Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Pristine Hydroxyapatite Synthesis
2.3. Substituted Hydroxyapatite Synthesis
2.4. Morphological and Structural Characterization
2.4.1. X-ray Diffraction
2.4.2. Dynamic Light Scattering (DLS) and Zeta Potential
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.5. In Vitro Interactions with Osteoblast Cells
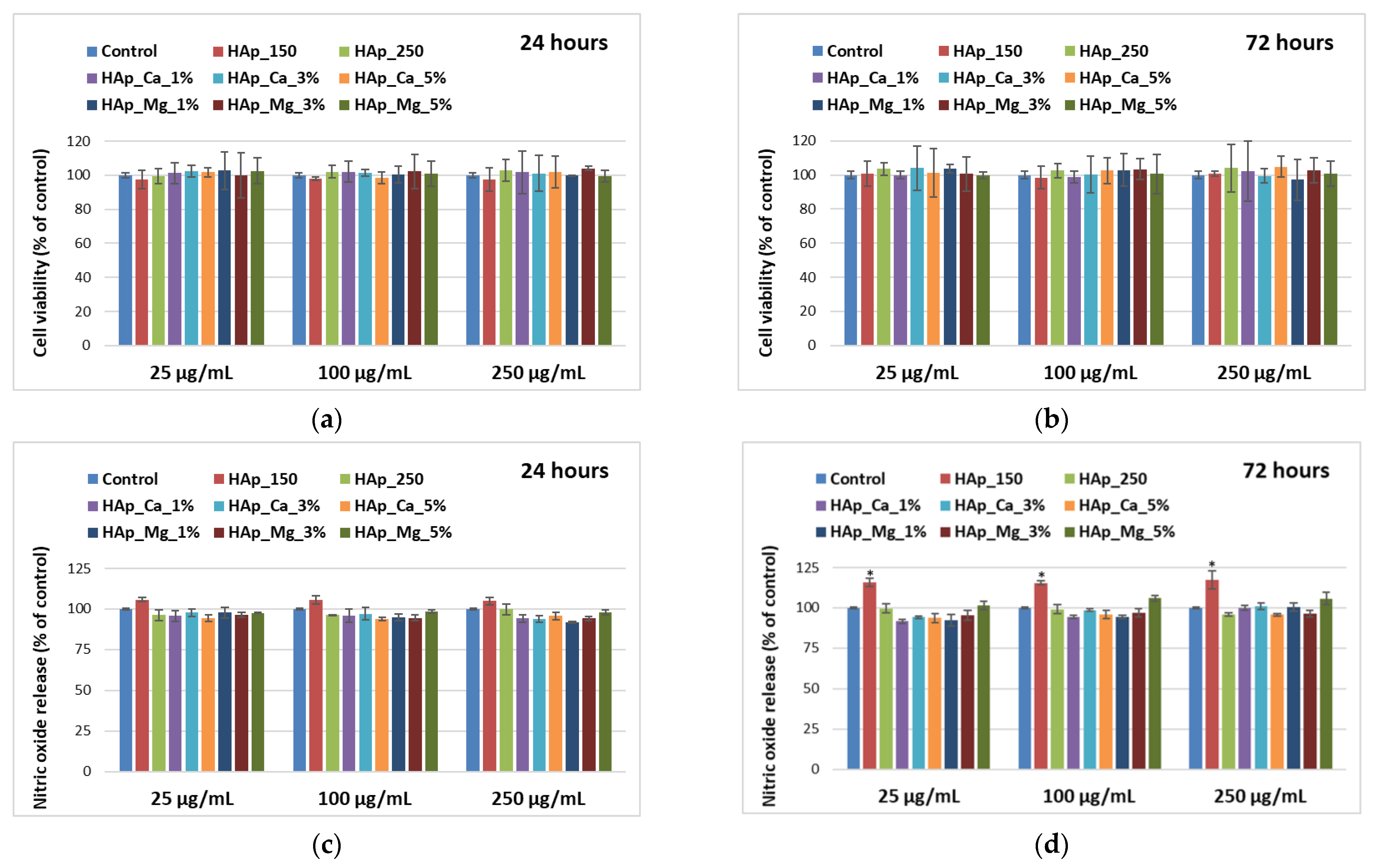
2.5.1. Cell Viability Assay
2.5.2. Griess Assay
