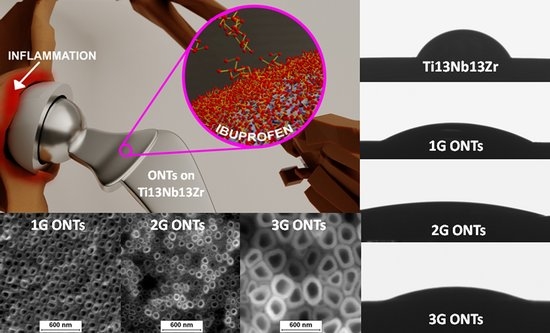
Biological Activity and Thrombogenic Properties of Oxide Nanotubes on the Ti-13Nb-13Zr Biomedical Alloy
Abstract
:1. Introduction
2. Materials and Methods
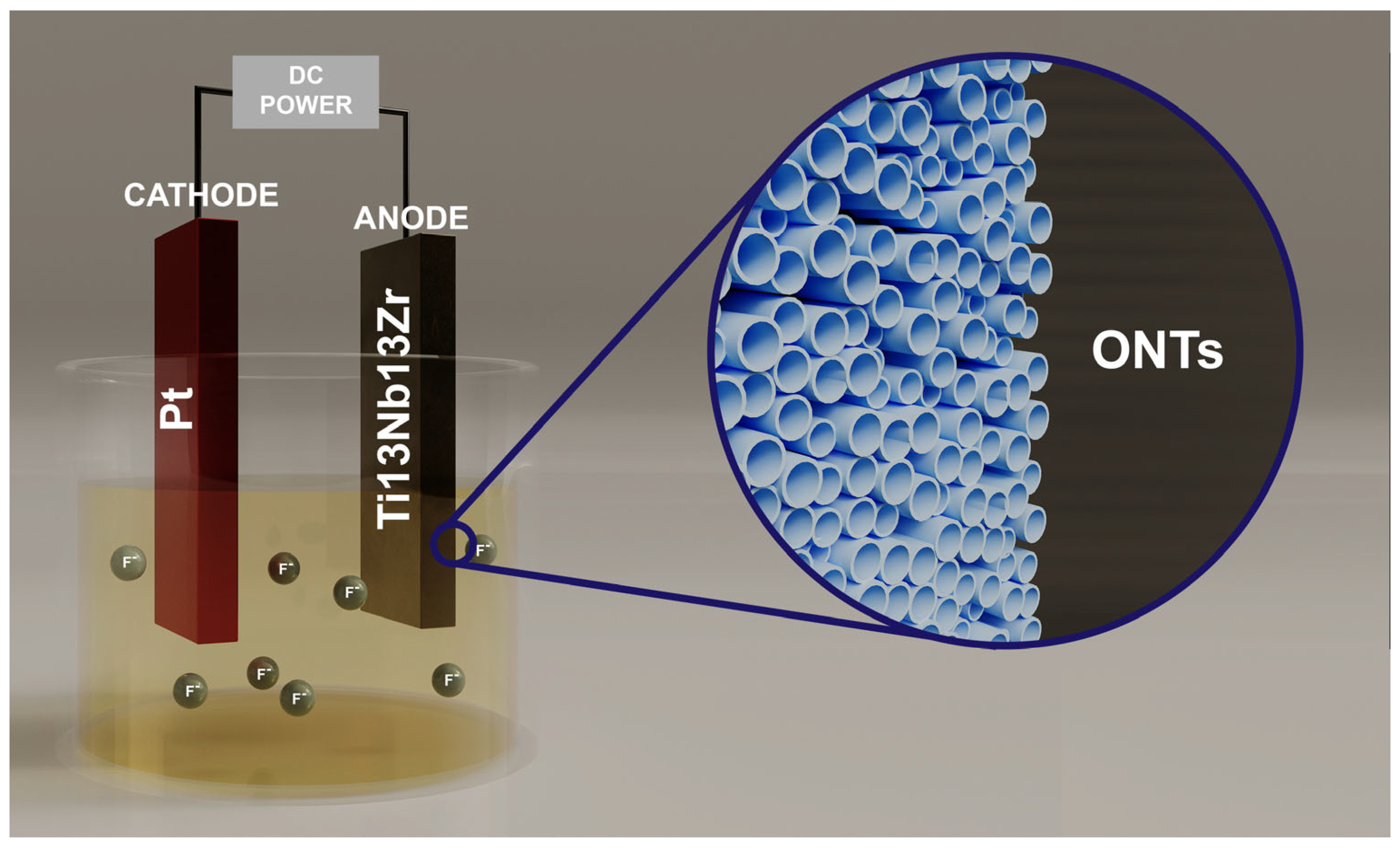
2.1. Preparation of Research Material
2.2. Material Characterization
2.3. Scanning Kelvin Probe Measurements
2.4. Wettability Measurements
2.5. In Vitro Hemocompatibility Test
2.6. Cell Culture and Cytotoxicity Assays
2.7. Qualitative In Vitro Cell Adhesion Assay
2.8. Thrombogenity Test
2.8.1. Blood Donation and Platelet-Reach Plasma Preparation
2.8.2. Qualitative Thrombogenicity Scale
2.8.3. Statistical Analysis Methods
2.9. Drug Delivery System
3. Results and Discussion
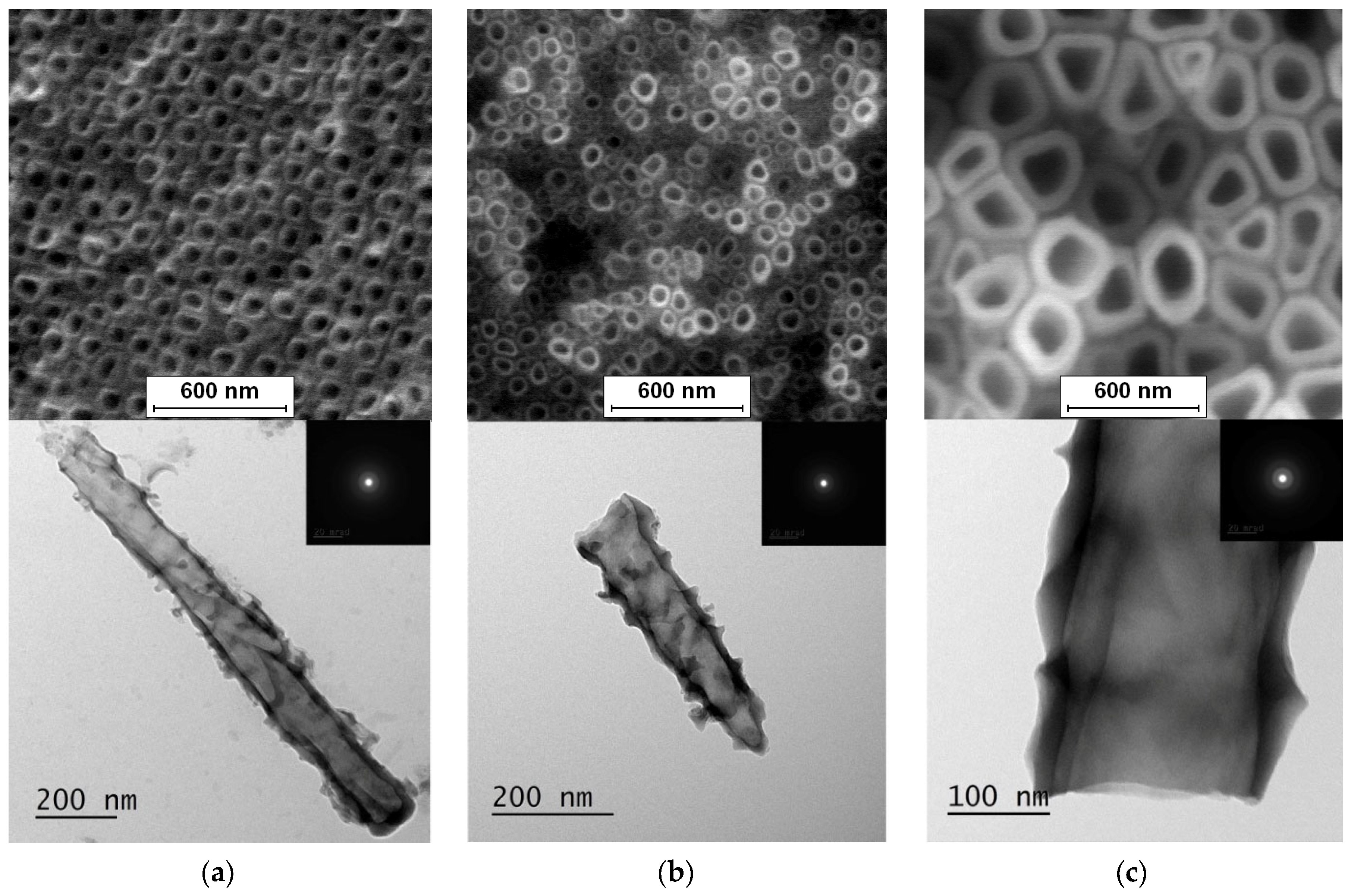
3.1. FE-SEM and TEM Characterization
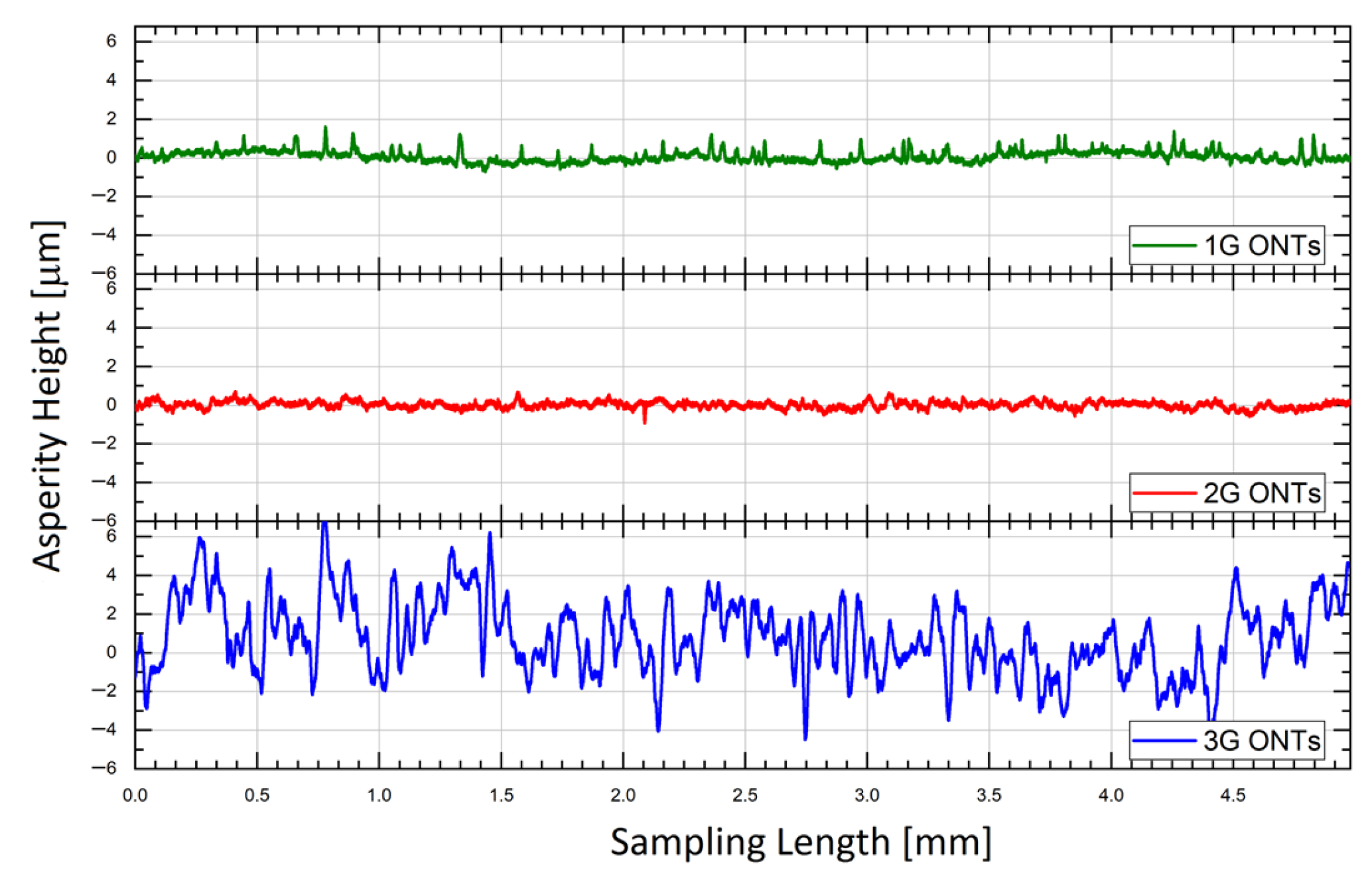
3.2. Roughness Profile Measurements
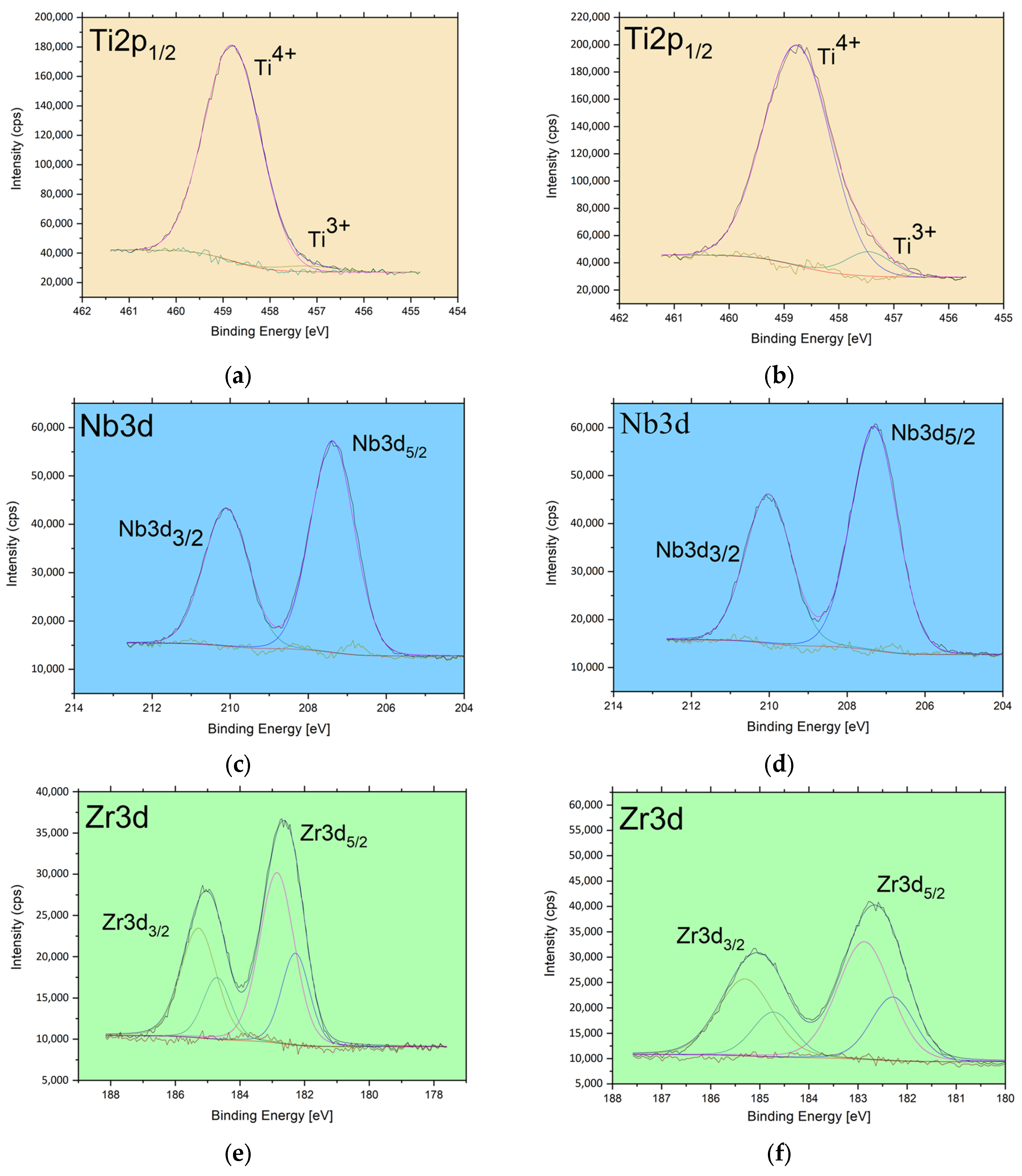
3.3. XPS Study of Chemical States
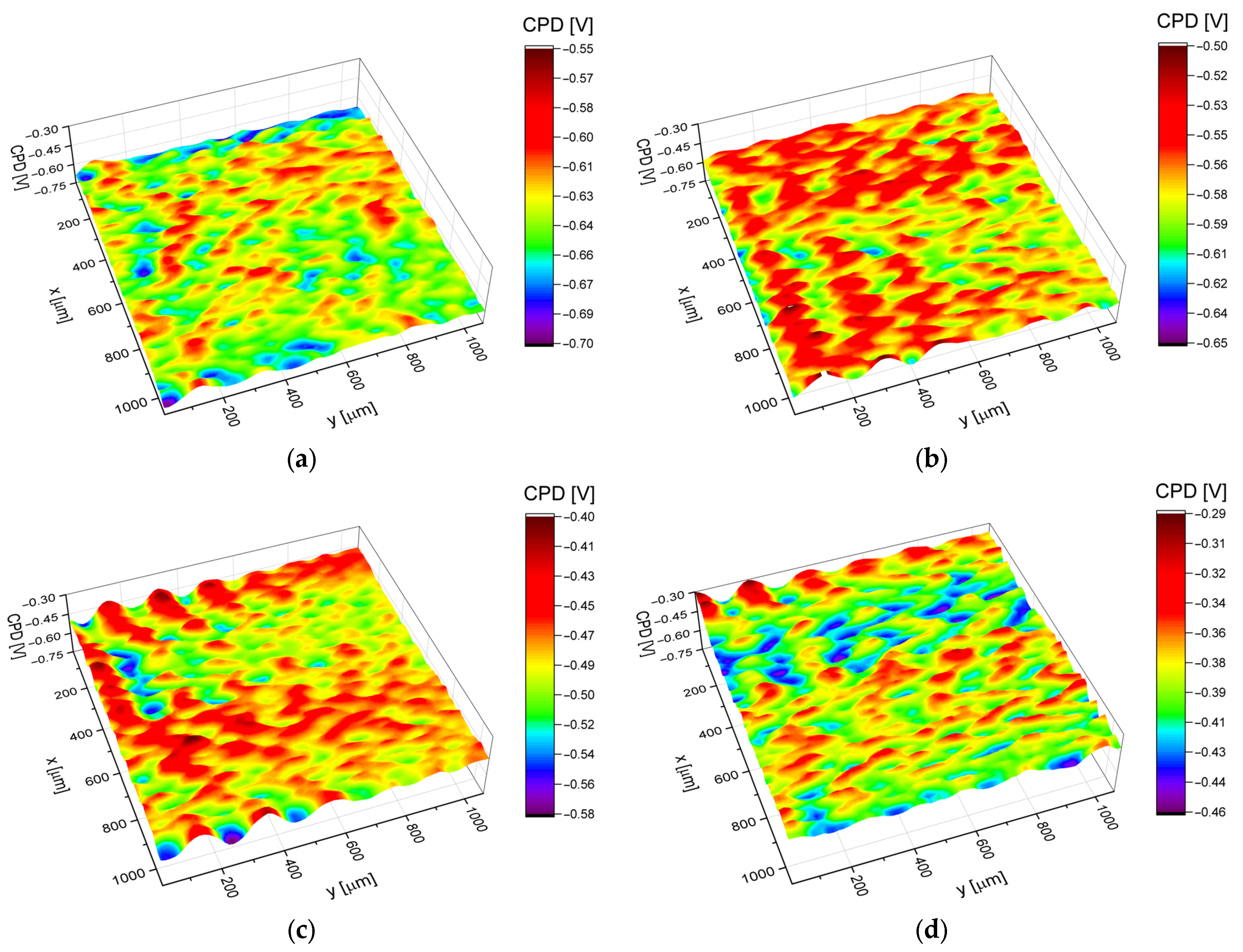
3.4. Electronic Properties
3.5. Surface Wettability
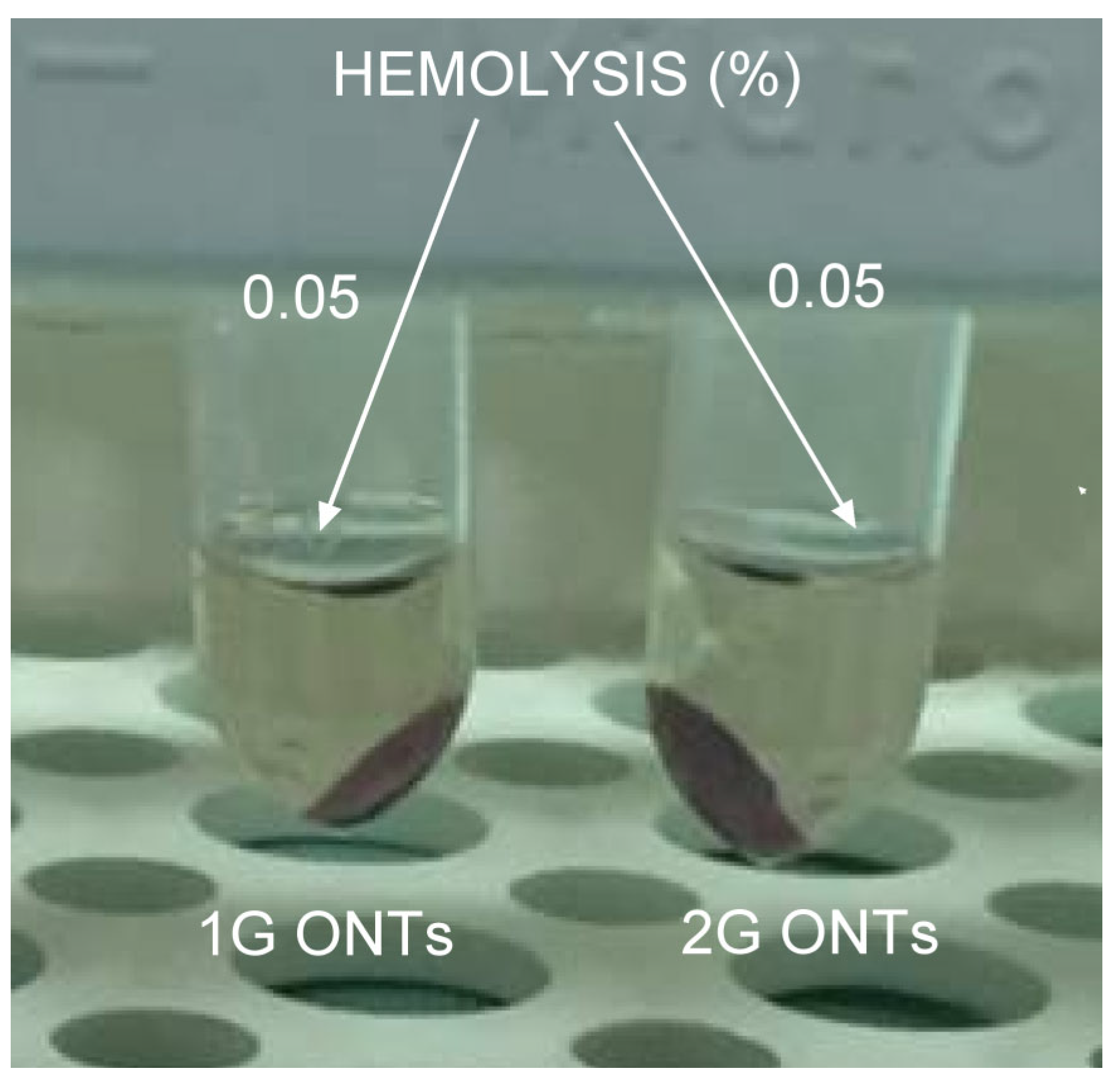
3.6. In Vitro Hemocompatibility Study
3.7. Cytotoxity and Cell Adhesion Assay
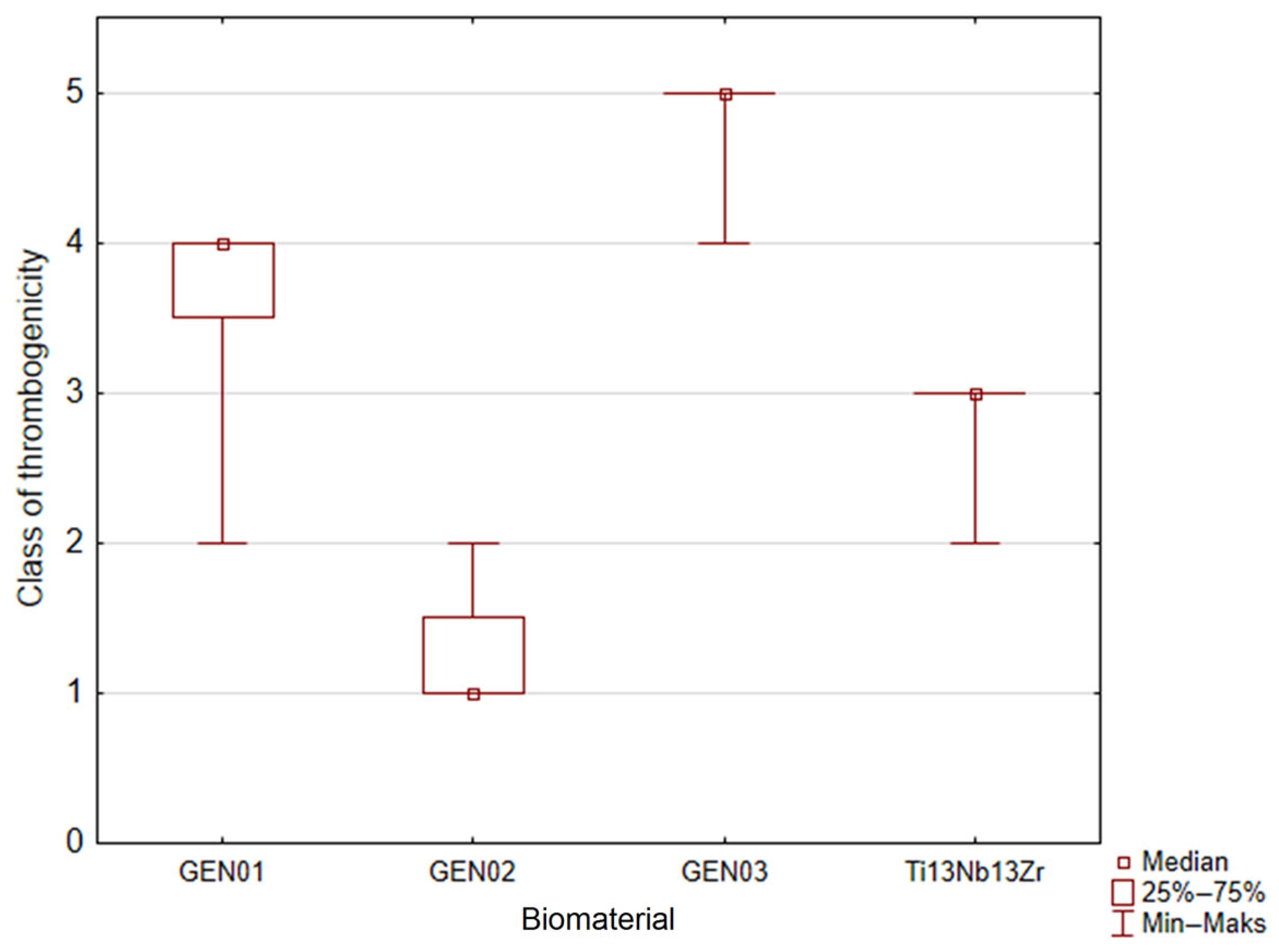
3.8. Thrombogenicity Test
3.9. Drug Release Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef]
- Branemark, P.I. The Osseointegration Book: From Calvarium to Calcaneus, 1st ed.; Quintessence: Berlin, Germany, 2007; ISBN 1850970904/978-1850970903. [Google Scholar]
