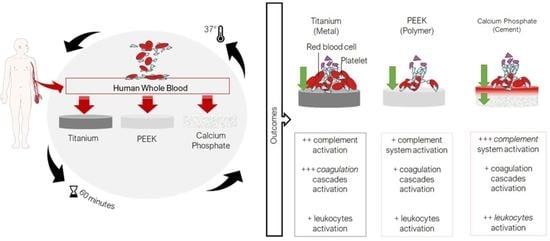
Human Whole Blood Interactions with Craniomaxillofacial Reconstruction Materials: Exploring In Vitro the Role of Blood Cascades and Leukocytes in Early Healing Events
Abstract
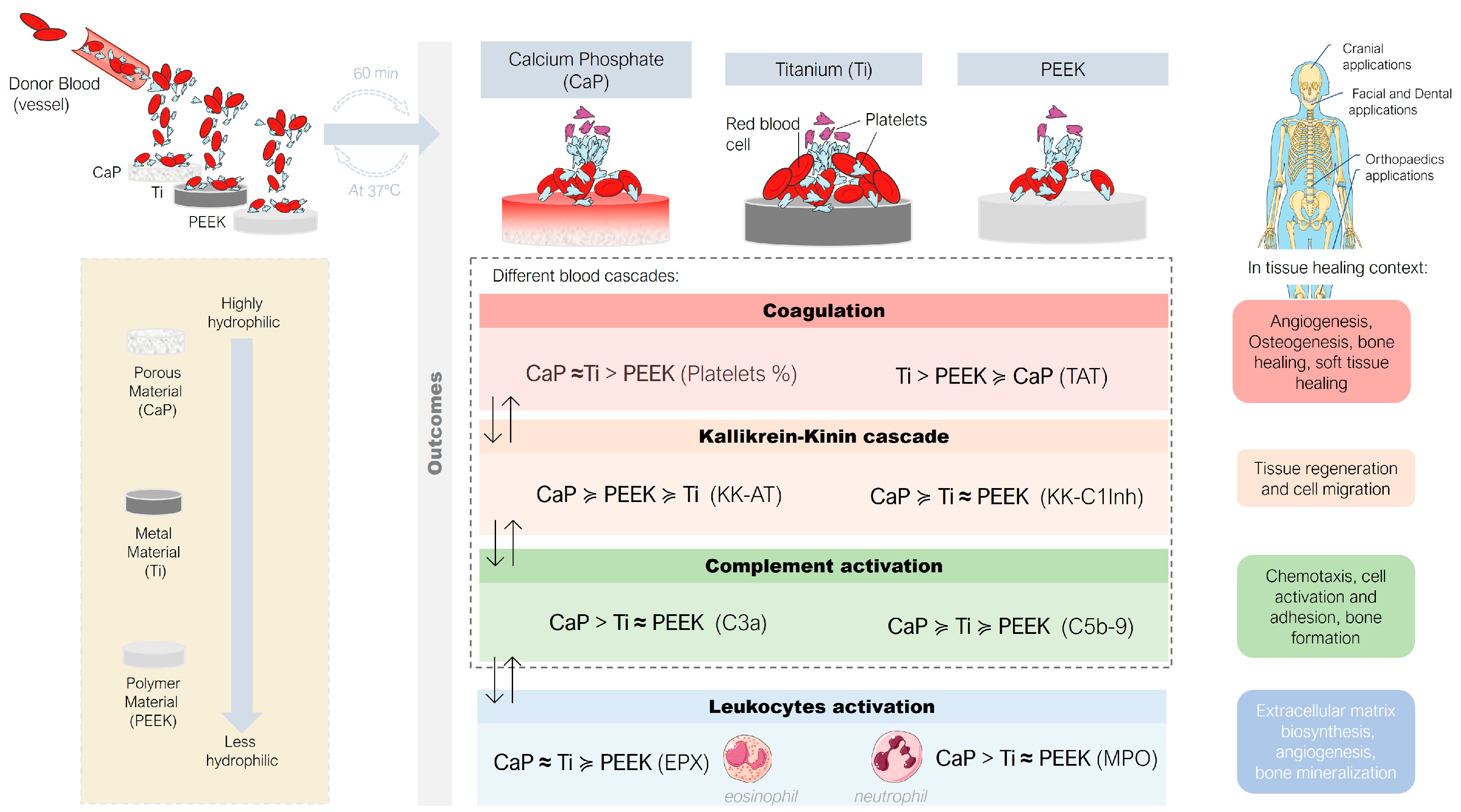
:1. Introduction
2. Materials and Methods
2.1. Material Manufacturing
2.2. Material Characterization
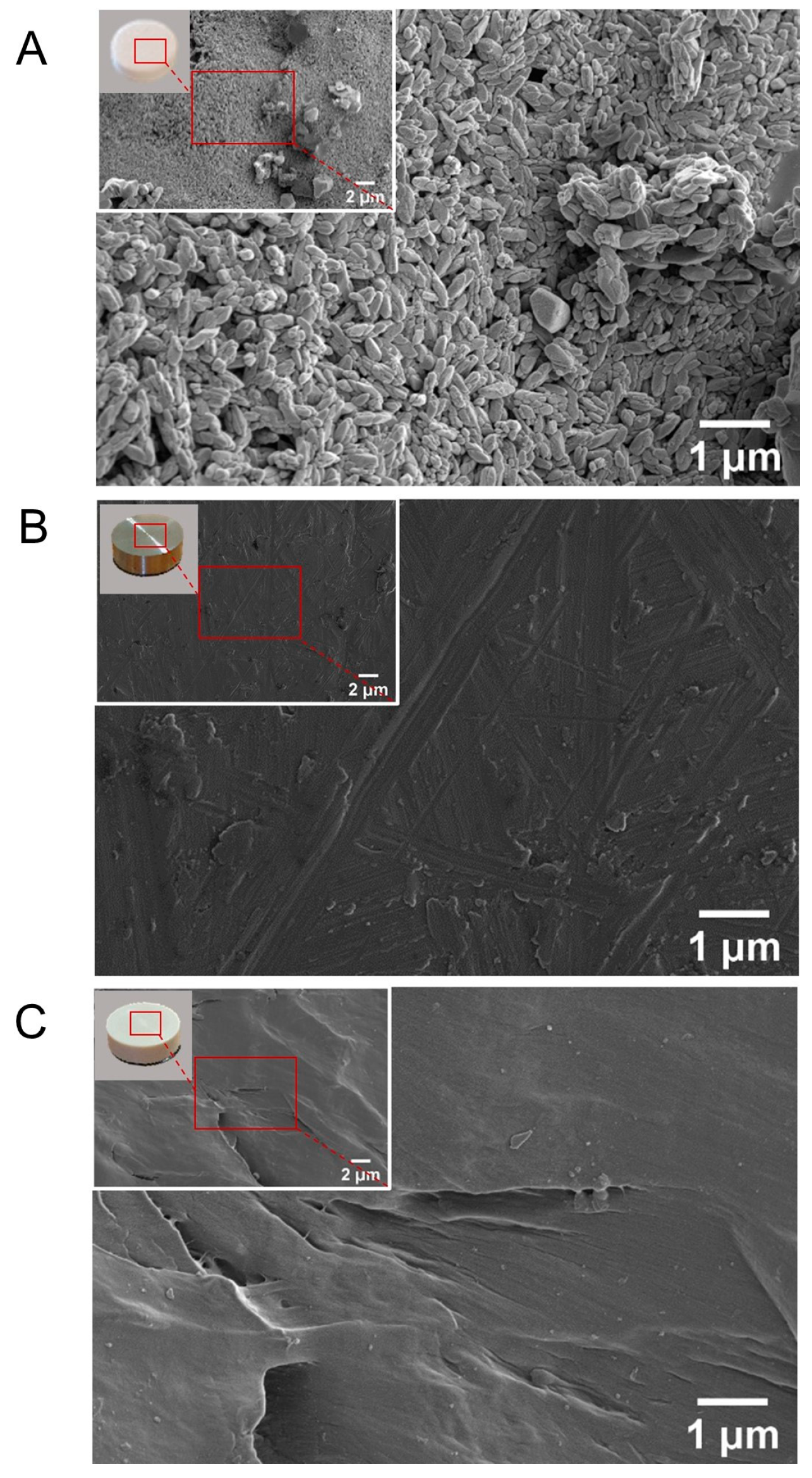
2.2.1. Surface Morphology
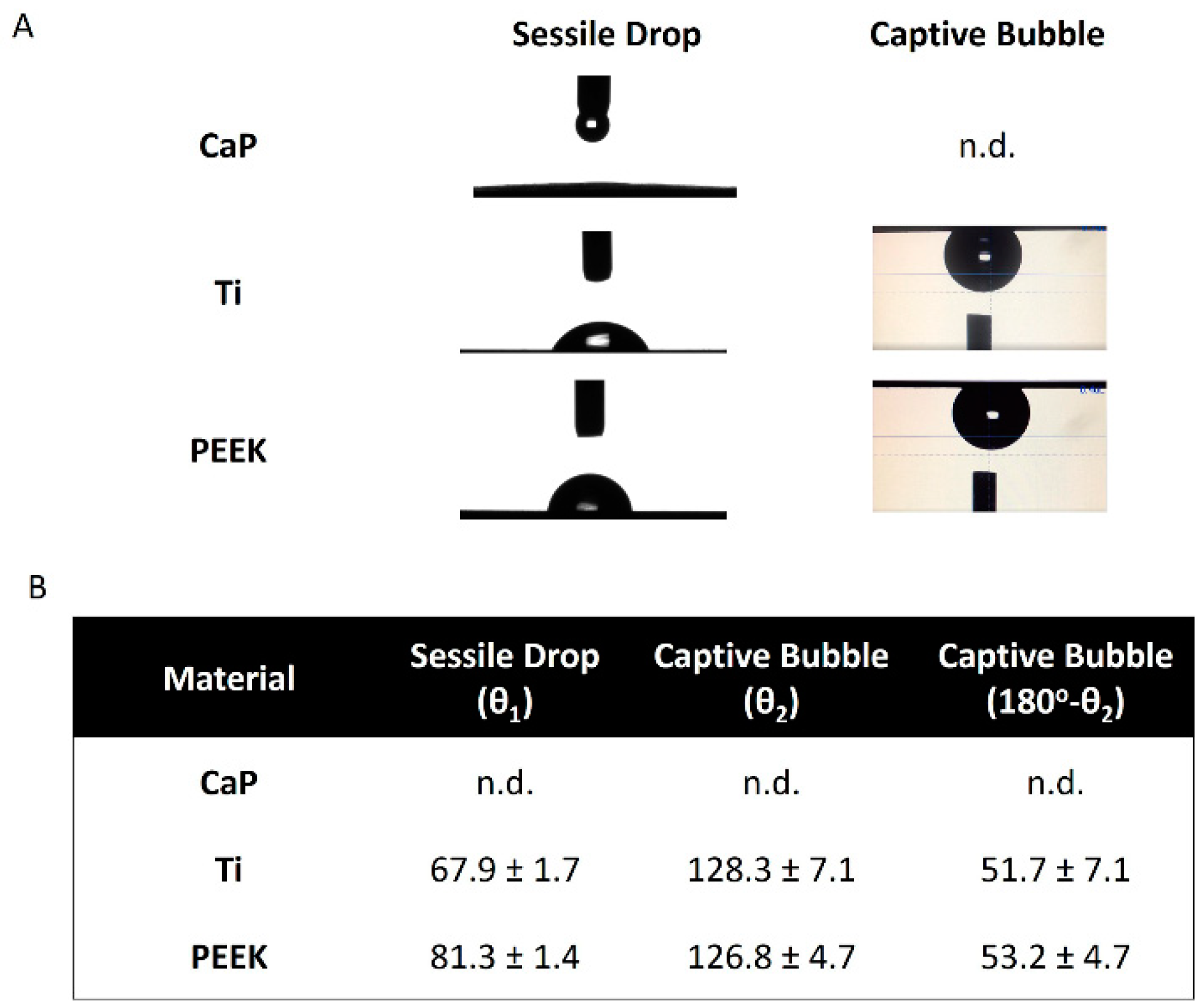
2.2.2. Wettability
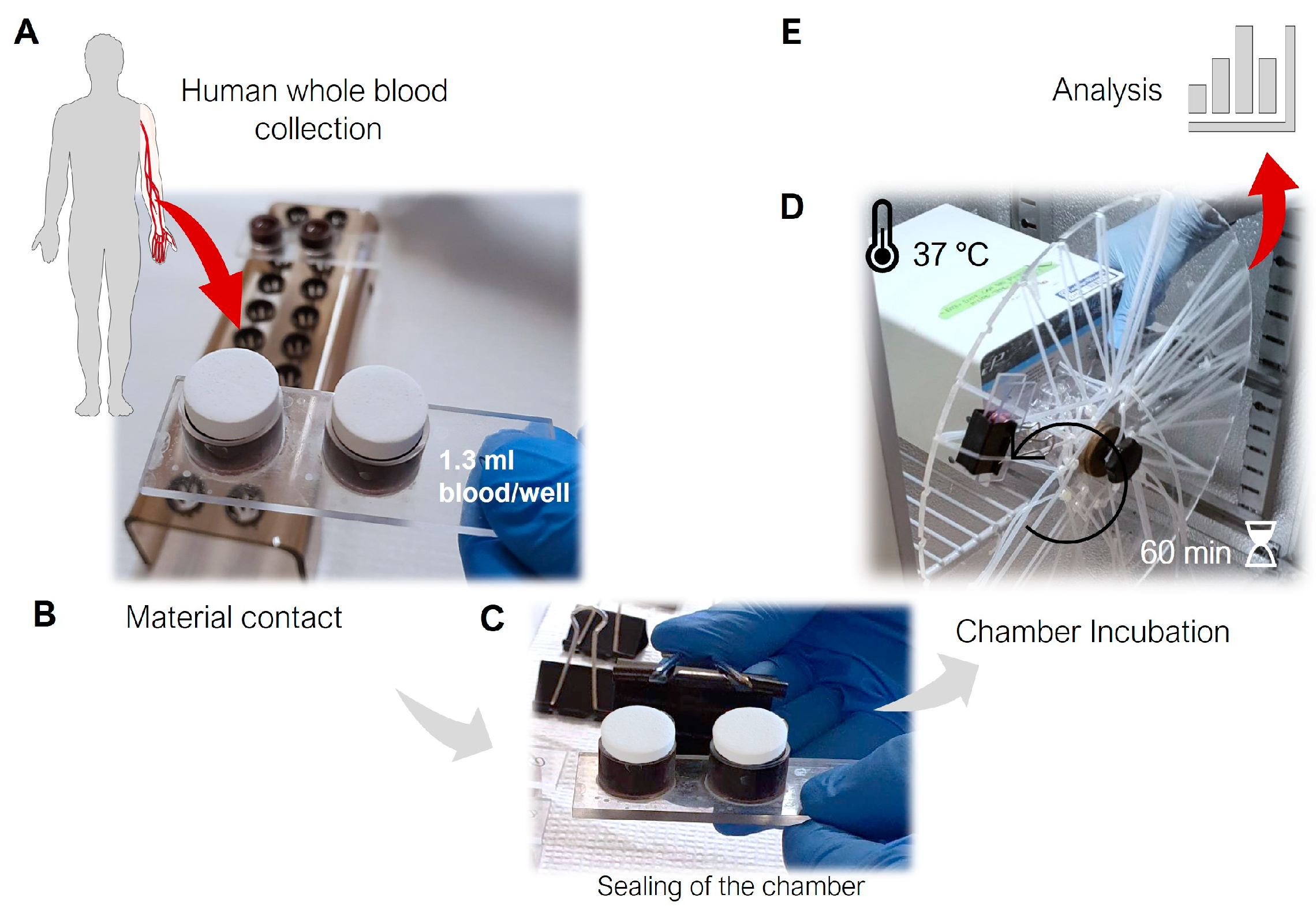
2.3. Study Design
2.4. Heparinization and Blood Collection
2.5. Processing of the Blood Samples
2.6. Analytical Procedures
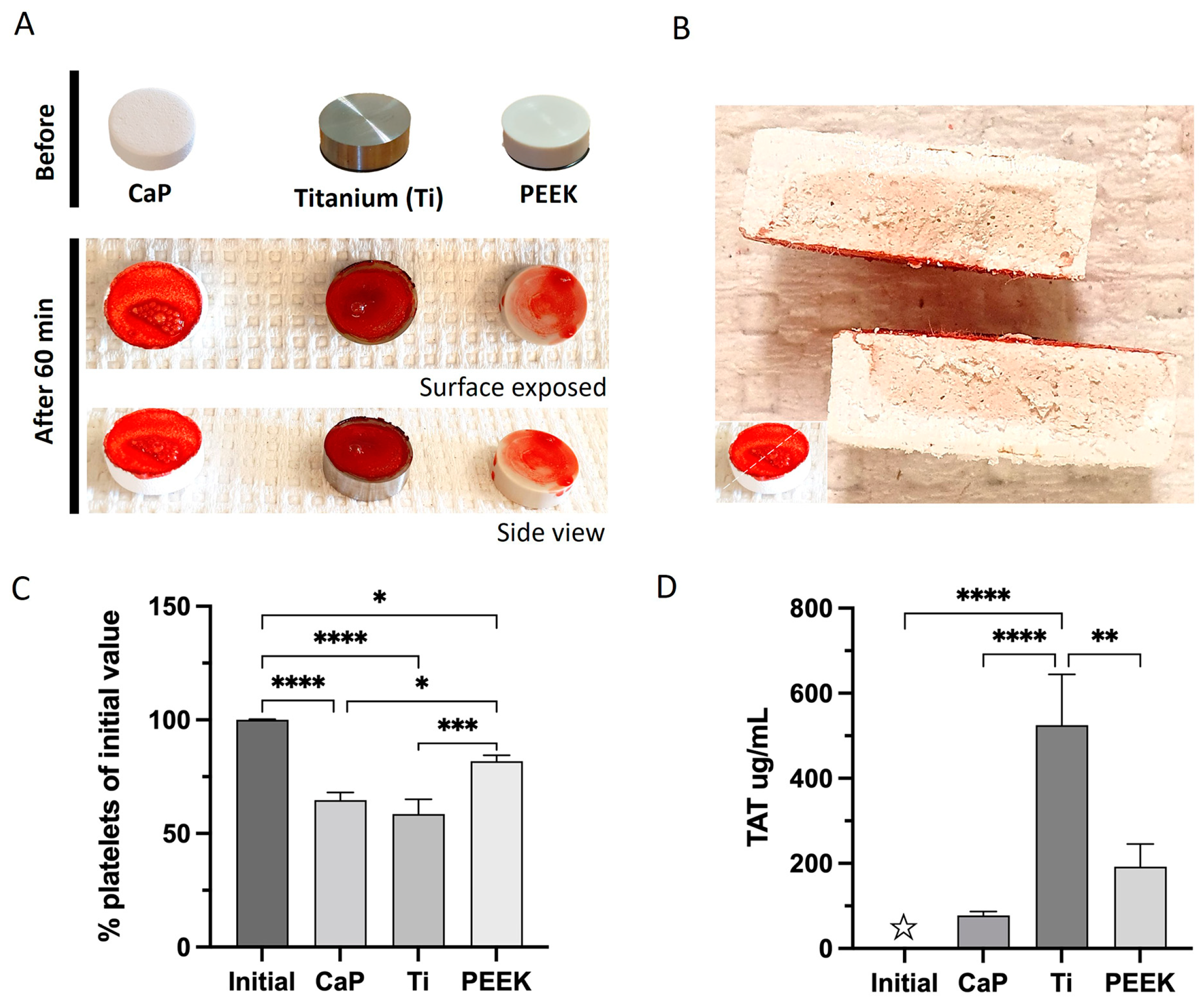
2.6.1. Macroscopic Visualization of Blood Interactions
2.6.2. Platelet Count
2.6.3. Coagulation and Contact Markers
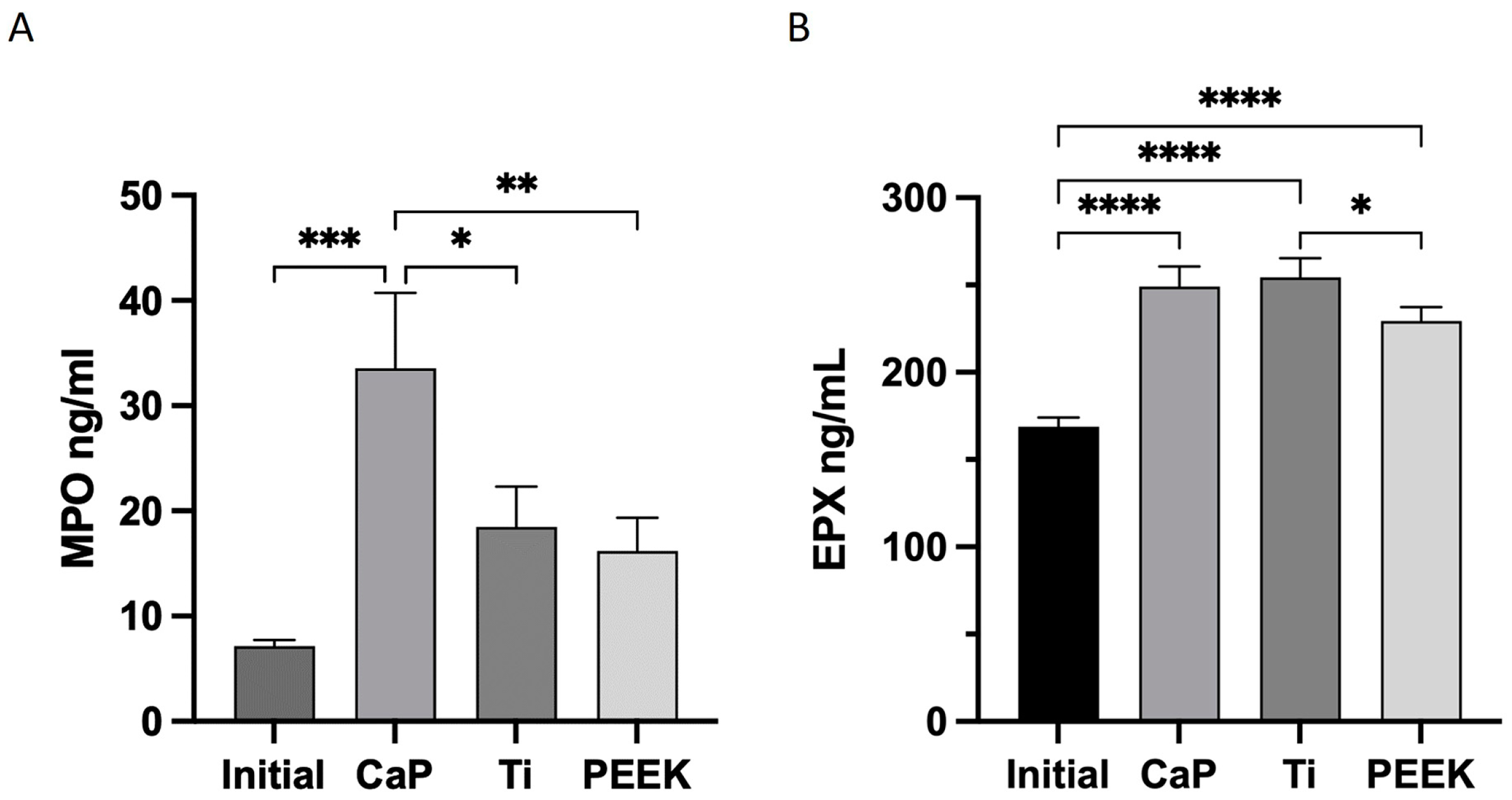
2.6.4. Myeloperoxidase (MPO) and EPX Release
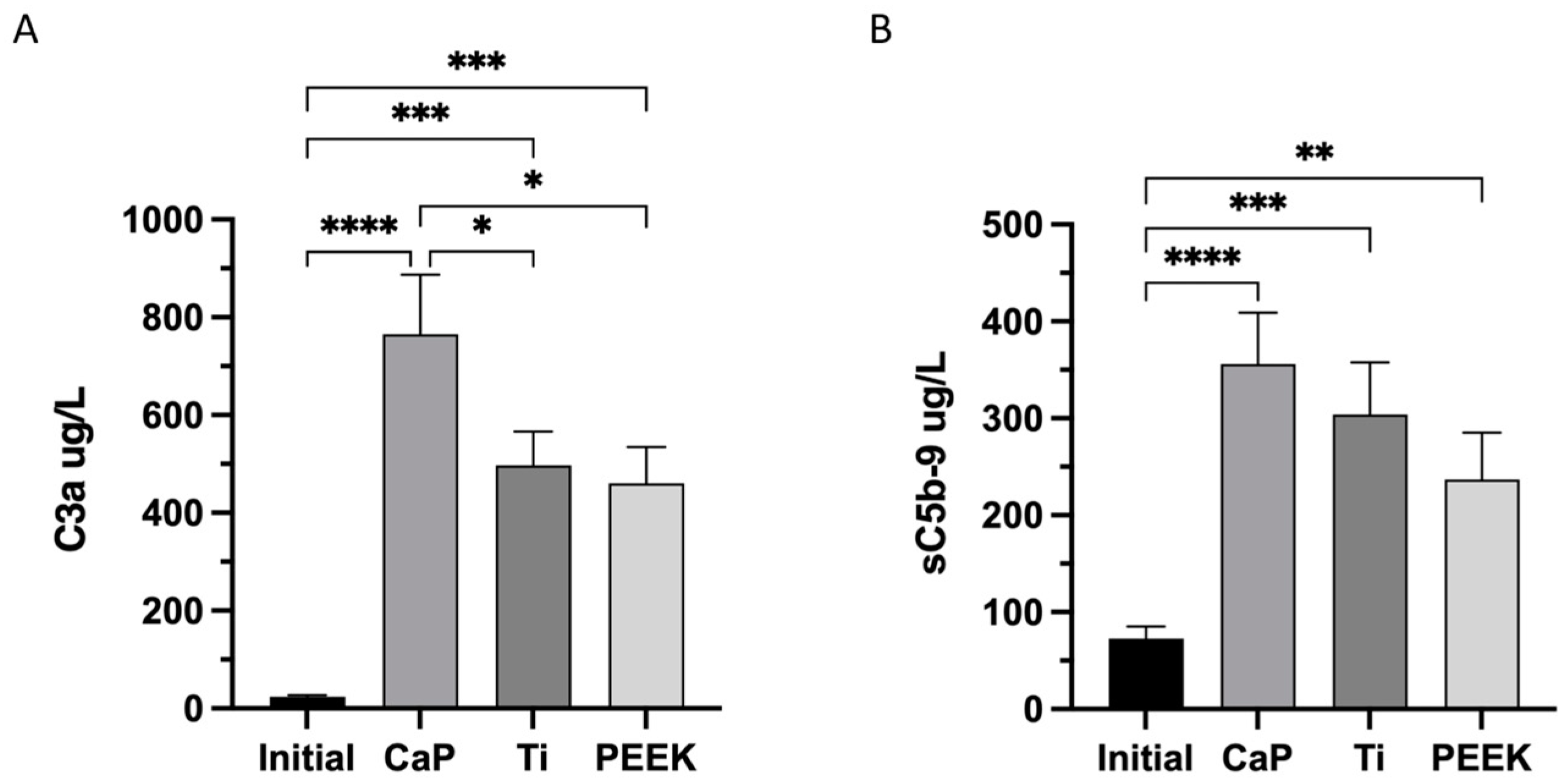
2.6.5. Complement Markers
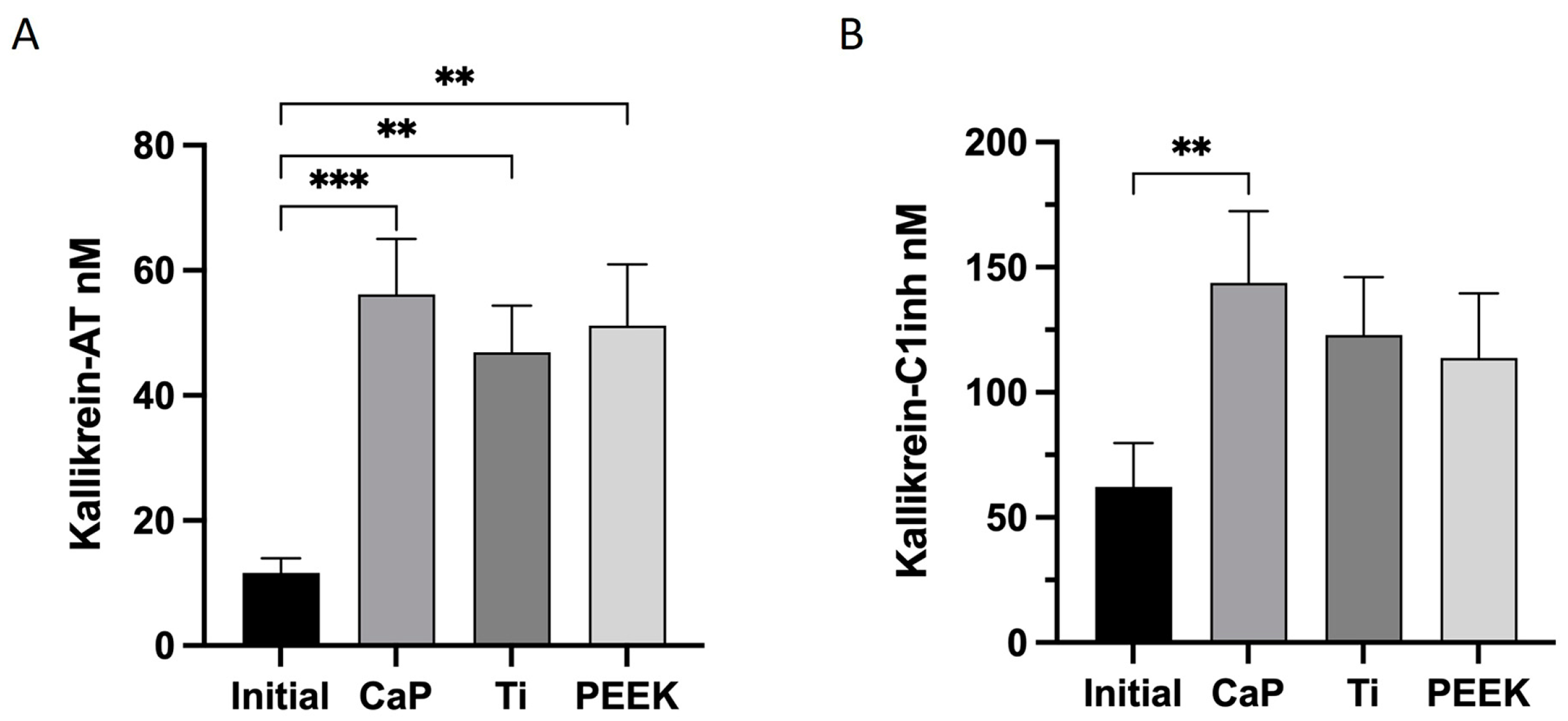
2.6.6. Kallikrein–Bradykinin (KK) Markers
2.7. Statistical Analysis
3. Results
3.1. Surface Topography Visualization
3.2. Wettability
3.3. Highest Coagulation to Ti
3.4. Highest Activation of Kallikrein–Bradykinin with CaP
3.5. Highest Activation of Complement C3a and C5b-9 Complexes in CaP
3.6. Highest Release of MPO with CaP
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Mödinger, Y.; Teixeira, G.; Neidlinger-Wilke, C.; Ignatius, A. Role of the Complement System in the Response to Orthopedic Biomaterials. Int. J. Mol. Sci. 2018, 19, 3367. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Engberg, A.E.; Jonsson, N.; Sandholm, K.; Nicholls, I.A.; Mollnes, T.E.; Fromell, K.; Nilsson, B.; Ekdahl, K.N. Reciprocal Relationship between Contact and Complement System Activation on Artificial Polymers Exposed to Whole Human Blood. Biomaterials 2016, 77, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Dzik, S. Complement and Coagulation: Cross Talk Through Time. Transfus. Med. Rev. 2019, 33, 199–206. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Nilsson, B.; Ekdahl, K.N.; Mollnes, T.E.; Lambris, J.D. Complement and Coagulation: Strangers or Partners in Crime? Trends Immunol. 2007, 28, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, Platelets, and Blood Coagulation at Biomaterial Interfaces. Colloids Surf. B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Gorbet, M.; Sperling, C.; Maitz, M.F.; Siedlecki, C.A.; Werner, C.; Sefton, M.V. The Blood Compatibility Challenge. Part 3: Material Associated Activation of Blood Cascades and Cells. Acta Biomater. 2019, 94, 25–32. [Google Scholar] [CrossRef]
