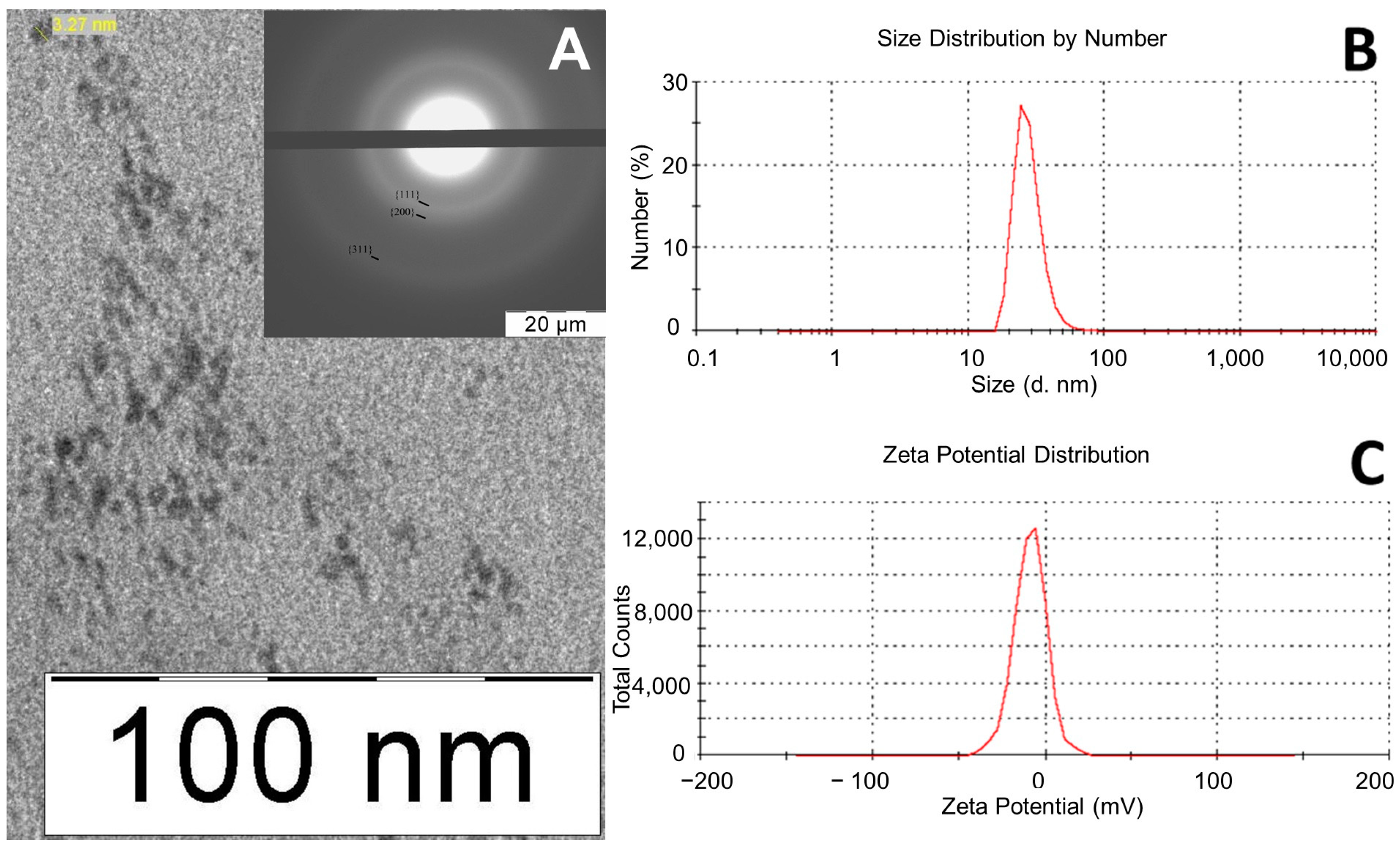
2.3. Synthesis and Characteristics of nPt
2.4. Determination of Pt Levels in Tissues
2.5. Morphological Examination of the Brain
2.6. Determination of Reduced Low-Molecular-Weight Aminothiols
2.7. Statistical Analysis
3. Results
3.1. nPt Characteristics
3.2. Morphological Examination of the Brain
3.3. Influence of nPts on CBF and MAP
3.4. Determination of Pt in Rat Tissues
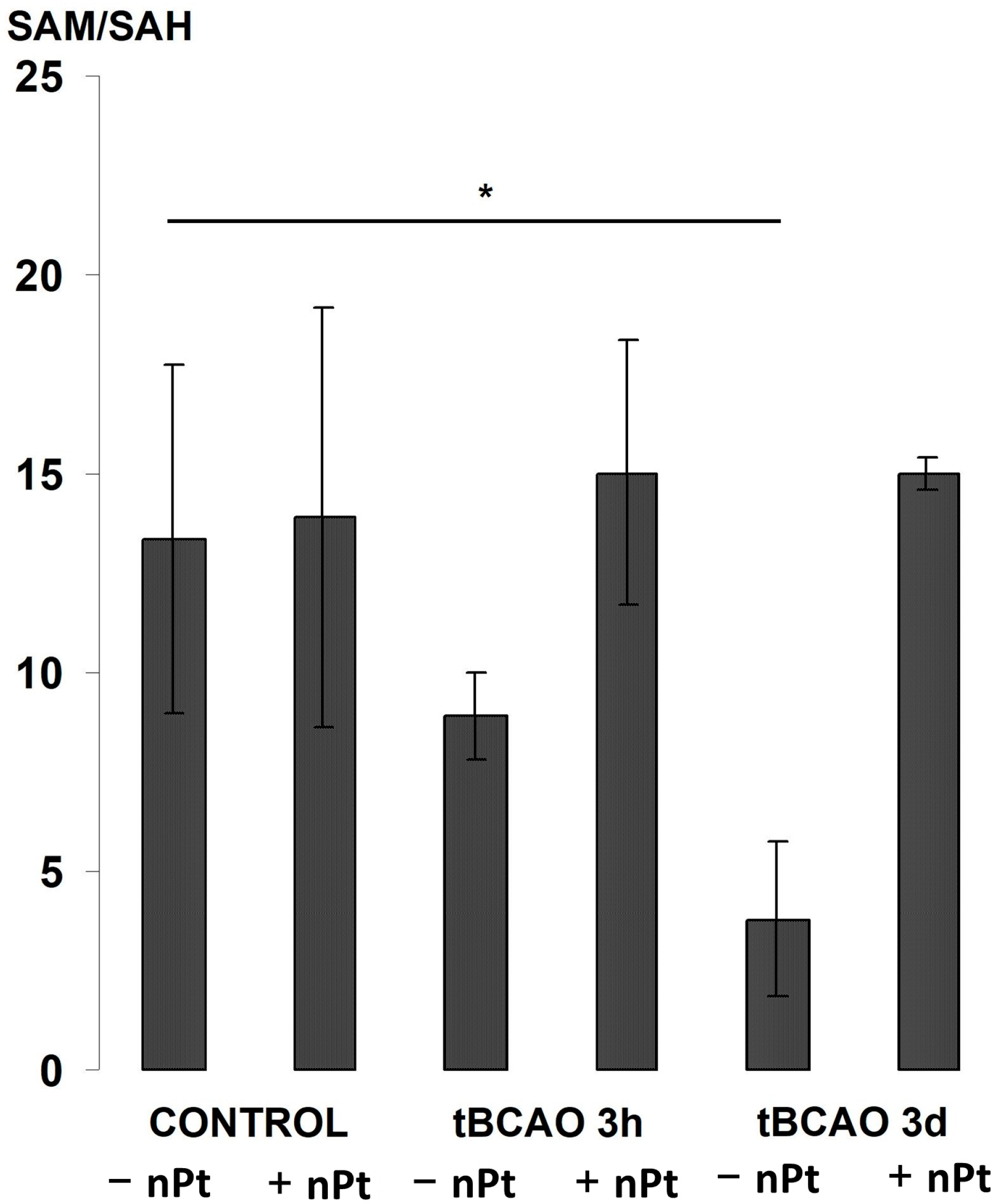
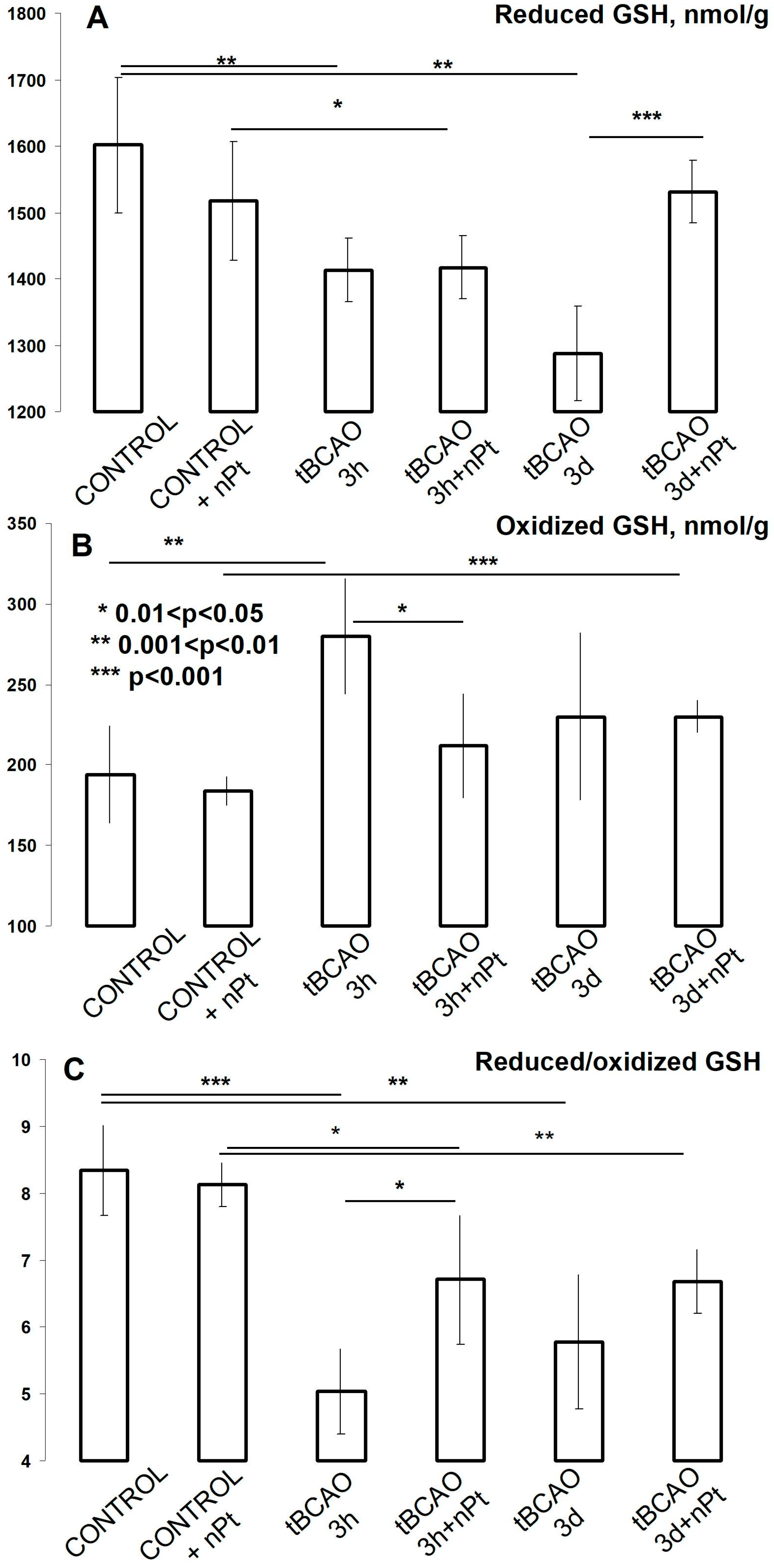
3.5. Effect of nPts on the Brain and Plasma Aminothiols
4. Discussion
4.1. nPt Synthesis
4.2. The Effect of nPts on Aminothiols
4.3. The Effect of nPts on CBF
4.4. Protective Action of nPts on Hippocampal Neurons during tBCAO
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandian, J.D.; Gall, S.L.; Kate, M.P.; Silva, G.S.; Akinyemi, R.O.; Ovbiagele, B.I.; Lavados, P.M.; Gandhi, D.; Thrift, A.G. Prevention of stroke: A global perspective. Lancet 2018, 392, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Llwyd, O.; Salinet, A.; Panerai, R.B.; Lam, M.Y.; Saeed, N.P.; Brodie, F.; Bor-Seng-Shu, E.; Robinson, T.G.; Nogueira, R.C. Cerebral Haemodynamics following Acute Ischaemic Stroke: Effects of Stroke Severity and Stroke Subtype. Cerebrovasc. Dis. Extra 2018, 8, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, N.; Ohyama, H.; Ichihara, S.; Takahashi, T.; Naya, T.; Kohno, M. Relation of postischemic delayed hypoperfusion and cerebral edema after transient forebrain ischemia. J. Stroke Cerebrovasc. Dis. 2007, 16, 103–108. [Google Scholar] [CrossRef]
- Hasan, T.F.; Hasan, H.; Kelley, R.E. Overview of Acute Ischemic Stroke Evaluation and Management. Biomedicines 2021, 9, 1486. [Google Scholar] [CrossRef]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 870141. [Google Scholar] [CrossRef]
- Berg, T. b1-blockers lower norepinephrine release by inhibiting presynaptic, facilitating b1-adrenoceptors in normotensive and hypertensive rats. Front. Neurol. 2014, 5, 51–61. [Google Scholar] [CrossRef]
