Adhesion and Surface Roughness of Apatite-Containing Carbomer and Improved Ionically Bioactive Resin Compared to Glass Ionomers
Abstract
:1. Introduction
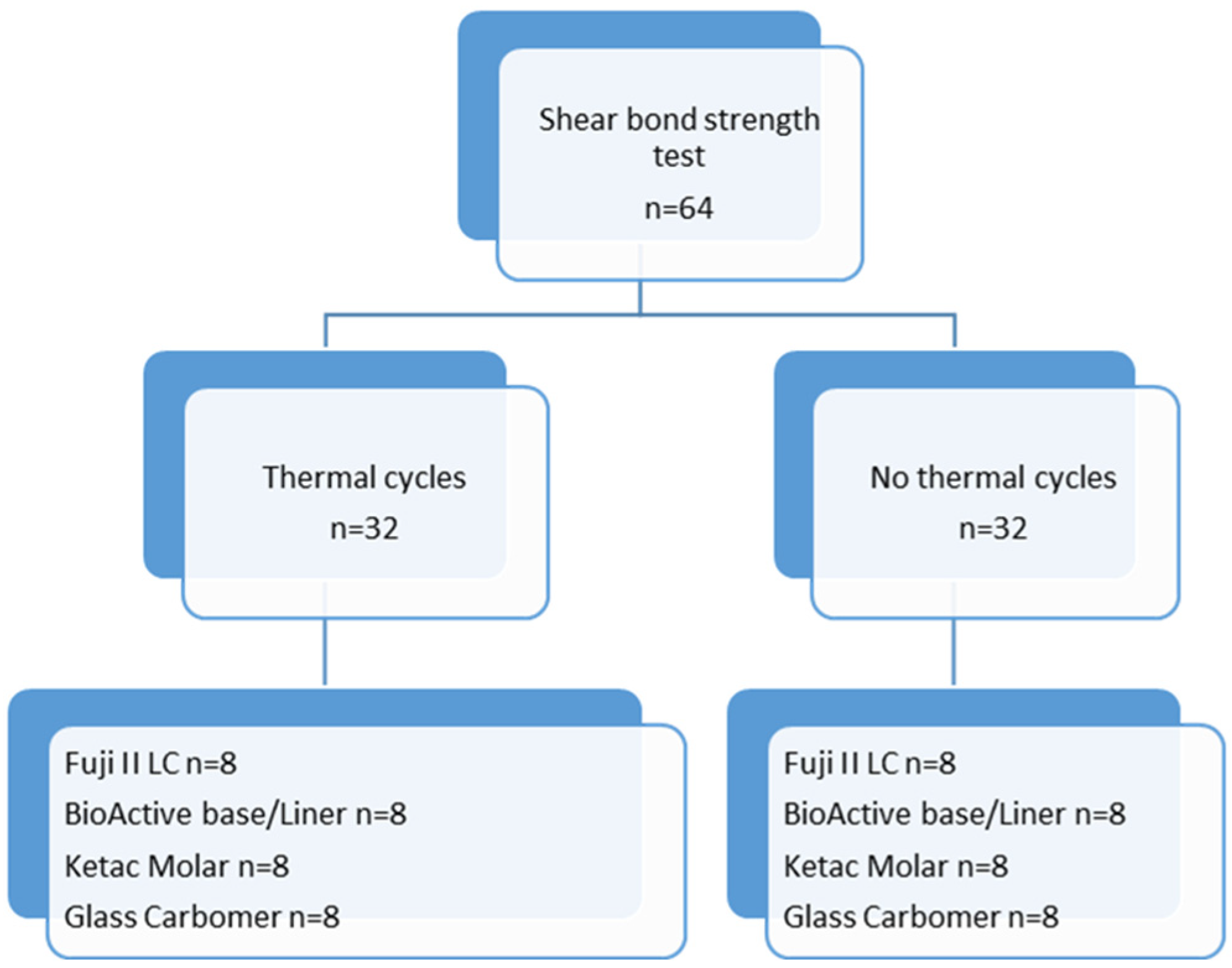
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Thermal Cycle Test
2.3. Shear Bond Strength Test
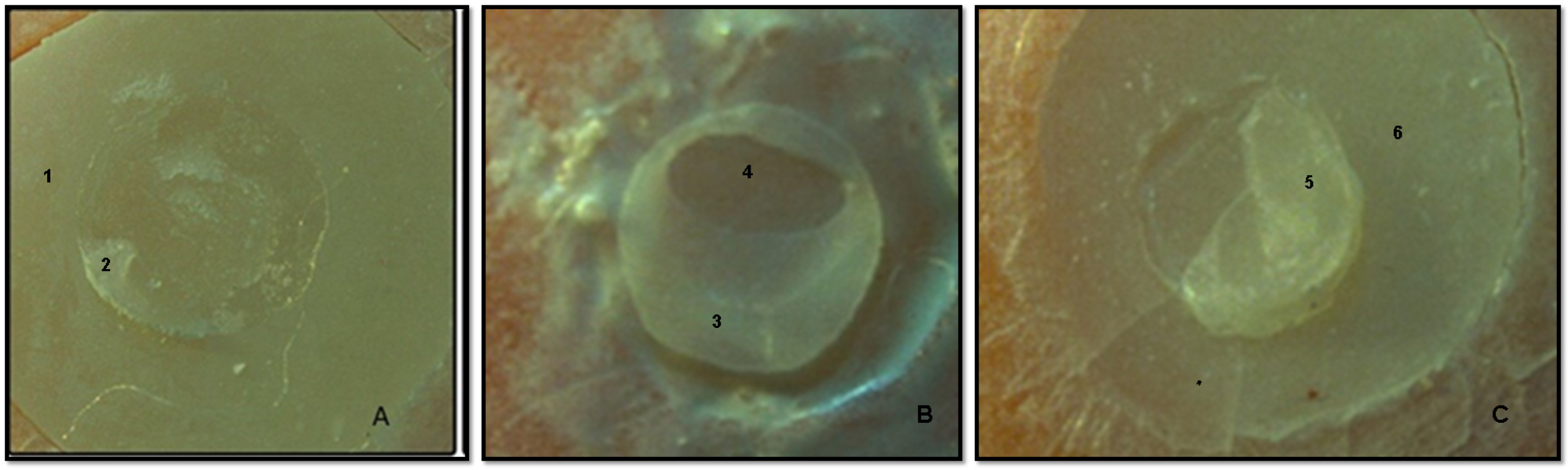
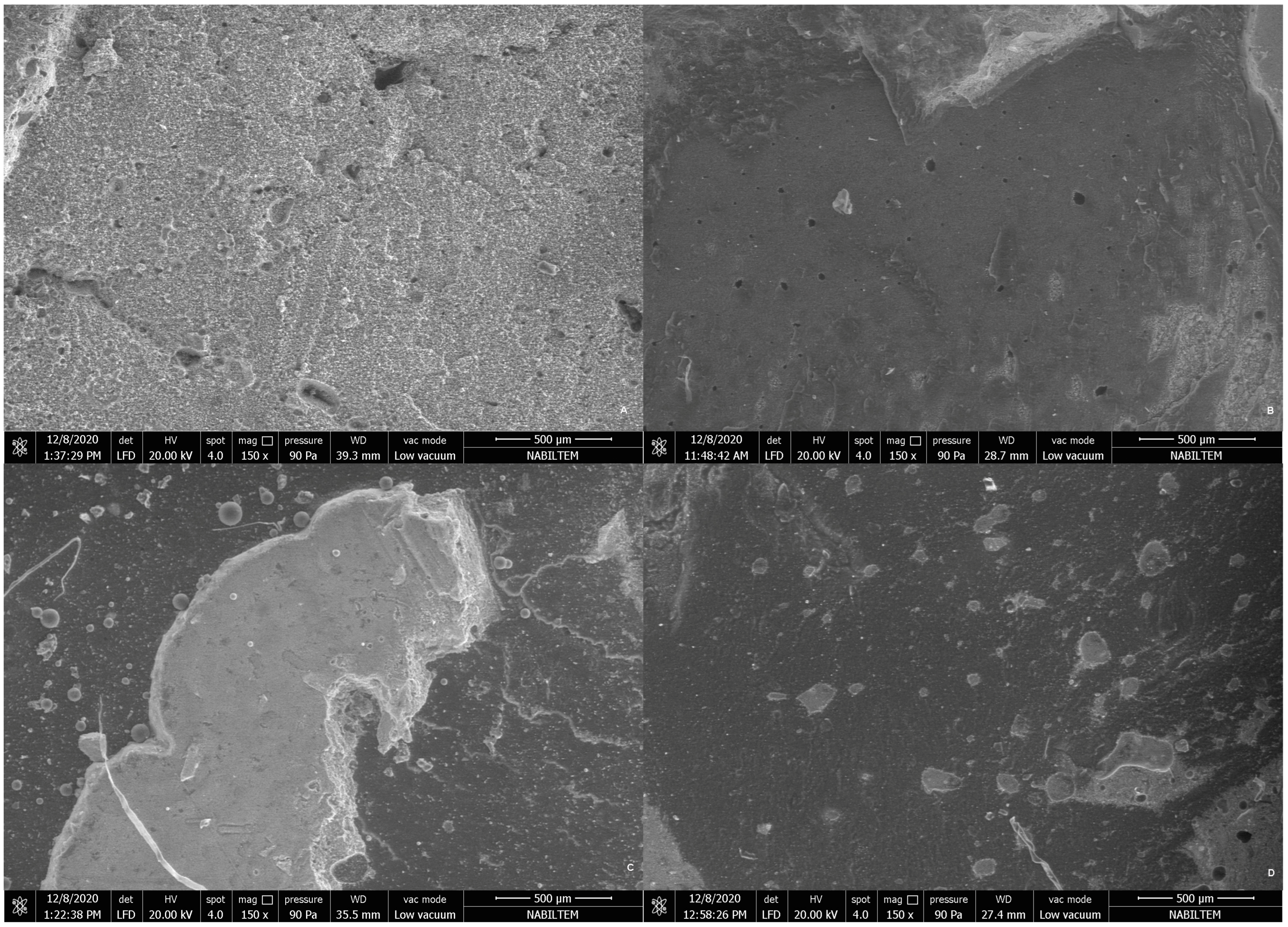
2.4. Stereomicroscopy and Scanning Electron Microscopy Analysis of Debonded Surfaces
- Type 1—cohesive failure inside the base material or composite;
- Type 2—adhesive failure at the interface of the base material and the composite;
- Type 3—mixed failure, combined failure (adhesive and cohesive).
2.5. Surface Roughness Test
2.6. Statistical Analysis
3. Results
3.1. Shear Bond Strength and Thermal Cycle Test
3.2. Surface Roughness
3.3. Stereomicroscopy and SEM Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanık, Ö.; Türkün, L.Ş. Recent Approaches in Restorative Glass Ionomer Cements. J. Ege Univ. Sch. Dent. 2016, 37, 54–65. [Google Scholar] [CrossRef]
- Hetrodt, F.; Lausch, J.; Meyer-Lueckel, H.; Conrads, G.; Apel, C. Evaluation of Restorative Materials Containing Preventive Additives in a Secondary Caries Model in Vitro. Caries Res. 2019, 53, 447–456. [Google Scholar] [CrossRef]
- Goodacre, C.J.; Eugene Roberts, W.; Munoz, C.A. Noncarious Cervical Lesions: Morphology and Progression, Prevalence, Etiology, Pathophysiology Clinical Guidelines for Restoration. J. Prosthodont. 2023, 32, e1–e18. [Google Scholar] [CrossRef]
- Sunnegårdh-Grönberg, K.; van Dijken, J.W.V.; Funegård, U.; Lindberg, A.; Nilsson, M. Selection of Dental Materials and Longevity of Replaced Restorations in Public Dental Health Clinics in Northern Sweden. J. Dent. 2009, 37, 673–678. [Google Scholar] [CrossRef]
- Reichl, F.X.; Seiss, M.; Kleinsasser, N.; Kehe, K.; Kunzelmann, K.H.; Thomas, P.; Spahl, W.; Hickel, R. Distribution and Excretion of BisGMA in Guinea Pigs. J. Dent. Res. 2008, 87, 378–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef] [PubMed]