- Kolev, M.; Le Friec, G.; Kemper, C. Complement—Tapping into New Sites and Effector Systems. Nat. Rev. Immunol. 2014, 14, 811–820. [Google Scholar] [CrossRef]
- Eriksson, O.; Mohlin, C.; Nilsson, B.; Ekdahl, K.N. The Human Platelet as an Innate Immune Cell: Interactions Between Activated Platelets and the Complement System. Front. Immunol. 2019, 10, 1590. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Huang, S.; Nilsson, B.; Teramura, Y. Complement Inhibition in Biomaterial- and Biosurface-Induced Thromboinflammation. Semin. Immunol. 2016, 28, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert. Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, T.; Boukhechba, F.; Clavé, A.; Bouvet-Gerbettaz, S.; Trojani, C.; Michiels, J.-F.; Laugier, J.-P.; Bouler, J.-M.; Carle, G.F.; Scimeca, J.-C.; et al. Biphasic Calcium Phosphate Microparticles for Bone Formation: Benefits of Combination with Blood Clot. Tissue Eng. Part. A 2010, 16, 3495–3505. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic Calcium Phosphate Ceramics for Bone Reconstruction: A Review of Biological Response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, D.; Li, B.; Hong, H.; Jiang, C.; Yuan, Y.; Wang, J.; Hu, R.; Li, B.; Liu, C. BMP-2/CPC Scaffold with Dexamethasone-Loaded Blood Clot Embedment Accelerates Clinical Bone Regeneration. Am. J. Transl. Res. 2022, 14, 2874–2893. [Google Scholar] [PubMed]
- Zuardi, L.R.; Silva, C.L.A.; Rego, E.M.; Carneiro, G.V.; Spriano, S.; Nanci, A.; de Oliveira, P.T. Influence of a Physiologically Formed Blood Clot on Pre-Osteoblastic Cells Grown on a BMP-7-Coated Nanoporous Titanium Surface. Biomimetics 2023, 8, 123. [Google Scholar] [CrossRef]
- Burkhardt, M.A.; Waser, J.; Milleret, V.; Gerber, I.; Emmert, M.Y.; Foolen, J.; Hoerstrup, S.P.; Schlottig, F.; Vogel, V. Synergistic Interactions of Blood-Borne Immune Cells, Fibroblasts and Extracellular Matrix Drive Repair in an in Vitro Peri-Implant Wound Healing Model. Sci. Rep. 2016, 6, 21071. [Google Scholar] [CrossRef]