- Dudek, K.; Dulski, M.; Łosiewicz, B. Functionalization of the NiTi Shape Memory Alloy Surface by HAp/SiO2/Ag Hybrid Coatings Formed on SiO2-TiO2 Glass Interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stróż, A.; Maszybrocka, J.; Goryczka, T.; Dudek, K.; Osak, P.; Łosiewicz, B. Influence of Anodizing Conditions on Biotribological and Micromechanical Properties of Ti–13Zr–13Nb Alloy. Materials 2023, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Łosiewicz, B.; Stróż, A.; Osak, P.; Maszybrocka, J.; Gerle, A.; Dudek, K.; Balin, K.; Łukowiec, D.; Gawlikowski, M.; Bogunia, S. Production, Characterization and Application of Oxide Nanotubes on Ti–6Al–7Nb Alloy as a Potential Drug Carrier. Materials 2021, 14, 6142. [Google Scholar] [CrossRef] [PubMed]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical properties, corrosion resistance and bioactivity of oxide layers formed by isothermal oxidation of Ti-6Al-7Nb alloy. Coatings 2021, 11, 505. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Jakubowicz, J. (Ed.) Ti-Based Biomaterials. In Ti-Based Biomaterials; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Stróż, A.; Goryczka, T.; Łosiewicz, B. Electrochemical formation of self-organized nanotubular oxide layers on niobium (Review). Curr. Nanosci. 2019, 15, 42–48. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Łosiewicz, B. Electrochemical synthesis of oxide nanotubes on biomedical Ti13Nb13Zr alloy with potential use as bone implant. AIP Conf. Proc. 2019, 2083, 030004. [Google Scholar] [CrossRef]
- Szklarska, M.; Dercz, G.; Simka, W.; Łosiewicz, B. Ac impedance study on the interfacial properties of passivated Ti13Zr13Nb alloy in physiological saline solution. Surf. Interface Anal. 2014, 46, 698–701. [Google Scholar] [CrossRef]