- Martinez-Revelles, S.; Jiménez-Altayó, F.; Caracuel, L.; Pérez-Asensio, F.J.; Planas, A.M.; Vila, E. Endothelial dysfunction in rat mesenteric resistance artery after transient middle cerebral artery occlusion. J. Pharmacol. Exp. Ther. 2008, 325, 363–369. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. (Landmark Ed.) 2022, 27, 105. [Google Scholar] [CrossRef]
- Toth, P.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.A.; Buki, A.; Koller, A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomecha-nisms, perspectives, and therapeutic implications. Am. J. Physiol. Heart. Circ. Physiol. 2016, 311, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, M.; Ricardo Pires, J.; Viseu, I.; Magalhães, P.; Gregório, H.; Afreixo, V.; Gregório, T. Edaravone for acute ischemic stroke—Systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2022, 219, 107299. [Google Scholar] [CrossRef] [PubMed]
- Sabetghadam, M.; Mazdeh, M.; Abolfathi, P.; Mohammadi, Y.; Mehrpooya, M. Evidence for a Beneficial Effect of Oral N-acetylcysteine on Functional Outcomes and Inflammatory Biomarkers in Patients with Acute Ischemic Stroke. Neuropsychiatr. Dis. Treat. 2020, 16, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Nguyen, D.T.; Kodibagkar, V.D.; Stabenfeldt, S.E. Nanoparticle-Based Therapeutics for Brain Injury. Adv. Healthc. Mater. 2018, 7, 1–31. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, Z.; Sha, X. Nanoparticles-mediated emerging approaches for effective treatment of ischemic stroke. Biomaterials 2021, 277, 121111. [Google Scholar] [CrossRef]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef]
- Hamasaki, T.; Kashiwagi, T.; Imada, T.; Nakamichi, N.; Aramaki, S.; Toh, K.; Morisawa, S.; Shimakoshi, H.; Hisaeda, Y.; Shirahata, S. Kinetic analysis of superoxide anion radical-scavenging and hydroxyl radical-scavenging activities of platinum nanoparticles. Langmuir: ACS J. Surf. Colloids 2008, 24, 7354–7364. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G.; et al. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Alexandrin, V.V.; Paltry, A.A.; Nikiforova, K.A.; Virus, E.D.; Luzyanin, B.P.; Maksimova, M.Y.; Piradov, M.A.; Kubatiev, A.A. Plasma low-molecular-weight thiol/disulphide homeostasis as an early indicator of global and focal cerebral ischaemia. Redox Rep. 2017, 22, 460–466. