Effect of Er:YAG Laser Exposure on the Amorphous Smear Layer in the Marginal Zone of the Osteotomy Site for Placement of Dental Screw Implants: A Histomorphological Study
Abstract
1. Introduction
1.1. Changes in Alveolar Bone When Using Different Osteotomy Techniques
1.2. Rotary Technique
1.3. Laser Technology
2. Objective
3. Materials and Methods
3.1. Materials
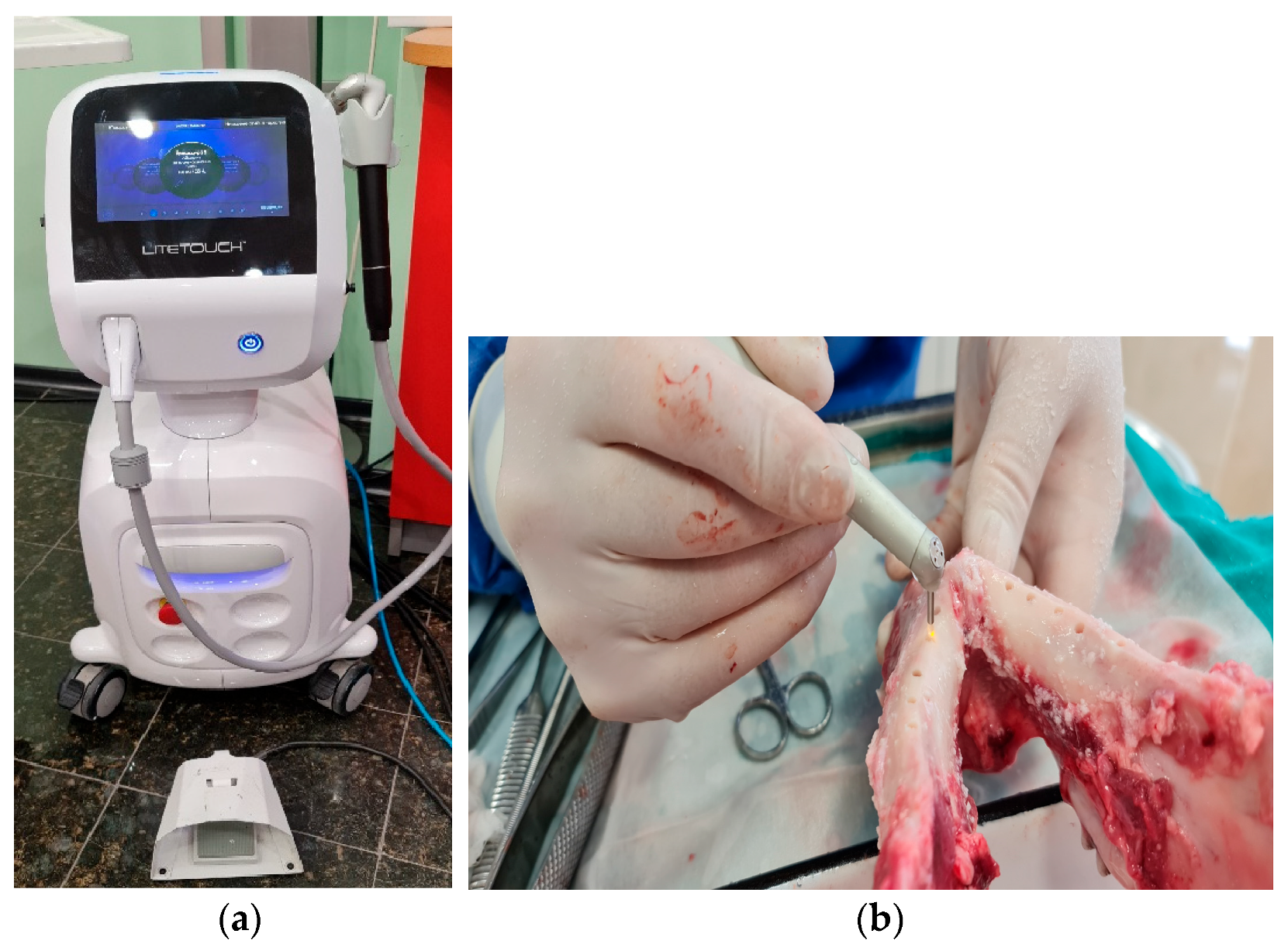
3.2. Study Design
3.2.1. Mandibular Osteotomy
- -
- Improved early bone–implant contact, which is an important factor for excellent primary stability;
- -
- Long-term bone–implant contact;
- -
- Accelerated and improved osseointegration;
- -
- Increased secondary stability.