- Stróż, A.; Luxbacher, T.; Dudek, K.; Chmiela, B.; Osak, P.; Łosiewicz, B. In Vitro Bioelectrochemical Properties of Second-Generation Oxide Nanotubes on Ti–13Zr–13Nb Biomedical Alloy. Materials 2023, 16, 1408. [Google Scholar] [CrossRef]
- Stróż, A.; Łosiewicz, B.; Zubko, M.; Chmiela, B.; Balin, K.; Dercz, G.; Gawlikowski, M.; Goryczka, T. Production, structure and biocompatible properties of oxide nanotubes on Ti13Nb13Zr alloy for medical applications. Mater. Charact. 2017, 132, 363–372. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Stróż, A.; Kubisztal, J.; Osak, P.; Zubko, M. EIS and LEIS Study on In Vitro Corrosion Resistance of Anodic Oxide Nanotubes on Ti–13Zr–13Nb Alloy in Saline Solution. Coatings 2023, 13, 875. [Google Scholar] [CrossRef]
- Sowa, M.; Piotrowska, M.; Widziołek, M.; Dercz, G.; Tylko, G.; Gorewoda, T.; Osyczka, A.M.; Simka, W. Bioactivity of coatings formed on Ti-13Nb-13Zr alloy using plasma electrolytic oxidation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of Titanium Implant Surface Topology on Bone Cell Attachment and Proliferation in vitro. Med. Devices 2022, 15, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Seramak, T.; Zieliński, A.; Serbiński, W.; Zasińska, K. Powder metallurgy of the porous Ti-13Nb-13Zr alloy of different powder grain size. Mater. Manuf. 2019, 34, 915–920. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Shivaram, A.; Bose, S.; Bandyopadhyay, A. Mechanical degradation of TiO2 nanotubes with and without nanoparticulate silver coating. J. Mech. Behav. Biomed. Mater. 