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Alexandrin, V.V.; Paltsyn, A.A.; Virus, E.D.; Nikiforova, K.A.; Bulgakova, P.O.; Sviridkina, N.B.; Apollonova, S.A.; Kubatiev, A.A. Metoprolol and Nebivolol Prevent the Decline of the Redox Status of Low-Molecular-Weight Aminothiols in Blood Plasma of Rats During Acute Cerebral Ischemia. J. Cardiovasc. Pharmacol. 2018, 72, 195–203. [Google Scholar] [CrossRef]
- Maksimova, M.Y.; Ivanov, A.V.; Virus, E.D.; Nikiforova, K.A.; Ochtova, F.R.; Suanova, E.T.; Kruglova, M.P.; Piradov, M.A.; Kubatiev, A.A. Impact of glutathione on acute ischemic stroke severity and outcome: Possible role of aminothiols redox status. Redox Rep. 2021, 26, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kesidou, E.; Bitsina, C.; Chatzisotiriou, A.; Theotokis, P.; Dandi, E.; Tata, D.A.; Spandou, E. N-Acetylcysteine Administration Attenuates Sensorimotor Impairments Following Neonatal Hypoxic-Ischemic Brain Injury in Rats. Int. J. Mol. Sci. 2022, 23, 16175. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, S.R. Neuroprotective effects of N-acetylcysteine via inhibition of matrix metalloproteinase in a mouse model of transient global cerebral ischemia. Brain Res. Bull. 2020, 154, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tao, J.; Duan, F.; Li, H.; Tan, H. HHcy Induces Pyroptosis and Atherosclerosis via the Lipid Raft-Mediated NOX-ROS-NLRP3 Inflammasome Pathway in apoE−/− Mice. Cells 2022, 11, 2438. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Dadar, M.; Lozynska, I.; Chirumbolo, S.; Lysiuk, R.; Lenchyk, L.; Upyr, T.; Severin, B. The role of B vitamins in stroke prevention. Crit. Rev. Food Sci. Nutr. 2022, 62, 5462–5475. [Google Scholar] [CrossRef] [PubMed]
- Pelka, J.; Gehrke, H.; Esselen, M.; Türk, M.; Crone, M.; Bräse, S.; Muller, T.; Blank, H.; Send, W.; Zibat, V.; et al. Cellular uptake of platinum nanoparticles in human colon carcinoma cells and their impact on cellular redox systems and DNA integrity. Chem. Res. Toxicol. 2009, 22, 649–659. [Google Scholar] [CrossRef]
- Qiu, M.; Xu, E.; Zhan, L. Epigenetic Regulations of Microglia/Macrophage Polarization in Ischemic Stroke. Front. Mol. Neurosci. 2021, 14, 697416. [Google Scholar] [CrossRef]