- -
- Marking the location to place the implant on the cortical bone using a round bone cutter.
- -
- Trepanning the bone with a pilot cutter to pre-determine the length of the implant, followed by a depth gauge check.
- -
- Preparing the implant site using cutters with successively increasing diameters to a diameter of 0.1–1.2 mm smaller than that of the implant. The speed of rotation of the tools was 600–800 revolutions per minute.
- -
- Taking in the cortical bone phase with the corresponding profile cutter.
- Laser energy: 400 mJ;
- Pulse frequency: 17 Hz;
- Water spray level: 6;
- Power: 6.80 W.
3.2.2. Histological Techniques
- Decalcification
- Embedding the material in paraffin blocks
- Hematoxylin and eosin staining
3.3. Statistical Analysis
4. Results
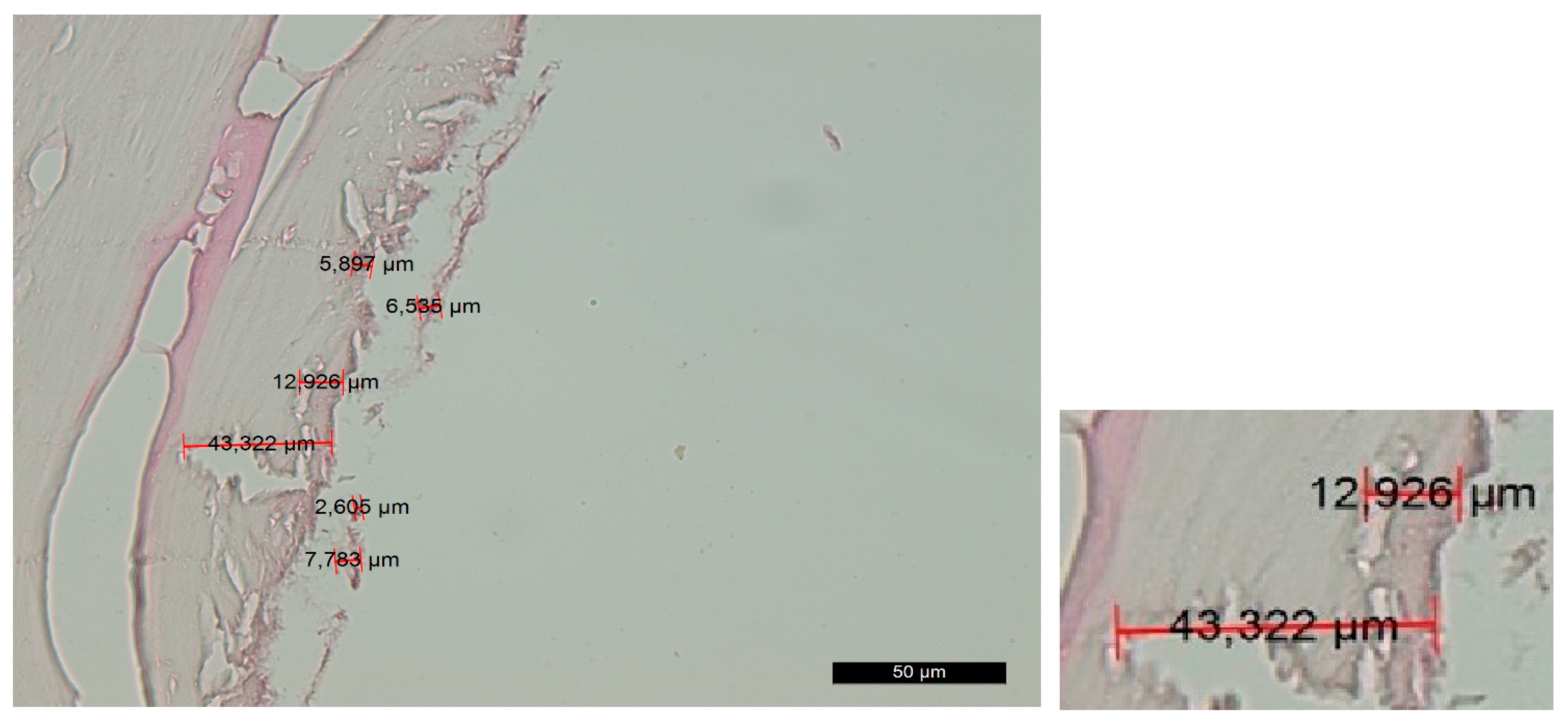
4.1. Histological Analysis
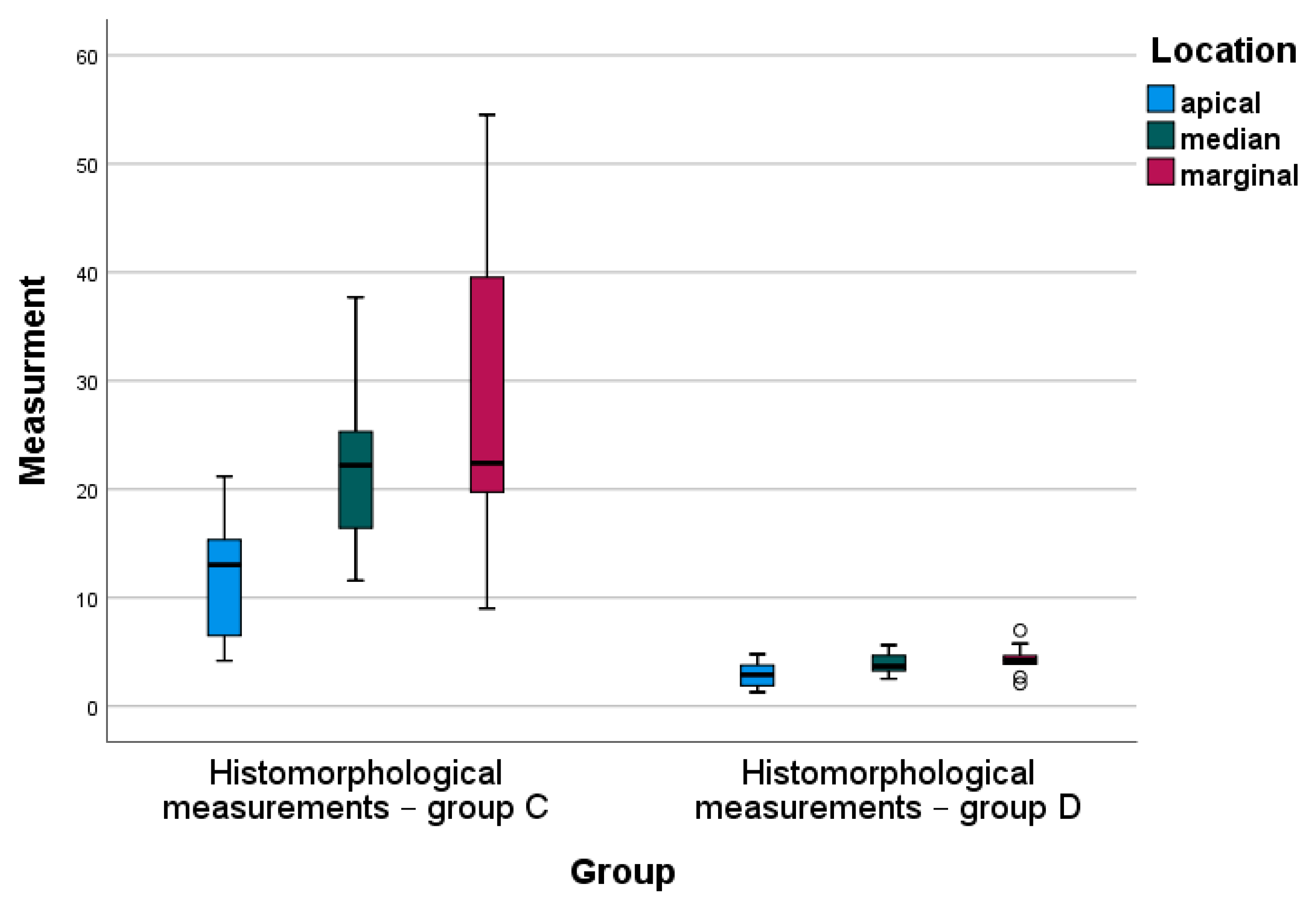
4.2. Statistical Analysis
5. Discussion
6. Conclusions
7. Patent
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.I. Introduction to osseointegration. In Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry, 1st ed.; Brånemark, P.I., Ed.; Quintessence Publishing Co.: Chicago, IL, USA, 1985; pp. 11–76. [Google Scholar]
- Zarb, G.; Albrektsson, T. Osseointegration: A requiem for the periodontal ligament. Int. J. Periodontics. Rest. Dent. 1991, 11, 88–91. [Google Scholar]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, 9. [Google Scholar] [CrossRef]
- Jayesh, R.S.; Dhinakarsamy, V. Osseointegration. J. Pharm. Bioall. Sci. 2015, 7, 226–229. [Google Scholar]
- Siebers, M.; Brugge, P.; Walboomers, X.; Jansen, J. Integrins as linker proteins between osteoblasts and bone replacing materials. A Crit. Rev. Biomater. 2005, 26, 137–146. [Google Scholar]
- Kasemo, B.; Lausmaa, J. Surface scoence aspects on inorganic biomaterials. CRC Crit. Rev. Biocomp. 1986, 2, 335–380. [Google Scholar]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26–38. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Impl. Res. 2011, 23, 1127–1135. [Google Scholar] [CrossRef]
- Chakkravarthy, V.; Sujin, P.J.; Lakshmanan, M.; Manojkumar, P.; Narayan, L.R.; Kumaran, M. Additive manufacturing of novel Ti-30Nb-2Zr biomimetic scaffolds for successful limb salvage Author links open overlay pane. Mater. Today Proc. 2022, 64, 1711–1716. [Google Scholar] [CrossRef]