2016, 59, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Schenk, R.K.; Buser, D. Osseointegration: A reality. Periodontology 2000 1998, 17, 22–35. [Google Scholar] [CrossRef]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone–implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control With Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1401–1412. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef] [Green Version]
- Rani, S.; Roy, S.C.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Kim, S.; Yoriya, S.; LaTempa, T.J.; Grimes, C.A. Synthesis and applications of electrochemically self-assembled titania nanotube arrays. Phys. Chem. Chem. Phys. 2010, 12, 2780–2800. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Boyd, B.J.; Birbilis, N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J. Control. Release 2014, 179, 63–75. [Google Scholar] [CrossRef]
- Al-Dubai, H.; Pittner, G.; Pittner, F.; Gabor, F. Biocompatible medical implant materials with binding sites for a biodegradable drug-delivery system. Nanotechnol. Sci. Appl. 2011, 4, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Tang, Y.; Wang, K.; Zhou, X.; Xiang, L. Nanostructured Titanium Implant Surface Facilitating Osseointegration from Protein Adsorption to Osteogenesis: The Example of TiO2 NTAs. Int. J. Nanomed. 2022, 17, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Smołka, A.; Rodak, K.; Dercz, G.; Dudek, K.; Łosiewicz, B. Electrochemical Formation of Self-Organized Nanotubular Oxide Layers on Ti13Zr13Nb Alloy for Biomedical Applications. Acta Phys. Pol. A 2014, 125, 932–935. [Google Scholar] [CrossRef]