- Thor, A. Porous Titanium Granules and Blood for Bone Regeneration around Dental Implants: Report of Four Cases and Review of the Literature. Case Rep. Dent. 2013, 2013, 410515. [Google Scholar] [CrossRef]
- Gallinetti, S.; Linder, L.K.B.; Åberg, J.; Illies, C.; Engqvist, H.; Birgersson, U. Titanium Reinforced Calcium Phosphate Improves Bone Formation and Osteointegration in Ovine Calvaria Defects: A Comparative 52 Weeks Study. Biomed. Mater. 2021, 16, 035031. [Google Scholar] [CrossRef]
- Billström, G.H.; Lopes, V.R.; Illies, C.; Gallinetti, S.; Åberg, J.; Engqvist, H.; Aparicio, C.; Larsson, S.; Linder, L.K.B.; Birgersson, U. Guiding Bone Formation Using Semi-onlay Calcium Phosphate Implants in an Ovine Calvarial Model. J. Tissue Eng. Regen. Med. 2022, 16, 435–447. [Google Scholar] [CrossRef]
- Ye, Z.; Kobe, A.C.; Sang, T.; Aparicio, C. Unraveling Dominant Surface Physicochemistry to Build Antimicrobial Peptide Coatings with Supramolecular Amphiphiles. Nanoscale 2020, 12, 20767–20775. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Nilsson Ekdahl, K.; Reynolds, H.; Larsson, R.; Nilsson, B. A New in Vitro Model to Study Interaction between Whole Blood and Biomaterials. Studies of Platelet and Coagulation Activation and the Effect of Aspirin. Biomaterials 1999, 20, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Sanchez, J.; Ekdahl, K.N.; Elgue, G.; Nilsson, B.; Larsson, R. Optimal Heparin Surface Concentration and Antithrombin Binding Capacity as Evaluated with Human Non-Anticoagulated Bloodin Vitro. J. Biomed. Mater. Res. 2003, 67, 458–466. [Google Scholar] [CrossRef]
- Hong, J.; Kurt, S.; Thor, A. A Hydrophilic Dental Implant Surface Exhibit Thrombogenic Properties In Vitro. Clin. Implant. Dent. Relat. Res. 2013, 15, 105–112. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, W.; Chen, J.; Yu, J.; Zhang, J.; Chen, J. The Application of Polyetheretherketone (PEEK) Implants in Cranioplasty. Brain Res. Bull. 2019, 153, 143–149. [Google Scholar] [CrossRef]
- Yan, Y.; Chibowski, E.; Szcześ, A. Surface Properties of Ti-6Al-4V Alloy Part I: Surface Roughness and Apparent Surface Free Energy. Mater. Sci. Eng. C 2017, 70, 207–215. [Google Scholar] [CrossRef]
- Rendu, F.; Brohard-Bohn, B. The Platelet Release Reaction: Granules’ Constituents, Secretion and Functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A. Autologous Platelets as a Source of Proteins for Healing and Tissue Regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Gudbrandsdottir, S.; Hasselbalch, H.C.; Nielsen, C.H. Activated Platelets Enhance IL-10 Secretion and Reduce TNF-α Secretion by Monocytes. J. Immunol. 2013, 191, 4059–4067. [Google Scholar] [CrossRef]