- Chakkravarthy, V.; Manojkumar, P.; Lakshmanan, M.; Eswar Prasad, K.; Dafale, R.; Chitra Vadhana, V.; Narayan, R.L. Comparing bio-tribocorrosion of selective laser melted Titanium-25% Niobium and conventionally manufactured Ti-6Al-4 V in in-flammatory conditions. J. Alloys Compd. 2023, 952, 169852. [Google Scholar] [CrossRef]
- Rajaei, A.; Kazemian, M.; Khandan, A. Investigation of mechanical stability of lithium disilicate ceramic reinforced with titanium nanoparticles. Nanomed. Res. J. 2022, 7, 350–359. [Google Scholar] [CrossRef]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. BioMed Res. Int. 2022, 2022, 11. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Eldomiaty, W.; Khabbaz, Y. Can Osseointegration Be Achieved Without Primary Stability? Dent. Clin. N. Am. 2019, 63, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Joos, G. Theoretical Physics, 3rd ed.; Dover Publications: New York, NY, USA, 1987; pp. 50–51. [Google Scholar]
- Peev, S. Bone Augmentation in Dental Implantology, 1st ed.; Quintessence Pub Co.: Varna, Bulgaria, 2016; pp. 20–21. [Google Scholar]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.R.; Albrektsson, T. Assessment of bone viability after heat trauma. Scand. J. Plast. Reconstr. Surg. 1984, 18, 261–268. [Google Scholar]
- Soldatos, N.; Pham, H.; Fakhouri, W.D.; Ngo, B.; Lampropoulos, P.; Tran, T.; Weltman, R. Temperature Changes during Implant Osteotomy Preparations in Human Cadaver Tibiae Comparing MIS® Straight Drills with Densah® Burs. Genes 2022, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, T.; Nevins, M.L.; Kim, D.M.; Nevins, M.; Wada, K.; Schenk, R.K.; Fiorellini, J.P. Osseus response following resective therapy with piezosurgey. Int. Periodontics Restor. Dent. 2005, 25, 543–549. [Google Scholar]
- Moslemi, N.; Shahnaz, A.; Masoumi, S.; Torabi, S.; Akbari, S. Laser-assisted osteotomy for implant site preparation: A literature review. Implant. Dent. 2017, 26, 129–136. [Google Scholar] [CrossRef]
- Marotti, J.; Neto, P.T.; de Campos, T.T.; Aranha, A.C.C.; Weingart, D.; Wolfart, S.; Haselhuhn, K. Recent Patents of Lasers in Implant Dentistry. Recent Pat. Biomed. Eng. 2011, 4, 103–109. [Google Scholar] [CrossRef]
- Stübinger, S.; Biermeier, K.; Bächi, B.; Ferguson, S.J.; Sader, R.; von Rechenberg, B. Comparison of Er:YAG laser, piezoelectric, and drill osteotomy for dental implant site preparation: A biomechanical and histological analysis in sheep. Lasers Surg. Med. 2010, 42, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Deng, F.; Wang, Z.; Zhao, Z.; Wang, J. Biomechanical and histomorphometric analyses of the osseointegration of microscrews with different surgical techniques in beagle dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, 644–650. [Google Scholar] [CrossRef]