- Hershfield, M.S.; Kredich, N.M.; Koller, C.A.; Mitchell, B.S.; Kurtzberg, J.; Kinney, T.R.; Falletta, J.M. S-adenosylhomocysteine catabolism and basis for acquired resistance during treatment of T-cell acute lymphoblastic leukemia with 2-deoxycoformycin alone and in combination with 9-beta-D-arabinofuranosyladenine. Cancer Res. 1983, 43, 3451–3458. [Google Scholar]
- Matsui, Y.; Kubo, Y.; Iwata, N. S-adenosyl-L-methionine prevents ischemic neuronal death. Eur. J. Pharmacol. 1987, 144, 211–216. [Google Scholar] [CrossRef]
- Sato, H.; Hariyama, H.; Moriguchi, K. S-adenosyl-L-methionine protects the hippocampal CA1 neurons from the ischemic neuronal death in rat. Biochem. Biophys. Res. Commun. 1988, 150, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, J.; Zhang, X.; Li, X.; Liu, Z.; Yang, Q.; Li, L. Epigenetic signature of chronic cerebral hypoperfusion and beneficial effects of S-adenosylmethionine in rats. Mol. Neurobiol. 2014, 50, 839–851. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.P.; Villalobos, M.A.; Cuerda, M.A.; Guerrero, A.; González-Correa, J.A.; Sánchez De La Cuesta, F. Effects of S-adenosyl-L-methionine on lipid peroxidation and glutathione levels in rat brain slices exposed to reoxygenation after oxygen-glucose deprivation. Neurosci. Lett. 2002, 318, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Ikeda, Y.; Matsuura, T.; Abe, K. Neurological and pathological improvements of cerebral infarction in mice with platinum nanoparticles. J. Neurosci. Res. 2011, 89, 125–133. [Google Scholar] [CrossRef]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Abe, K. Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience 2012, 221, 47–55. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Aleksandrin, V.V.; Ivanov, A.V.; Virus, E.D.; Bulgakova, P.O.; Kubatiev, A.A. Application of wavelet analysis to detect dysfunction in cerebral blood flow autoregulation during experimental hyperhomocysteinaemia. Lasers Med. Sci. 2018, 33, 1327–1333. [Google Scholar] [CrossRef]
- Sandell, E.B. Colorimetric Determination of Traces of Metals; Interselenct Publishers Inc.: Geneva, Switzerland, 1959. [Google Scholar]