- Smołka, A.; Dercz, G.; Rodak, K.; Łosiewicz, B. Evaluation of Corrosion Resistance of Nanotubular Oxide Layers on the Ti13Zr13Nb Alloy in Physiological Saline Solution. Arch. Metall. Mater. 2015, 60, 2681–2686. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Stróż, D.; Łosiewicz, B. Electrochemical Formation of Second Generation TiO2 Nanotubes on Ti13Nb13Zr Alloy for Biomedical Applications. Acta Phys. Pol. A 2016, 130, 1079–1080. [Google Scholar] [CrossRef]
- Aïnouche, L.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Bradai, D. Interfacial Barrier Layer Properties of Three Generations of TiO2 Nanotube Arrays. Electrochim. Acta 2014, 133, 597–609. [Google Scholar] [CrossRef]
- Panaitescu, E.; Richter, C.; Menon, L. A Study of Titania Nanotube Synthesis in Chloride-Ion-Containing Media. J. Electrochem. Soc. 2008, 155, E7. [Google Scholar] [CrossRef] [Green Version]
- ASTM F756-17; Standard Practice For Assessment Of Hemolytic Properties Of Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Gawlikowski, M.; Major, R.; Mika, B.; Komorowski, D.; Janiczak, K.; Tkacz, E.; Tamulewicz, A.; Piaseczna, N. Semi-Quantitative Method of Assessing the Thrombogenicity of Biomaterials Intended for Long-Term Blood Contact. Materials 2023, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Handzlik, P.; Gutkowski, K. Synthesis of oxide nanotubes on Ti13Nb13Zr alloy by the electrochemical method. J. Porous Mater. 