- Fernandes, K.R.; Zhang, Y.; Magri, A.M.P.; Renno, A.C.M.; van den Beucken, J.J.J.P. Biomaterial Property Effects on Platelets and Macrophages: An in Vitro Study. ACS Biomater. Sci. Eng. 2017, 3, 3318–3327. [Google Scholar] [CrossRef]
- Hong, J.; Andersson, J.; Ekdahl, K.N.; Elgue, G.; Axén, N.; Larsson, R.; Nilsson, B. Titanium Is a Highly Thrombogenic Biomaterial: Possible Implications for Osteogenesis. Thromb. Haemost. 1999, 82, 58–64. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Gonçalves, I.C.; Martins, M.C.L.; Barbosa, M.A.; Ratner, B.D. Fibrinogen Adsorption, Platelet Adhesion and Activation on Mixed Hydroxyl-/Methyl-Terminated Self-Assembled Monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef]
- Hulsart-Billström, G.; Janson, O.; Engqvist, H.; Welch, K.; Hong, J. Thromboinflammation as Bioactivity Assessment of H2O2-Alkali Modified Titanium Surfaces. J. Mater. Sci. Mater. Med. 2019, 30, 66. [Google Scholar] [CrossRef] [PubMed]
- Calciolari, E.; Hamlet, S.; Ivanovski, S.; Donos, N. Pro-Osteogenic Properties of Hydrophilic and Hydrophobic Titanium Surfaces: Crosstalk between Signalling Pathways in in Vivo Models. J. Periodontal Res. 2018, 53, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.H. The Contact Activation and Kallikrein/Kinin Systems: Pathophysiologic and Physiologic Activities. J. Thromb. Haemost. 2016, 14, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Da Soley, B.S.; Morais, R.L.T.d.; Pesquero, J.B.; Bader, M.; Otuki, M.F.; Cabrini, D.A. Kinin Receptors in Skin Wound Healing. J. Dermatol. Sci. 2016, 82, 95–105. [Google Scholar] [CrossRef]
- Ehrenfeld, P.; Millan, C.; Matus, C.E.; Figueroa, J.E.; Burgos, R.A.; Nualart, F.; Bhoola, K.D.; Figueroa, C.D. Activation of Kinin B1 Receptors Induces Chemotaxis of Human Neutrophils. J. Leukoc. Biol. 2006, 80, 117–124. [Google Scholar] [CrossRef]
- Sheng, Z.; Yao, Y.; Li, Y.; Yan, F.; Huang, J.; Ma, G. Bradykinin Preconditioning Improves Therapeutic Potential of Human Endothelial Progenitor Cells in Infarcted Myocardium. PLoS ONE 2013, 8, e81505. [Google Scholar] [CrossRef]