- Jeong, S.M.; Yoo, J.H.; Fang, Y.; Choi, B.-H.; Son, J.-S.; Oh, J.-H. The effect of guided flapless implant procedure on heat generation from implant drilling. J. Craniomaxillofac. Surg. 2014, 42, 725–729. [Google Scholar] [PubMed]
- Deliverska, E.; Yordanov, B. Osseodensification as an Alternative Approach in DentalImplantology for Implant Sites with Insufficient Available Bone. J. IMAB 2019, 25, 2606–2610. [Google Scholar] [CrossRef]
- Sasaki, K.M.; Aoki, A.; Ichinose, S.; Yoshino, T.; Yamada, S.; Ishikawa, I. Scanning electron microscopy and Fourier transformed infrared spectroscopy analysis of bone removal using Er:YAG and CO2 lasers. J. Periodontol. 2002, 73, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Delgado-Peña, J.; Maté-Sánchez, J.E.; Mareque Bueno, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Novel hybrid drilling protocol. Evaluation for the implant healing. Thermal changes, crestal bone loss, and bone-to-implant contact. Clin. Oral Implant. Res. 2015, 26, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Barrak, I.; Joób-Fancsaly, Á.; Braunitzer, G.; Varga, E., Jr.; Boa, K.; Piffkó, J. Intraosseous heat generation during osteotomy performed freehand and through template with an integrated metal guide sleeve: An in vitro study. Implant. Dent. 2018, 27, 342–350. [Google Scholar] [CrossRef]
- Cordioli, G.; Majzoub, Z. Heat generation during implant site preparation: An in vitro study. Int. J. Oral Maxillofac. Implant. 1997, 12, 186–193. [Google Scholar]
- Misir, A.F.; Sumer, M.; Yenisey, M.; Ergioglu, E. Effect of surgical drill guide on heat generated from implant drilling. J. Oral Maxillofac. Surg. 2009, 67, 2663–2668. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Adell, R. Temperatures during drilling for the placement of implants using the osseointegration technique. J. Oral Maxillofac. Surg. 1986, 44, 4–7. [Google Scholar] [CrossRef]
- Tehemar, S.H. Factors affecting heat generation during implant site preparation: A review of biologic observations and future considerations. Int. J. Oral Maxillofac. Implant. 1999, 14, 127–136. [Google Scholar]
- Gabrić, D.; Aumiler, D.; Vuletić, M.; Gjorgievska, E.; Blašković, M.; Mladenov, M.; Pavlić, V. Thermal Evalu-ation by Infrared Thermography Measurement of Osteotomies Performed with Er:YAG Laser, Piezosurgery and Surgical Drill-An Animal Study. Materials 2021, 14, 3051. [Google Scholar] [CrossRef]