2019, 26, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Łosiewicz, B.; Skwarek, S.; Stróż, A.; Osak, P.; Dudek, K.; Kubisztal, J.; Maszybrocka, J. Production and Characterization of the Third-Generation Oxide Nanotubes on Ti-13Zr-13Nb Alloy. Materials 2022, 15, 2321. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of Autoclaving Time on Corrosion Resistance of Sandblasted Ti G4 in Artificial Saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef] [PubMed]
- Ossowska, A.; Sobieszczyk, S.; Supernak, M.; Zielinski, A. Morphology and properties of nanotubular oxide layer on the “Ti–13Zr–13Nb” alloy. Surf. Coat. Technol. 2014, 258, 1239–1248. [Google Scholar] [CrossRef]
- Suzuki, K.; Aoki, K.; Ohya, K. Effects of surface roughness of titanium implants on bone remodeling activity of femur in rabbits. Bone 1997, 21, 507–514. [Google Scholar] [CrossRef]
- Schmidt, C.; Kaspar, D.; Sarkar, M.R.; Claes, L.E.; Ignatius, A.A. A scanning electron microscopy study of human osteoblast morphology on five orthopedic metals. J. Biomed. Mater. 2002, 63, 252–261. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Korolkov, I.V.; Koretskaya, T.P.; Chepeleva, E.V.; Kuz’min, N.B.; Fedorenko, A.D.; Pischur, D.P.; Guselnikova, T.Y.; Maksimovskii, E.A.; et al. Application of Biocompatible Noble Metal Film Materials to Medical Implants: TiNi Surface Modification. Coatings 2023, 13, 222. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database; NIST Standard Reference Database 20, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000.