- Gorseta, K.; Borzabadi-Farahani, A.; Moshaverinia, A.; Glavina, D.; Lynch, E. Effect of Different Thermo-Light Polymerization on Flexural Strength of Two Glass Ionomer Cements and a Glass Carbomer Cement. J. Prosthet. Dent. 2017, 118, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Gavic, L.; Gorseta, K.; Borzabadi-Farahani, A.; Tadin, A.; Glavina, D.; van Duinen, R.; Lynch, E. Influence of Thermo-Light Curing with Dental Light-Curing Units on the Microhardness of Glass-Ionomer Cements. Int. J. Periodontics Restor. Dent. 2016, 36, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F.G.; Sampaio, C.S.; Fucio, S.B.P.; Carlo, H.L.; Correr-Sobrinho, L.; Puppin-Rontani, R.M. Effect of Chemical and Mechanical Degradation on Surface Roughness of Three Glass Ionomers and a Nanofilled Resin Composite. Oper. Dent. 2012, 37, 509–517. [Google Scholar] [CrossRef]
- Menne-Happ, U.; Ilie, N. Effect of Gloss and Heat on the Mechanical Behaviour of a Glass Carbomer Cement. J. Dent. 2013, 41, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, M.; Borzabadi-Farahani, A.; Sameni, A.; Moshaverinia, A.; Ansari, S. Effects of Incorporation of Nano-Fluorapatite Particles on Microhardness, Fluoride Releasing Properties, and Biocompatibility of a Conventional Glass Ionomer Cement (GIC). Dent. Mater. J. 2016, 35, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, E.; Bossù, M.; Giovannetti, A.; La Torre, G.; Guerra, F.; Polimeni, A. Surface Roughness of Glass Ionomer Cements Indicated for Uncooperative Patients According to Surface Protection Treatment. Ann. Stomatol. 2013, 4, 250–258. [Google Scholar]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Bona, Á.; Pinzetta, C.; Rosa, V. Effect of Acid Etching of Glass Ionomer Cement Surface on the Microleakage of Sandwich Restorations. J. Appl. Oral Sci. 2007, 15, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bilgrami, A.; Maqsood, A.; Alam, M.K.; Ahmed, N.; Mustafa, M.; Alqahtani, A.R.; Alshehri, A.; Alqahtani, A.A.; Alghannam, S. Evaluation of Shear Bond Strength between Resin Composites and Conventional Glass Ionomer Cement in Class II Restorative Technique—An In Vitro Study. Materials 2022, 15, 4293. [Google Scholar] [CrossRef] [PubMed]
- Kakaboura, A.; Fragouli, M.; Rahiotis, C.; Silikas, N. Evaluation of Surface Characteristics of Dental Composites Using Profilometry, Scanning Electron, Atomic Force Microscopy and Gloss-Meter. J. Mater. Sci. Mater. Med. 2007, 18, 155–163. [Google Scholar] [CrossRef]