- Karandasheva, V.K.; Turanov, A.N.; Orlova, T.A.; Lezhnev, A.E.; Nosenko, S.V.; Zolotareva, N.I.; Moskvitina, I.R. Use of the Inductively Coupled Plasma Mass Spectrometry for Element Analysis of Environmental Objects. Inorg. Mater. 2008, 44, 1491–1500. [Google Scholar] [CrossRef]
- Ostrovskaya, L.A.; Korman, D.B.; Burmiy, J.P.; Kuzmin, V.A.; Bluhterova, N.V.; Fomina, M.M.; Abzaeva, K.A. An Experimental Study of the Pharmacokinetics of the Antitumor Drug Aurumacryl. Biophysics 2018, 63, 469–476. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: New York, NY, USA, 2007. [Google Scholar]
- Cremer, J.E.; Seville, M.P. Regional brain blood flow, blood volume, and haematocrit values in the adult rat. J. Cereb. Blood. Flow. Metab. 1983, 3, 254–256. [Google Scholar] [CrossRef]
- Vitello, D.J.; Ripper, R.M.; Fettiplace, M.R.; Weinberg, G.L.; Vitello, J.M. Blood Density Is Nearly Equal to Water Density: A Validation Study of the Gravimetric Method of Measuring Intraoperative Blood Loss. J. Vet. Med. 2015, 2015, 152730. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Holt-Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Rahman, S.; Rahman, M.S.; Ali, M.A.; Nandi, N.C.; Noor, P.; Ahmed, K.N.; Akhter, S. Optimized reduction conditions for the microfluidic synthesis of 1.3–0.3 nm Pt clusters. J. Nanostruct. Chem. 2016, 6, 49–56. [Google Scholar] [CrossRef]
- Frassetto, S.S.; Schetinger, M.R.; Webber, A.; Sarkis, J.J.; Netto, C.A. Ischemic preconditioning reduces peripheral oxidative damage associated with brain ischemia in rats. Brazil. J. Med. Bio. Res. 1999, 32, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Lysko, P.G.; Lysko, K.A.; Yue, T.L.; Webb, C.L.; Gu, J.L.; Feuerstein, G. Neuroprotective effects of carvedilol, a new antihypertensive agent, in cultured rat cerebellar neurons and in gerbil global brain ischemia. Stroke 1992, 23, 1630–1635. [Google Scholar] [CrossRef]
- Nikiforova, K.A.; Alexandrin, V.V.; Bulgakova, P.O.; Ivanov, A.V.; Virus, E.D.; Kubatiev, A.A. Influ-ence of carvedilol on the redox-status of low-molecular-weight aminothyols in blood plasma during acute cerebral ischemia inrats. Pathol. Physiol. Exp. Ther. 2018, 62, 12–18. [Google Scholar]