- Bekassy, Z.; Lopatko Fagerström, I.; Bader, M.; Karpman, D. Crosstalk between the Renin–Angiotensin, Complement and Kallikrein–Kinin Systems in Inflammation. Nat. Rev. Immunol. 2022, 22, 411–428. [Google Scholar] [CrossRef]
- Klein, C.P.; de Groot, K.; van Kamp, G. Activation of Complement C3 by Different Calcium Phosphate Powders. Biomaterials 1983, 4, 181–184. [Google Scholar] [CrossRef]
- Ehrnthaller, C.; Huber-Lang, M.; Nilsson, P.; Bindl, R.; Redeker, S.; Recknagel, S.; Rapp, A.; Mollnes, T.; Amling, M.; Gebhard, F.; et al. Complement C3 and C5 Deficiency Affects Fracture Healing. PLoS ONE 2013, 8, e81341. [Google Scholar] [CrossRef]
- Ignatius, A.; Schoengraf, P.; Kreja, L.; Liedert, A.; Recknagel, S.; Kandert, S.; Brenner, R.E.; Schneider, M.; Lambris, J.D.; Huber-Lang, M. Complement C3a and C5a Modulate Osteoclast Formation and Inflammatory Response of Osteoblasts in Synergism with IL-1β. J. Cell Biochem. 2011, 112, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.; Ehrnthaller, C.; Brenner, R.E.; Kreja, L.; Schoengraf, P.; Lisson, P.; Blakytny, R.; Recknagel, S.; Claes, L.; Gebhard, F.; et al. The Anaphylatoxin Receptor C5aR Is Present During Fracture Healing in Rats and Mediates Osteoblast Migration In Vitro. J. Trauma Inj. Infect. Crit. Care 2011, 71, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Park, K.; Ito, M.; Ikeda, K.; Takeshita, S. Osteoclast-Derived Complement Component 3a Stimulates Osteoblast Differentiation. J. Bone Miner. Res. 2014, 29, 1522–1530. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Kovtun, A.; Ignatius, A. The Role of Complement in Trauma and Fracture Healing. Semin. Immunol. 2013, 25, 73–78. [Google Scholar] [CrossRef]
- Omar, O.; Engstrand, T.; Kihlström Burenstam Linder, L.; Åberg, J.; Shah, F.A.; Palmquist, A.; Birgersson, U.; Elgali, I.; Pujari-Palmer, M.; Engqvist, H.; et al. In Situ Bone Regeneration of Large Cranial Defects Using Synthetic Ceramic Implants with a Tailored Composition and Design. Proc. Natl. Acad. Sci. USA 2020, 117, 26660–26671. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, P.; Lopes, V.R.; Billström, G.H.; Gallinetti, S.; Illies, C.; Linder, L.K.B.; Birgersson, U. Targeted ToF-SIMS Analysis of Macrophage Content from a Human Cranial Triphasic Calcium Phosphate Implant. ACS Appl. Bio Mater. 2021, 4, 6791–6798. [Google Scholar] [CrossRef]