- Lardani, L.; Derchi, G.; Marchio, V.; Carli, E. One-Year Clinical Performance of ActivaTM Bioactive-Restorative Composite in Primary Molars. Children 2022, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Beresescu, L.; Kovacs, M.; Vlasa, A.; Stoica, A.M.; Benedek, C.; Pop, M.; Bungardean, D.; Eșian, D. Retention Ability of a Glass Carbomer Pit and Fissure Sealant. Int. J. Environ. Res. Public Health 2022, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Vennat, E.; Le Goff, S.; Ruscassier, N.; Attal, J.P.; Dursun, E. Shear Bond Strength and Interface Analysis between a Resin Composite and a Recent High-Viscous Glass Ionomer Cement Bonded with Various Adhesive Systems. Clin. Oral Investig. 2019, 23, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Schwengberg, S.; Bohlen, H.; Kleinsasser, N.; Kehe, K.; Seiss, M.; Walther, U.I.; Hickel, R.; Reichl, F.X. In Vitro Embryotoxicity Assessment with Dental Restorative Materials. J. Dent. 2005, 33, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W.; Czarnecka, B. The Biocompatibility of Resin-Modified Glass-Ionomer Cements for Dentistry. Dent. Mater. 2008, 24, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Chrószcz-Porębska, M.W.; Barszczewska-Rybarek, I.M.; Kazek-Kęsik, A.; Ślęzak-Prochazka, I. Cytotoxicity and Microbiological Properties of Copolymers Comprising Quaternary Ammonium Urethane-Dimethacrylates with Bisphenol A Glycerolate Dimethacrylate and Triethylene Glycol Dimethacrylate. Materials 2023, 16, 3855. [Google Scholar] [CrossRef] [PubMed]
- Ozer, S.; Sen Tunc, E.; Gonulol, N. Bond Strengths of Silorane- and Methacrylate-Based Composites to Various Underlying Materials. Biomed. Res. Int. 2014, 2014, 782090. [Google Scholar] [CrossRef] [PubMed]
- Koc Vural, U.; Gokalp, S.; Kiremitci, A. Effect of Cavity Lining on the Restoration of Root Surface Carious Lesions: A Split-Mouth, 5-Year Randomized Controlled Clinical Trial. Clin. Oral Investig. 2020, 24, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Çilingir, A.; Dulger, K. Microleakage Evaluation of Expired and Non-Expired Resin Composites and Bonding Agents: In Vitro Study. J. Adv. Oral Res. 2022, 13, 113–119. [Google Scholar] [CrossRef]