- Aleksi’c, V.; Aoki, A.; Iwasaki, K.; Takasaki, A.A.; Wang, C.Y.; Abiko, Y.; Ishikawa, I.; Izumi, Y. Low-level Er:YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med. Sci. 2010, 25, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur.Cell Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Fried, N.M.; Fried, D. Comparison of Er:YAG and 9.6-microm TE CO2 lasers for ablation of skull tissue. Lasers Surg. Med. 2001, 28, 335–343. [Google Scholar] [CrossRef]
- Sasaki, K.M.; Aoki, A.; Ichinose, S.; Ishikawa, I. Ultrastructural Analysis of Bone Tissue Irradiated by Er:YAG Laser. Lasers Surg. Med. 2002, 31, 322–332. [Google Scholar] [CrossRef]
- O’Donnell, R.J.; Deutsch, T.F.; Flotte, R.J.; Lorente, C.A.; Tomford, W.W.; Mankin, H.J.; Schomacker, K.T. Effect of Er:YAG laser holes on osteoinduction in demineral-ized rat calvarial allografts. J. Orthop. Res. 1996, 14, 108–113. [Google Scholar] [CrossRef]
- Romanos, G.E.; Gutknecht, N.; Dieter, S.; Schwarz, F.; Crespi, R.; Sculean, A. Laser wavelengths and oral im-plantology. Lasers Med. Sci. 2009, 24, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.K.; Tomov, G. The use of the LiteTouch Er:YAG laser in peri-implantitis treatment. Laser 2012, 2, 22–28. [Google Scholar]
- Sculean, A.; Schwarz, F.; Berakdar, M.; Windisch, P.; Arweiler, N.B.; Romanos, G.E. Healing of intrabony defects following surgical treatment with or without an Er:YAG laser. J. Clin. Periodontol. 2004, 31, 604–608. [Google Scholar] [CrossRef]
- Tim, C.R.; Pinto, K.N.; Rossi, B.R.; Fernandes, K.; Matsumoto, M.A.; Parizotto, N.A.; Renno, A.C. Low-level laser therapy enhances the expression of osteogenic factors during bone repair in rats. Lasers Med. Sci. 2014, 29, 147–156. [Google Scholar] [CrossRef]
- Stübinger, S. Advances in bone surgery: The Er:YAG laser in oral surgery and implant dentistry. Clin. Cosmet. Investig. Dent. 2010, 30, 47–62. [Google Scholar] [CrossRef]
- Seymen, G.; Turgut, Z.; Berk, G.; Bodur, A. Implant Bed Preparation with an Er, Cr: YSGG Laser Using Stereolithographic Surgical Guide. J. Lasers Med. Sci. 2013, 4, 25–32. [Google Scholar]
- Walsh, J.T., Jr.; Deutsch, T.F. Er:YAG laser ablation of tissue: Measurement of ablation rates. Lasers Surg. Med. 1989, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.T., Jr.; Flotte, T.J.; Deutsch, T.F. Er:YAG laser ablation of tissue: Effect of pulse duration and tissue type on thermal damage. Lasers Surg. Med. 1989, 9, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Keller, U.; Hibst, R.; Mohr, W. Experimental animal studies on laser osteotomy using the erbium:YAG laser system. Dtsch. Z. Mund. Kiefer. Gesichtschir. 1991, 15, 197–199. (In German) [Google Scholar] [PubMed]