- Zhang, Q.G.; Tian, H.; Li, H.C.; Zhang, G.Y. Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci. Lett. 2006, 408, 159–164. [Google Scholar] [CrossRef]
- van den Brule, J.M.D.; van der Hoeven, J.G.; Hoedemaekers, C.W.E. Cerebral Perfusion and Cerebral Autoregulation after Cardiac Arrest. Biomed. Res. Int. 2018, 2018, 4143636. [Google Scholar] [CrossRef]
- Villalba, N.; Sackheim, A.M.; Nunez, I.A.; Hill-Eubanks, D.C.; Nelson, M.T.; Wellman, G.C.; Freeman, K. Traumatic Brain Injury Causes Endothelial Dysfunction in the Systemic Microcirculation through Arginase-1-Dependent Uncoupling of Endothelial Nitric Oxide Synthase. J. Neurotrauma 2017, 34, 192–203. [Google Scholar] [CrossRef]
- Kim, C.D.; Shin, H.K.; Lee, H.S.; Lee, J.H.; Lee, T.H.; Hong, K.W. Gene transfer of Cu/Zn SOD to cerebral vessels prevents FPI-induced CBF autoregulatory dysfunction. Am. J. Physiol. Heart. Circ. Physiol. 2002, 282, 1836–1842. [Google Scholar] [CrossRef]
- Brzezinska, A.K.; Gebremedhin, D.; Chilian, W.M.; Kalyanaraman, B.; Elliott, S.J. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am. J. Physiol. Heart. Circ. Physiol. 2000, 278, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.J.; Fisher, C.M.; Jiang, X.; Chong, Y.; Zhang, H.; Guo, H.; Zhang, Q.; Zheng, J.; Knolhoff, A.M.; Croley, T.R.; et al. Platinum nanoparticles: An avenue for enhancing the release of nitric oxide from S-nitroso-N-acetylpenicillamine and S-nitrosoglutathione. Nanoscale 2018, 10, 11176–11185. [Google Scholar] [CrossRef] [PubMed]
- Elmazoglu, Z.; Kayhan, H.; Santamaría, A.; Rangel-López, E.; Uğur, P.K.; Ceylan, A.; Aschner, M.; Karasu, Ç. Platinum nanoparticles Protect Against Lipopolysaccharide-Induced Inflammation in Microglial BV-2 Cells via Decreased Oxidative Damage and Increased Phagocytosis. Neurochem. Res. 2021, 46, 3325–3341. [Google Scholar] [CrossRef] [PubMed]
- Loan, T.T.; Do, L.T.; Yoo, H. Different Cellular Effects of Platinum Nanoparticles on RAW 264.7 Cells. J. Nanosci. Nanotechnol. 2019, 19, 709–712. [Google Scholar] [CrossRef]
- Foley, N.; Marshall, S.; Pikul, J.; Salter, K.; Teasell, R. Hypermetabolism following moderate to severe traumatic acute brain injury: A systematic review. J. Neurotrauma 2008, 25, 1415–1431. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Brassard, P. Why is the neural control of cerebral autoregulation so controversial? F1000 Prime Rep. 2014, 6, 14–19. [Google Scholar] [CrossRef]