- Nag, G.; Nha, K.; Rd, A. Microleakage Testing. Ann. Dent. Univ. Malaya 1997, 4, 31–37. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, T.H.; Kao, C.T.; Ding, S.J. Effect of Conditioners on Bond Durability of Resin Composite to Nd:YAP Laser-Irradiated Dentin. Dent. Mater. J. 2006, 25, 463–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.S.; Ontiveros, J.C.; English, J.D.; Wirthlin, J.O.; Cozad, B.E.; Harrington, D.A.; Kasper, F.K. Effect of Material and Pad Abrasion on Shear Bond Strength of 3D-Printed Orthodontic Brackets. Orthod. Craniofac. Res. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sakai, V.T.; Kawai, E.S.; Buzalaf, M.A.R.; Atta, M.T. Effect of Adhesive Systems Associated with Resin-Modified Glass Ionomer Cements. J. Oral Rehabil. 2006, 33, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Mitsuo, H.; Oshima, S.; Gonçalves Mota, E.; Lima Grossi, M. Influence of Chisel Width on Shear Bond Strength of Composite to Enamel Influência Da Largura Do Cinzel Sobre a Resistência Ao Cisalhamento Da União Esmalte/Resina Composta. Rev. Odonto Ciência 2009, 24, 19–21. [Google Scholar]
- Bani, M.; Öztaş, N. Cam Iyonomer Içerikli Farklı Restoratif Materyallerin Yüzey Pürüzlülüklerinin Değerlendirilmesi. Acta Odontol. Turc. 2013, 30, 13–17. [Google Scholar]
- Kam Hepdeniz, Ö.; Seçkin Kelten, Ö.; Gürdal, O. Cam Iyonomer Içerikli Dört Farklı Restoratif Materyalin Yüzey Pürüzlülüklerinin Değerlendirilmesi. SDÜ Sağlık Bilim. Derg. 2019, 10, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Roeder, L.B.; Lei, L.; Powers, J.M. Effect of Surface Roughness on Stain Resistance of Dental Resin Composites. J. Esthet. Restor. Dent. 2005, 17, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.B.; Lopes, L.I.G.; Bergstrom, T.G.; da Silva, A.S.S.; Lopes, R.T.; de Almeida Neves, A. Porosity and Pore Size Distribution in High-Viscosity and Conventional Glass Ionomer Cements: A Micro-Computed Tomography Study. Restor. Dent. Endod. 2021, 46, e57. [Google Scholar] [CrossRef]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical Properties and Microstructures of Glass-Ionomer Cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Botta, A.C.; Duarte, S.; Paulin Filho, P.I.; Gheno, S.M.; Powers, J.M. Surface Roughness of Enamel and Four Resin Composites. Am. J. Dent. 2009, 22, 252–254. [Google Scholar] [PubMed]