- Hibst, R. Mechanical effects of erbium: YAG laser bone ablation. Lasers Surg. Med. 1992, 12, 125–130. [Google Scholar] [CrossRef]
- Lewandrowski, K.U.; Lorente, C.; Schomacker, K.T.; Flotte, T.J.; Wilkes, J.W.; Deutsch, T.F. Use of the Er:YAG laser for improved plating in maxillofacial surgery: Comparison of bone healing in laser and drill osteoto-mies. Lasers Surg. Med. 1996, 19, 40–45. [Google Scholar] [CrossRef]
- Kesler, G.; Romanos, G.; Koren, R. Use of Er:YAG laser to improve osseointegration of titanium alloy im-plants-a comparison of bone healing. Int. J. Oral. Maxillofac. Implants 2006, 21, 375–379. [Google Scholar]
- Lee, S.Y.; Piao, C.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Kim, T.H.; Kim, M.J.; Kwon, H.B.; Kim, S.K. A comparison of bone bed preparation with laser and conventional drill on the relationship between implant stability quotient (ISQ) values and implant insertion variables. J. Adv. Prosthodont. 2010, 2, 148–153. [Google Scholar] [CrossRef]
- Świder, K.; Dominiak, M. Er:YAG and diode laser application in implant bed preparation and implant uncovering: A case report. Dent. Med. Probl. 2019, 56, 111–116. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implants. Res. 2003, 14, 251–262. [Google Scholar] [CrossRef]
- Dyakov, L.; Lozanov, L.; Angelov, A.; Stoykov, D. Manual for Exercises in Veterinary-Medical Pathohistology, 1st ed; Blackwell Publishing: Sofia, Bulgaria, 1989; pp. 15–17. [Google Scholar]
- El-Montaser, M.; Devlin, H.; Dickinson, M.R.; Sloan, P.; Lloyd, R.E. Osseointegration of titanium metal im-plants in erbium-YAG laser-prepared bone. Implant. Dent. 1999, 8, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Oliver, W.; Herten, M.; Sager, M.; Chaker, A.; Becker, J. Influence of implant bed preparation using an Er:YAG laser on the osseointegration of titanium implants: A histomorphometrical study in dogs. J. Oral Rehabil. 2007, 34, 273–281. [Google Scholar] [PubMed]
- Belcheva, A.; Tomov, G. In Hard dental tissues in normality and pathology. In Electron Microscopic Atlas, 1st ed; Panov, V., Rangelov, B., Eds.; Springer: Plovdiv, Bulgaria, 2018; p. 49. [Google Scholar]