- ter Laan, M.; van Dijk, J.M.; Elting, J.W.; Staal, M.J.; Absalom, A.R. Sympathetic regulation of cerebral blood flow in humans: A review. Br. J. Anaesth. 2013, 111, 361–367. [Google Scholar] [CrossRef]
- Dreier, J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011, 17, 439–447. [Google Scholar] [CrossRef]
- McBean, D.E.; Kelly, P.A. Rodent models of global cerebral ischemia: A comparison of two-vessel occlusion and four-vessel occlusion. Gen. Pharmacol. 1998, 30, 431–444. [Google Scholar] [CrossRef]
- Zeng, Y.S.; Xu, Z.C. Co-existence of necrosis and apoptosis in rat hippocampus following transient forebrain ischemia. Neurosci. Res. 2000, 37, 113–125. [Google Scholar] [CrossRef]
- Martone, M.E.; Hu, B.R.; Ellisman, M.H. Alterations of hippocampal postsynaptic densities following transient ischemia. Hippocampus 2000, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug. Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, S.; Aoshiba, K.; Kajita, M.; Miyamoto, Y.; Nagai, A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther. 2009, 22, 340–349. [Google Scholar] [CrossRef]
- Zhang, L.; Laug, L.; Münchgesang, W.; Pippel, E.; Gösele, U.; Brandsch, M.; Knez, M. Reducing stress on cells with apoferritin-encapsulated platinum nanoparticles. Nano Lett. 2010, 10, 219–223. [Google Scholar] [CrossRef]
- Nejdl, L.; Kudr, J.; Moulick, A.; Hegerova, D.; Ruttkay-Nedecky, B.; Gumulec, J.; Cihalova, K.; Smerkova, K.; Dostalova, S.; Krizkova, S.; et al. Platinum nanoparticles induce damage to DNA and inhibit DNA replication. PLoS ONE 2017, 12, e0180798. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.H.; Kim, J.H. Anticancer Properties of Platinum Nanoparticles and Retinoic Acid: Combination Therapy for the Treatment of Human Neuroblastoma Cancer. Int. J. Mol. Sci. 2020, 21, 6792. [Google Scholar] [CrossRef]