- Bayrak, G.D.; Sandalli, N.; Selvi-Kuvvetli, S.; Topcuoglu, N.; Kulekci, G. Effect of Two Different Polishing Systems on Fluoride Release, Surface Roughness and Bacterial Adhesion of Newly Developed Restorative Materials. J. Esthet. Restor. Dent. 2017, 29, 424–434. [Google Scholar] [CrossRef]
- Manihani, A.K.D.S.; Mulay, S.; Beri, L.; Shetty, R.; Gulati, S.; Dalsania, R. Effect of Total-Etch and Self-Etch Adhesives on the Bond Strength of Composite to Glass-Ionomer Cement/Resin-Modified Glass-Ionomer Cement in the Sandwich Technique—A Systematic Review. Dent. Res. J. 2021, 18, 72. [Google Scholar]
- Kiran, K.M.; Gopal, T.; Nujella, S.B.P.; Choudary, M.T.; Reddy, S.P. Comparison of Shear Bond Strength of Aesthetic Restorative Materials. Contemp. Clin. Dent. 2012, 3, 22–26. [Google Scholar] [CrossRef]
- Ertaş, E.; Ulusoy, N. Üç Farklı Hibrit Kompozit Rezinin Iki Hibrit Iyonomer ve Bir Konvansiyonel Cam-Iyonomer Simana Bağlanma Güçlerinin Değerlendirilmesi. Ankara Üniversitesi Diş Hekim. Fakültesi Derg. 2002, 29, 105–114. [Google Scholar]
- Sibal, G.K. Comparative Evaluation of Shear Bond Strength of Various Glass Ionomer Cements to Dentin of Primary Teeth: An in Vitro Study. Int. J. Clin. Pediatr. Dent. 2016, 9, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The Effect of Thermal Cycling on the Bovine Dentine Shear Bond Strength of Current Adhesive Systems. J. Oral Rehabil. 2004, 31, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Leloup, G.; D’Hoore, W.; Bouter, D.; Degrange, M.; Vreven, J. Meta-Analytical Review of Factors Involved in Dentin Adherence. J. Dent. Res. 2001, 80, 1605–1614. [Google Scholar] [CrossRef]
- Panahandeh, N.; Torabzadeh, H.; Ghassemi, A.; Mahdian, M.; Akbarzadeh Bagheban, A.; Moayyedi, S. Effect of Bonding Application Time on Bond Strength of Composite Resin to Glass Ionomer Cement. J. Dent. 2015, 12, 859–867. [Google Scholar]




| Materials | Manufacturer | Composition | Lot |
|---|---|---|---|
| Conventional glass–ionomer cement, Ketac Molar Easymix | 3M ESPE, St Paul, MN, USA | Powder: aluminum–calcium–lanthanum fluorosilicate glass, acrylic acid, maleic acid; Liquid: poly(alkenoic acid) tartaric acid, water. | 6702046 |
| Resin-modified glass–ionomer cement, Fuji II LC | GC, Tokyo, Japan | Powder: fluoroaluminosilicate glass, HEMA, urethane dimethacrylate, water, photoinitiator (camphoroquinone); Liquid: poly(acrylic acid). | 191209A |