- Guillet, C.; Birgersson, U.; Engstrand, T.; Åberg, J.; Lopes, V.R.; Thor, A.; Engqvist, H.; Forterre, F. Bone Formation beyond the Skeletal Envelope Using Calcium Phosphate Granules Packed into a Collagen Pouch-a Pilot Study. Biomed. Mater. 2023, 18, 035007. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Cambier, S.; Proost, P.; Marques, P.E. Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Front. Cell Dev. Biol. 2021, 9, 624025. [Google Scholar] [CrossRef]
- Thien, A.; King, N.K.K.; Ang, B.T.; Wang, E.; Ng, I. Comparison of Polyetheretherketone and Titanium Cranioplasty after Decompressive Craniectomy. World Neurosurg. 2015, 83, 176–180. [Google Scholar] [CrossRef]
- Zwirner, J.; Götze, O.; Begemann, G.; Kapp, A.; Kirchhoff, K.; Werfel, T. Evaluation of C3a Receptor Expression on Human Leucocytes by the Use of Novel Monoclonal Antibodies. Immunology 1999, 97, 166–172. [Google Scholar] [CrossRef]
- Jhunjhunwala, S. Neutrophils at the Biological–Material Interface. ACS Biomater. Sci. Eng. 2018, 4, 1128–1136. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- DeNichilo, M.O.; Shoubridge, A.J.; Panagopoulos, V.; Liapis, V.; Zysk, A.; Zinonos, I.; Hay, S.; Atkins, G.J.; Findlay, D.M.; Evdokiou, A. Peroxidase Enzymes Regulate Collagen Biosynthesis and Matrix Mineralization by Cultured Human Osteoblasts. Calcif. Tissue Int. 2016, 98, 294–305. [Google Scholar] [CrossRef] [PubMed]
- DeNichilo, M.O.; Panagopoulos, V.; Rayner, T.E.; Borowicz, R.A.; Greenwood, J.E.; Evdokiou, A. Peroxidase Enzymes Regulate Collagen Extracellular Matrix Biosynthesis. Am. J. Pathol. 2015, 185, 1372–1384. [Google Scholar] [CrossRef]
- Panagopoulos, V.; Zinonos, I.; Leach, D.A.; Hay, S.J.; Liapis, V.; Zysk, A.; Ingman, W.v.; DeNichilo, M.O.; Evdokiou, A. Uncovering a New Role for Peroxidase Enzymes as Drivers of Angiogenesis. Int. J. Biochem. Cell Biol. 2015, 68, 128–138. [Google Scholar] [CrossRef]
- Panagopoulos, V.; Liapis, V.; Zinonos, I.; Hay, S.; Leach, D.A.; Ingman, W.; DeNichilo, M.O.; Atkins, G.J.; Findlay, D.M.; Zannettino, A.C.W.; et al. Peroxidase Enzymes Inhibit Osteoclast Differentiation and Bone Resorption. Mol. Cell Endocrinol. 2017, 440, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, S.; Li, H.; Si, S.; Wang, Z. Myeloperoxidase Controls Bone Turnover by Suppressing Osteoclast Differentiation Through Modulating Reactive Oxygen Species Level. J. Bone Min. Res. 2021, 36, 591–603. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Zheng, X.; Jin, L.; Xiang, N.; Zhang, M.; Chen, Z. Eosinophils Attenuate Arthritis by Inducing M2 Macrophage Polarization via Inhibiting the IκB/P38 MAPK Signaling Pathway. Biochem. Biophys. Res. Commun. 2019, 508, 894–901. [Google Scholar] [CrossRef]