- Gondal, M.A.; Fasasi, T.A.; Baig, U.; Mekki, A. Effects of Oxidizing Media on the Composition, Morphology and Optical Properties of Colloidal Zirconium Oxide Nanoparticles Synthesized via Pulsed Laser Ablation in Liquid Technique. J. Nanosci. Nanotechnol. 2018, 18, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Kubisztal, J.; Łosiewicz, B.; Dybał, P.; Kozik, V.; Bąk, A. Water-Induced Corrosion Damage of Carbon Steel in Sulfolane. Energies 2020, 13, 4580. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Bogunia, S.; Ratajczak, P.; Aniołek, K. Effect of Temperature on Electrochemically Assisted Deposition and Bioactivity of CaP Coatings on CpTi Grade 4. Materials 2021, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
- Kubisztal, J.; Łosiewicz, B.; Dybal, P.; Kozik, V.; Bak, A. Temperature-Related Corrosion Resistance of AISI 1010 Carbon Steel in Sulfolane. Materials 2020, 13, 2563. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nanotube formation on TiZr as scaffolds for cell growth. Bioelectrochemistry 2014, 98, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, S.; Ungureanu, C.; Kirchgeorg, R.; Schmuki, P.; Demetrescu, I. Various sized nanotubes on TiZr for antibacterial surfaces. Appl. Surf. Sci. 2013, 270, 190–196. [Google Scholar] [CrossRef]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglič, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B Biointerfaces 2015, 129, 47–53. [Google Scholar] [CrossRef]
- Shin, D.H.; Shokuhfar, T.; Choi, C.K.; Lee, S.H.; Friedrich, C. Wettability changes of TiO2 nanotube surfaces. Nanotechnology 2011, 22, 315704. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; Heyde, H.; van der Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Citeau, A.; Guicheux, J.; Vinatier, C.; Layrolle, P.; Nguyen, T.P.; Pilet, P.; Daculsi, G. In vitro biological effects of titanium rough surface obtained by calcium phosphate grid blasting. Biomaterials 2005, 26, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef] [PubMed]
- Aninwene, G.E.I.; Yao, C.; Webster, T.J. Enhanced osteoblast adhesion to drug-coated anodized nanotubular titanium surfaces. Int. J. Nanomed. 2008, 3, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Daraio, C.; Chen, L.H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res. A 2006, 78A, 97–103. [Google Scholar] [CrossRef]
- Bauer, S.; Pittrof, A.; Tsuchiya, H.; Schmuki, P. Size-effects in TiO2 nanotubes: Diameter dependent anatase/rutile stabilization. Electrochem. Commun. 2011, 13, 538–541. [Google Scholar] [CrossRef]
- Shankland, N.; Wilson, C.C.; Florence, A.J.; Cox, P.J. Refinement of Ibuprofen at 100 K by Single-Crystal Pulsed Neutron Diffraction Conmaent. Acta Cryst. 1997, C53, 951–954. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An Overview of Clinical Pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–161. [Google Scholar] [CrossRef]
- Al-Janabi, A.A. In vitro antibacterial activity of Ibuprofen and acetaminophen. J. Glob. Infect. Dis. 2010, 2, 105–108. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- Ren, Z.; Romar, H.; Varila, T.; Xu, X.; Wang, Z.; Sillanpää, M.; Leiviskä, T. Ibuprofen degradation using a Co-doped carbon matrix derived from peat as a peroxymonosulphate activator. Environ. Res. 2021, 193, 110564. [Google Scholar] [CrossRef]
- Jia, H.; Kerr, L.L. Sustained ibuprofen release using composite poly(lactic-co-glycolic acid)/titanium dioxide nanotubes from Ti implant surface. J. Pharm. Sci. 2013, 102, 2341–2348. [Google Scholar] [CrossRef]
- Jarosz, M.; Pawlik, A.; Szuwarzyński, M.; Jaskuła, M.; Sulka, G.D. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloids Surf. B Biointerfaces 2016, 143, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Oyetade, O.A.; Martincigh, B.S.; Skelton, A.A. The Interplay between Electrostatic and Hydrophobic Interactions in the pH-Dependent Adsorption of Ibuprofen onto Acid-Functionalized Multiwalled Carbon Nanotubes. J. Phys. Chem. C 2018, 122, 22556–22568. [Google Scholar] [CrossRef]
- Kurtoglu, Y.E.; Mishra, M.K.; Kannan, S.; Kannan, R.M. Drug release characteristics of PAMAM dendrimer-drug conjugates with different linkers. Int. J. Pharm. 2010, 384, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Bhaskar, K.; Srinivasa Rao, G.; Dhanaraju, M.D. Ibuprofen-loaded ethylcellulose/polystyrene microspheres: An approach to get prolonged drug release with reduced burst effect and low ethylcellulose content. J. Microencapsul. 2003, 20, 289–302. [Google Scholar] [CrossRef]




| Class | Description | Example |
|---|---|---|