| Improved resin-modified glass–ionomer cement, BioACTIVE Base/Liner | PULPDENT, Corporation, Watertown, MA, USA | Urethane dimethacrylate, bis 2-methacryloyloxy ethyl phosphate, barium glass, poly(acrylic acid) maleic acid, copolymer, sodium fluoride, coloring agent, photoinitiator. | 191009 |
| Glass carbomer cement, GCP Glass Fill | GCP Dental, Leiden, Holland | Powder: fluoroaluminosilicate glass, apatite; Liquid: poly(acids). | 71808616 |
| Universal composite, Essentia | GC, Tokyo, Japan | UDMA, Bis-MEPP, Bis-EMA, Bis-GMA, TEGDMA, barium glass, silicon dioxide, coloring agent, photoinitiator. | 191003A |
| Universal adhesive resin, G-Premio Bond | GC, Tokyo, Japan | MDP, 4-MET, MEPS, dimethacrylate monomer, acetone, water, silicon dioxide, photoinitiator. | 1910244 |
| Sealant, GCP gloss | GCP Dental, Leiden, Holland | Modified polysiloxane. | 1607101 |
| (a) | |||
| Before TC Mean ± SD | After TC Mean ± SD | p | |
| Fuji II LC | 35.68 ± 6.74 | 21.62 ± 4.99 | 0.000 * |
| BioActive | 33.17 ± 5.02 | 30.93 ± 7.26 | 0.484 |
| Ketac Molar | 19.84 ± 7.66 | 14.73 ± 4.81 | 0.133 |
| Glass Carbomer | 11.71 ± 2.96 | 8.87 ± 2.87 | 0.072 |
| p | 0.000 * | 0.000 * | |
| (b) | |||
| 1 With TC | 2 Without TC | ||
| Fuji II LC | BioActive | 0.829 | 0.064 |
| Ketac Molar | 0.000 * | 0.080 | |
| Glass Carbomer | 0.000 * | 0.000 * | |
| BioActive | Fuji II LC | 0.829 | 0.064 |
| Ketac Molar | 0.001 * | 0.001 * | |
| Glass Carbomer | 0.000 * | 0.000 * | |
| Ketac Molar | Fuji II LC | 0.000 * | 0.080 |
| BioActive | 0.001 * | 0.001 * | |
| Glass Carbomer | 0.046 | 0.073 | |
| Glass Carbomer | Fuji II LC | 0.000 * | 0.000 * |
| BioActive | 0.000 * | 0.000 * | |
| Ketac Molar | 0.046 * | 0.073 | |
| (a) | ||
| Roughness Mean ± SD | ||
| Fuji II LC | 0.81 ± 0.26 | |
| BioActive | 1.06 ± 0.38 | |
| Ketac Molar | 0.79 ± 0.21 | |
| Glass Carbomer | 2.03 ± 0.41 | |
| p | 0.000 * | |
| (b) | ||
| Roughness | ||
| Fuji II LC | BioActive | 0.061 |
| Ketac Molar | 1.000 | |
| Glass Carbomer | 0.000 * | |
| BioActive | Fuji II LC | 0.061 |
| Ketac Molar | 0.023 * | |
| Glass Carbomer | 0.000 * | |
| Ketac Molar | Fuji II LC | 1.000 |
| BioActive | 0.023 * | |
| Glass Carbomer | 0.000 * | |
| Glass Carbomer | Fuji II LC | 0.000 * |