- Borelli, V.; Vita, F.; Shankar, S.; Soranzo, M.R.; Banfi, E.; Scialino, G.; Brochetta, C.; Zabucchi, G. Human Eosinophil Peroxidase Induces Surface Alteration, Killing, and Lysis of Mycobacterium tuberculosis. Infect. Immun. 2003, 71, 605–613. [Google Scholar] [CrossRef]
- Kwarcinski, J.; Boughton, P.; Ruys, A.; Doolan, A.; van Gelder, J. Cranioplasty and Craniofacial Reconstruction: A Review of Implant Material, Manufacturing Method and Infection Risk. Appl. Sci. 2017, 7, 276. [Google Scholar] [CrossRef]
- Henry, J.; Amoo, M.; Taylor, J.; O’Brien, D.P. Complications of Cranioplasty in Relation to Material: Systematic Review, Network Meta-Analysis and Meta-Regression. Neurosurgery 2021, 89, 383–394. [Google Scholar] [CrossRef]
- Phan, K.; Hogan, J.A.; Assem, Y.; Mobbs, R.J. PEEK-Halo Effect in Interbody Fusion. J. Clin. Neurosci. 2016, 24, 138–140. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, J.M.; Schwartz, Z.; Boyan, B.D. Implant Materials Generate Different Peri-Implant Inflammatory Factors. Spine 2015, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, J.; Chen, C.; Wang, Y.; Hao, Z.; Chen, T.; Wang, J.; Li, J. Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review. J. Funct. Biomater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Barbasz, A.; Kozik, A. The Assembly and Activation of Kinin-Forming Systems on the Surface of Human U-937 Macrophage-like Cells. Biol. Chem. 2009, 390, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, J.M.; Oberle, A.; Oechslin, C.; Bohner, M.; Frei, C.; Boecken, I.; von Rechenberg, B. Assessment of the Suitability of a New Brushite Calcium Phosphate Cement for Cranioplasty—An Experimental Study in Sheep. J. Cranio-Maxillofac. Surg. 2005, 33, 37–44. [Google Scholar] [CrossRef]
- Kihlström Burenstam Linder, L.; Birgersson, U.; Lundgren, K.; Illies, C.; Engstrand, T. Patient-Specific Titanium-Reinforced Calcium Phosphate Implant for the Repair and Healing of Complex Cranial Defects. World Neurosurg. 2019, 122, e399–e407. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, T.; Kihlström, L.; Lundgren, K.; Trobos, M.; Engqvist, H.; Thomsen, P. Bioceramic Implant Induces Bone Healing of Cranial Defects. Plast. Reconstr. Surg. Glob. Open 2015, 3, e491. [Google Scholar] [CrossRef]