| 0 | contains samples characterized by minimal thrombogenicity: separated platelets of low diverse level, no platelet aggregates | 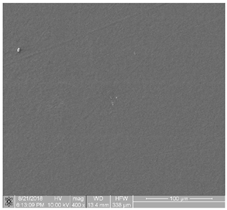 |
| 1 | contains samples characterized by very low-degree thrombogenicity: a dozen or so adhered blood platelets not creating aggregates |  |
| 2 | contains samples characterized by very low-degree thrombogenicity: several dozen of visible platelets which can be present as single, separated aggregates with a small area | 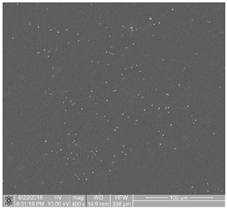 |
| 3 | contains samples characterized by average-degree thrombogenicity: biological material mainly comprises aggregates larger than individual blood platelets |  |
| 4 | contains samples characterized by high-degree thrombogenicity: sample is covered with highly differentiated biological material, and the individual objects are connected with each other without possibility of separating them | 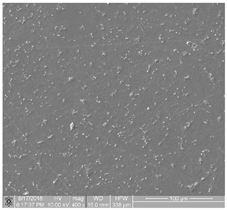 |
| 5 | contains samples characterized by very high-degree thrombogenicity: platelets are highly differentiated and form numerous aggregates, which are connected with each other without possibility of separating and counting objects | 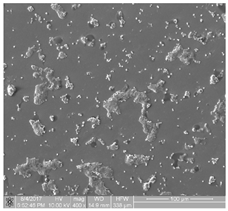 |
| Electrolyte | Anodization Parameters | ONT Internal Diameter (nm) | ONT Outer Diameter (nm) | ONTs Length (μm) |
|---|---|---|---|---|
| 0.5% HF | E = 20 V, t = 120 min | 71(7) | 87(10) | 0.94(9) |
| 1M (NH4)2SO4 +2% NH4F | E = 20 V, t = 120 min | 61(6) | 103(10) | 3.9(4) |
| 1M C2H6O2 +4% NH4F | E = 50 V, t = 80 min | 169(17) | 342(34) | 9.7(9) |
| Parameter | Ti-13Nb-13Zr | 1G ONTs | 2G ONTs | 3G ONTs |
|---|---|---|---|---|
| CPDav (mVKP) | −634.2 | −566.5 | −480.4 | −386.7 |
| CPDrms (mVKP) | 17.8 | 19.1 | 23.7 | 24.7 |
| CPDal (µm) | 63.61 | 31.37 | 43.12 | 52.29 |
| CPDsk | −0.20 | 0.06 | −0.15 | 0.07 |
| CPDku | −0.09 | 0.05 | 0.28 | −0.02 |
| Material | Percent Viability (%) | System Suitability |
|---|---|---|
| Positive control | 0.54 | No Cytotoxic Potential |
| Negative control | 98.33 | No Cytotoxic Potential |
| Ti-13Nb-13Zr (1×) | 92.26 | No Cytotoxic Potential |
| Ti-13Nb-13Zr (2×) | 97.18 | No Cytotoxic Potential |
| Ti-13Nb-13Zr (3×) | 97.49 | No Cytotoxic Potential |
| Ti-13Nb-13Zr (4×) | 99.63 | No Cytotoxic Potential |
| 1G ONTs (1×) | 100.6 | No Cytotoxic Potential |
| 1G ONTs (2×) | 85.29 | No Cytotoxic Potential |
| 1G ONTs (3×) | 103.8 | No Cytotoxic Potential |
| 1G ONTs (4×) | 98.67 | No Cytotoxic Potential |
| 2G ONTs (1×) | 87.91 | No Cytotoxic Potential |
| 2G ONTs (2×) | 99.59 | No Cytotoxic Potential |
| 2G ONTs (3×) | 106.3 | No Cytotoxic Potential |
| 2G ONTs (4×) | 106.2 | No Cytotoxic Potential |
| 3G ONTs (1×) | 88.45 | No Cytotoxic Potential |
| 3G ONTs (2×) | 89.97 | No Cytotoxic Potential |
| 3G ONTs (3×) | 95.02 | No Cytotoxic Potential |
| 3G ONTs (4×) | 96.63 | No Cytotoxic Potential |
| Material | Level of Adhesion |
|---|---|
| Ti-13Nb-13Zr | High |
| 1G ONTs | High |
| 2G ONTs | Medium |
| 3G ONTs | Medium |
| Expert 2 | ||||||
|---|---|---|---|---|---|---|
| Expert 1 | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |
| Class 1 | 5 | 0 | 0 | 0 | 0 | |
| Class 2 | 1 | 4 | 0 | 0 | 0 | |
| Class 3 | 0 | 1 | 8 | 1 | 0 | |
| Class 4 | 0 | 0 | 1 | 9 | 1 | |
| Class 5 | 0 | 0 | 0 | 0 | 7 | |
| 1G ONTs | 2G ONTs | 3G ONTs | Ti-13Nb-13Zr | |
|---|---|---|---|---|
| 1G ONTs | 0.0030 | 0.1520 | 0.5908 | |
| 2G ONTs | 0.0030 | 0.0000 | 0.4582 | |
| 3G ONTs | 0.1520 | 0.0000 | 0.0017 | |
| Ti-13Nb-13Zr | 0.5908 | 0.4582 | 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stróż, A.; Gawlikowski, M.; Balin, K.; Osak, P.; Kubisztal, J.; Zubko, M.; Maszybrocka, J.; Dudek, K.; Łosiewicz, B. Biological Activity and Thrombogenic Properties of Oxide Nanotubes on the Ti-13Nb-13Zr Biomedical Alloy. J. Funct. Biomater. 2023, 14, 375. https://doi.org/10.3390/jfb14070375
Stróż A, Gawlikowski M, Balin K, Osak P, Kubisztal J, Zubko M, Maszybrocka J, Dudek K, Łosiewicz B. Biological Activity and Thrombogenic Properties of Oxide Nanotubes on the Ti-13Nb-13Zr Biomedical Alloy. Journal of Functional Biomaterials. 2023; 14(7):375. https://doi.org/10.3390/jfb14070375
Chicago/Turabian StyleStróż, Agnieszka, Maciej Gawlikowski, Katarzyna Balin, Patrycja Osak, Julian Kubisztal, Maciej Zubko, Joanna Maszybrocka, Karolina Dudek, and Bożena Łosiewicz. 2023. "Biological Activity and Thrombogenic Properties of Oxide Nanotubes on the Ti-13Nb-13Zr Biomedical Alloy" Journal of Functional Biomaterials 14, no. 7: 375. https://doi.org/10.3390/jfb14070375