| BioActive | 0.000 * | |
| Ketac Molar | 0.000 * | |
| Group | Fracture Type | Test | Test Statistics | |
|---|---|---|---|---|
| With TC | Without TC | |||
| Ketac Molar | Adhesive | 6 (%75) a,b | 3 (%37.5) a,b | χ2 = 1.000; p = 0.317; V = 0.333 |
| Mixed | 1 (%12.5) a,b | 2 (%25) a | χ2 = 0.333; p = 0.564; V = 0.191 | |
| Cohesive | 1 (%12.5) a | 3 (%37.5) a | χ2 = 1.000; p = 0.317; V = 0.333 | |
| Fuji II LC | Adhesive | 4 (%50) a,b | 2 (%25) a,b | χ2 = 0.667; p = 0.414; V = 0.272 |
| Mixed | 2 (%25) a,b | 2 (%25) a | χ2 = 0.001; p = 0.999; V = 0.001 | |
| Cohesive | 2 (%25) a | 4 (%50) a | χ2 = 0.667; p = 0.414; V = 0.272 | |
| BioActive | Adhesive | 1 (%12.5) a | 0 (%0) a | χ2 = 0.333; p = 0.564; V = 0.211 |
| Mixed | 6 (%75.0) a | 4 (%50) a | χ2 = 0.400; p = 0.527; V = 0.192 | |
| Cohesive | 1 (%12.5) a | 4 (%50) a | χ2 = 1.800; p = 0.180; V = 0.407 | |
| Glass Carbomer | Adhesive | 7 (%87.5) b | 6 (%75) b | χ2 = 0.077; p = 0.782; V = 0.092 |
| Mixed | 0 (%0) a | 1 (%12.5) a | χ2 = 0.333; p = 0.564; V = 0.192 | |
| Cohesive | 1 (%12.5) a | 1 (%12.5) a | χ2 = 0.001; p = 0.999; V = 0.001 | |
| Test statistics | χ2 = 15.906; p = 0.014; V = 0.476 | χ2 = 14.409; p = 0.025; V = 0.409 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Işık, H.Y.; Çilingir, A. Adhesion and Surface Roughness of Apatite-Containing Carbomer and Improved Ionically Bioactive Resin Compared to Glass Ionomers. J. Funct. Biomater. 2023, 14, 367. https://doi.org/10.3390/jfb14070367
Işık HY, Çilingir A. Adhesion and Surface Roughness of Apatite-Containing Carbomer and Improved Ionically Bioactive Resin Compared to Glass Ionomers. Journal of Functional Biomaterials. 2023; 14(7):367. https://doi.org/10.3390/jfb14070367
Chicago/Turabian StyleIşık, Handan Yıldırım, and Aylin Çilingir. 2023. "Adhesion and Surface Roughness of Apatite-Containing Carbomer and Improved Ionically Bioactive Resin Compared to Glass Ionomers" Journal of Functional Biomaterials 14, no. 7: 367. https://doi.org/10.3390/jfb14070367
APA StyleIşık, H. Y., & Çilingir, A. (2023). Adhesion and Surface Roughness of Apatite-Containing Carbomer and Improved Ionically Bioactive Resin Compared to Glass Ionomers. Journal of Functional Biomaterials, 14(7), 367. https://doi.org/10.3390/jfb14070367