- Gulino, M.; Santos, S.D.; Pêgo, A.P. Biocompatibility of Platinum Nanoparticles in Brain ex vivo Models in Physiological and Pathological Conditions. Front. Neurosci. 2021, 15, 787518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Chong, Y.; Wamer, W.G.; Xia, Q.; Cai, L.; Nie, Z.; Fu, P.P.; Yin, J.-J. Platinum nanoparticles: Efficient and stable catechol oxidase mimetics. ACS Appl. Mater. Interfaces 2015, 7, 19709–19717. [Google Scholar] [CrossRef] [PubMed]
- You, J.-G.; Liu, Y.-W.; Lu, C.-Y.; Tseng, W.-L.; Yu, C.-J. Colorimetric assay of heparin in plasma based on the inhibition of oxidase-like activity of citrate-capped platinum nanoparticles. Biosens. Bioelectron. 2017, 92, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Gu, J.L.; Cao, J.M. The acute toxic effects of platinum nanoparticles on ion channels, transmembrane potentials of cardiomyocytes in vitro and heart rhythm in vivo in mice. Int. J. Nanomed. 2019, 14, 5595–5609. [Google Scholar] [CrossRef]



| Rat No. | Tissue Type | |||
|---|---|---|---|---|
| Liver | Blood | Hippocampus (Whole) | Hippocampus (without Blood) | |
| Median | 167 | 44 | 0.83 | 0.74 |
| Min. | 26 | 2.9 | <0.3 (N = 3) | <0.3 (N = 3) |
| Max. | 467 | 97 | 1.5 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippov, A.G.; Alexandrin, V.V.; Ivanov, A.V.; Paltsyn, A.A.; Sviridkina, N.B.; Virus, E.D.; Bulgakova, P.O.; Burmiy, J.P.; Kubatiev, A.A. Neuroprotective Effect of Platinum Nanoparticles Is Not Associated with Their Accumulation in the Brain of Rats. J. Funct. Biomater. 2023, 14, 348. https://doi.org/10.3390/jfb14070348
Filippov AG, Alexandrin VV, Ivanov AV, Paltsyn AA, Sviridkina NB, Virus ED, Bulgakova PO, Burmiy JP, Kubatiev AA. Neuroprotective Effect of Platinum Nanoparticles Is Not Associated with Their Accumulation in the Brain of Rats. Journal of Functional Biomaterials. 2023; 14(7):348. https://doi.org/10.3390/jfb14070348
Chicago/Turabian StyleFilippov, Alexander Gennadievich, Valery Vasil’evich Alexandrin, Alexander Vladimirovich Ivanov, Alexander Alexandrovich Paltsyn, Nadezhda Borisovna Sviridkina, Edward Danielevich Virus, Polina Olegovna Bulgakova, Joanna Petrovna Burmiy, and Aslan Amirkhanovich Kubatiev. 2023. "Neuroprotective Effect of Platinum Nanoparticles Is Not Associated with Their Accumulation in the Brain of Rats" Journal of Functional Biomaterials 14, no. 7: 348. https://doi.org/10.3390/jfb14070348