- Sundblom, J.; Xheka, F.; Casar-Borota, O.; Ryttlefors, M. Bone Formation in Custom-Made Cranioplasty: Evidence of Early and Sustained Bone Development in Bioceramic Calcium Phosphate Implants. Patient Series. J. Neurosurg. Case Lessons 2021, 1, CASE20133. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Kolar, P.; Pfaff, M.; Perka, C.; Buttgereit, F.; Duda, G.; Lienau, J. Cellular Composition of the Initial Fracture Hematoma Compared to a Muscle Hematoma: A Study in Sheep. J. Orthop. Res. 2009, 27, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential Proteomic Analysis Distinguishes Tissue Repair Biomarker Signatures in Wound Exudates Obtained from Normal Healing and Chronic Wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, V.R.; Birgersson, U.; Manivel, V.A.; Hulsart-Billström, G.; Gallinetti, S.; Aparicio, C.; Hong, J. Human Whole Blood Interactions with Craniomaxillofacial Reconstruction Materials: Exploring In Vitro the Role of Blood Cascades and Leukocytes in Early Healing Events. J. Funct. Biomater. 2023, 14, 361. https://doi.org/10.3390/jfb14070361
Lopes VR, Birgersson U, Manivel VA, Hulsart-Billström G, Gallinetti S, Aparicio C, Hong J. Human Whole Blood Interactions with Craniomaxillofacial Reconstruction Materials: Exploring In Vitro the Role of Blood Cascades and Leukocytes in Early Healing Events. Journal of Functional Biomaterials. 2023; 14(7):361. https://doi.org/10.3390/jfb14070361
Chicago/Turabian StyleLopes, Viviana R., Ulrik Birgersson, Vivek Anand Manivel, Gry Hulsart-Billström, Sara Gallinetti, Conrado Aparicio, and Jaan Hong. 2023. "Human Whole Blood Interactions with Craniomaxillofacial Reconstruction Materials: Exploring In Vitro the Role of Blood Cascades and Leukocytes in Early Healing Events" Journal of Functional Biomaterials 14, no. 7: 361. https://doi.org/10.3390/jfb14070361
APA StyleLopes, V. R., Birgersson, U., Manivel, V. A., Hulsart-Billström, G., Gallinetti, S., Aparicio, C., & Hong, J. (2023). Human Whole Blood Interactions with Craniomaxillofacial Reconstruction Materials: Exploring In Vitro the Role of Blood Cascades and Leukocytes in Early Healing Events. Journal of Functional Biomaterials, 14(7), 361. https://doi.org/10.3390/jfb14070361