| Location | Deformation in Implant Cavity— Standard Drills (in Microns) | Deformation in Implant Cavity— Er-YAG Laser (in Microns) | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | N | Mean | Std. Deviation | ||
| Apical | 10 | 64.52 | 28.717 | 10 | 19.50 | 11.142 | 0.000 |
| Median | 10 | 79.12 | 29.836 | 10 | 18.52 | 7.874 | 0.000 |
| Marginal | 10 | 106.65 | 20.700 | 10 | 29.52 | 11.533 | 0.000 |
| Location | Histomorphological Measurements— Group A | Histomorphological Measurements— Group B | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | N | Mean | Std. Deviation | ||
| Apical | 15 | 44.90 | 27.78 | 15 | 14.24 | 3.73 | 0.000 |
| Median | 15 | 77.08 | 42.22 | 15 | 19.21 | 7.19 | 0.000 |
| Marginal | 15 | 104.16 | 61.14 | 15 | 29.00 | 8.08 | 0.000 |
| Location | Histomorphological Measurements— Group C | Histomorphological Measurements— Group D | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | Std. Deviation | N | Mean | Std. Deviation | ||
| Apical | 10 | 12.29 | 5.45 | 10 | 2.84 | 1.11 | 0.000 |
| Median | 10 | 22.09 | 7.18 | 10 | 3.85 | 0.98 | 0.000 |
| Marginal | 10 | 27.91 | 13.92 | 10 | 4.29 | 1.40 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanazirski, N.; Vladova, D.; Neychev, D.; Raycheva, R.; Kanazirska, P. Effect of Er:YAG Laser Exposure on the Amorphous Smear Layer in the Marginal Zone of the Osteotomy Site for Placement of Dental Screw Implants: A Histomorphological Study. J. Funct. Biomater. 2023, 14, 376. https://doi.org/10.3390/jfb14070376
Kanazirski N, Vladova D, Neychev D, Raycheva R, Kanazirska P. Effect of Er:YAG Laser Exposure on the Amorphous Smear Layer in the Marginal Zone of the Osteotomy Site for Placement of Dental Screw Implants: A Histomorphological Study. Journal of Functional Biomaterials. 2023; 14(7):376. https://doi.org/10.3390/jfb14070376
Chicago/Turabian StyleKanazirski, Nikolay, Diyana Vladova, Deyan Neychev, Ralitsa Raycheva, and Petya Kanazirska. 2023. "Effect of Er:YAG Laser Exposure on the Amorphous Smear Layer in the Marginal Zone of the Osteotomy Site for Placement of Dental Screw Implants: A Histomorphological Study" Journal of Functional Biomaterials 14, no. 7: 376. https://doi.org/10.3390/jfb14070376
APA StyleKanazirski, N., Vladova, D., Neychev, D., Raycheva, R., & Kanazirska, P. (2023). Effect of Er:YAG Laser Exposure on the Amorphous Smear Layer in the Marginal Zone of the Osteotomy Site for Placement of Dental Screw Implants: A Histomorphological Study. Journal of Functional Biomaterials, 14(7), 376. https://doi.org/10.3390/jfb14070376