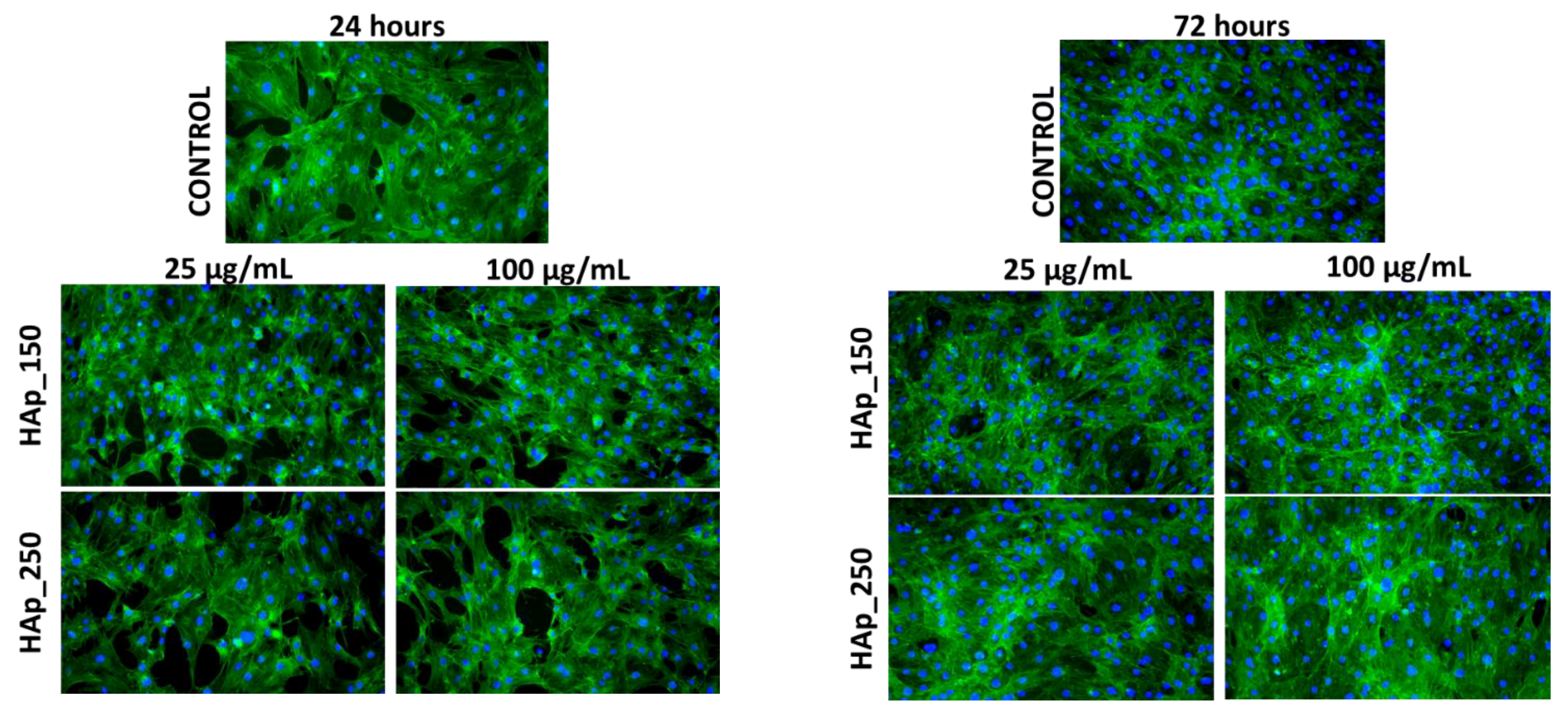
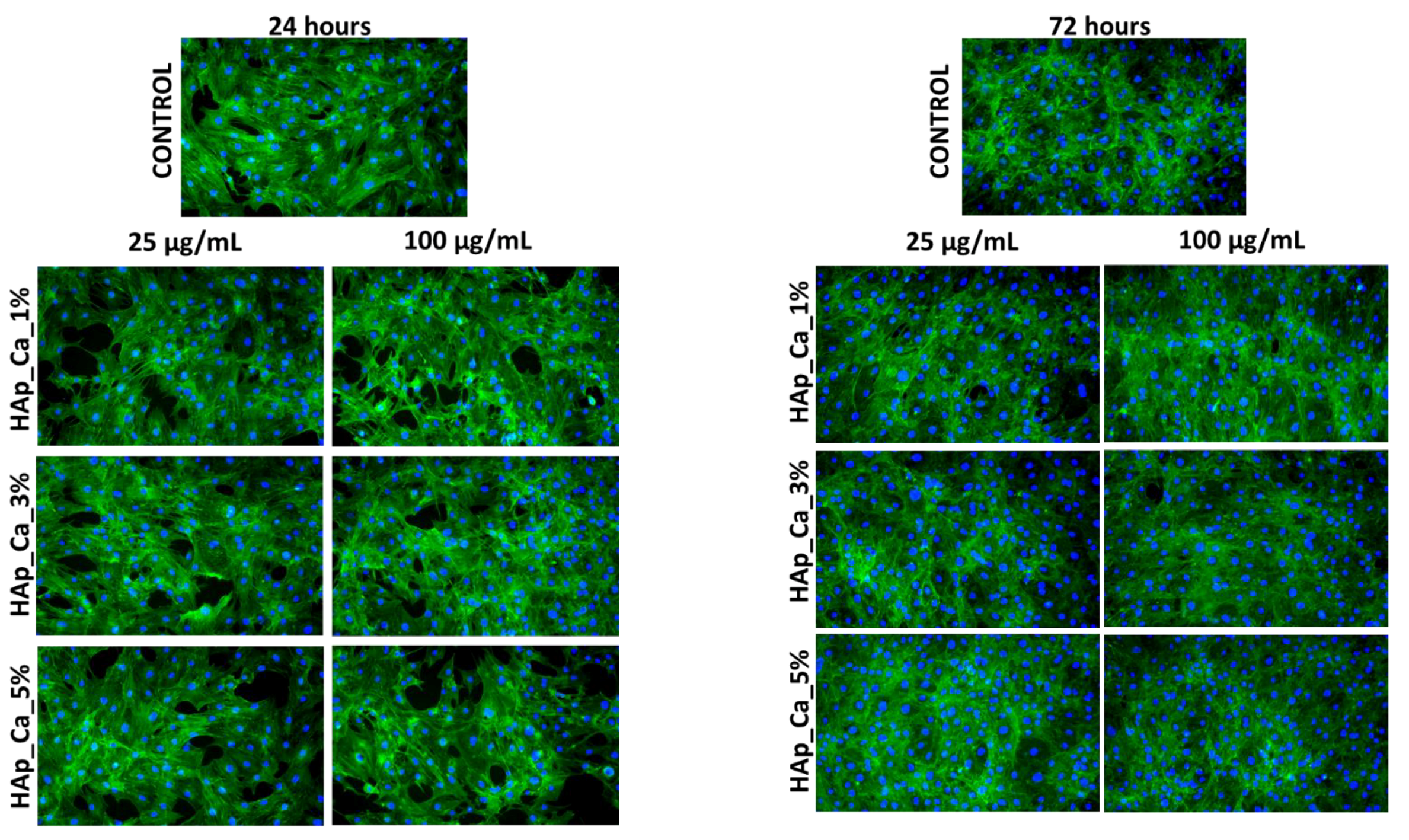
2.5.3. Fluorescence Microscopy
2.6. Microbiological Evaluation
2.6.1. MIC (Minimum Inhibitory Concentration) Method
2.6.2. Development of Monospecific Biofilms
3. Results
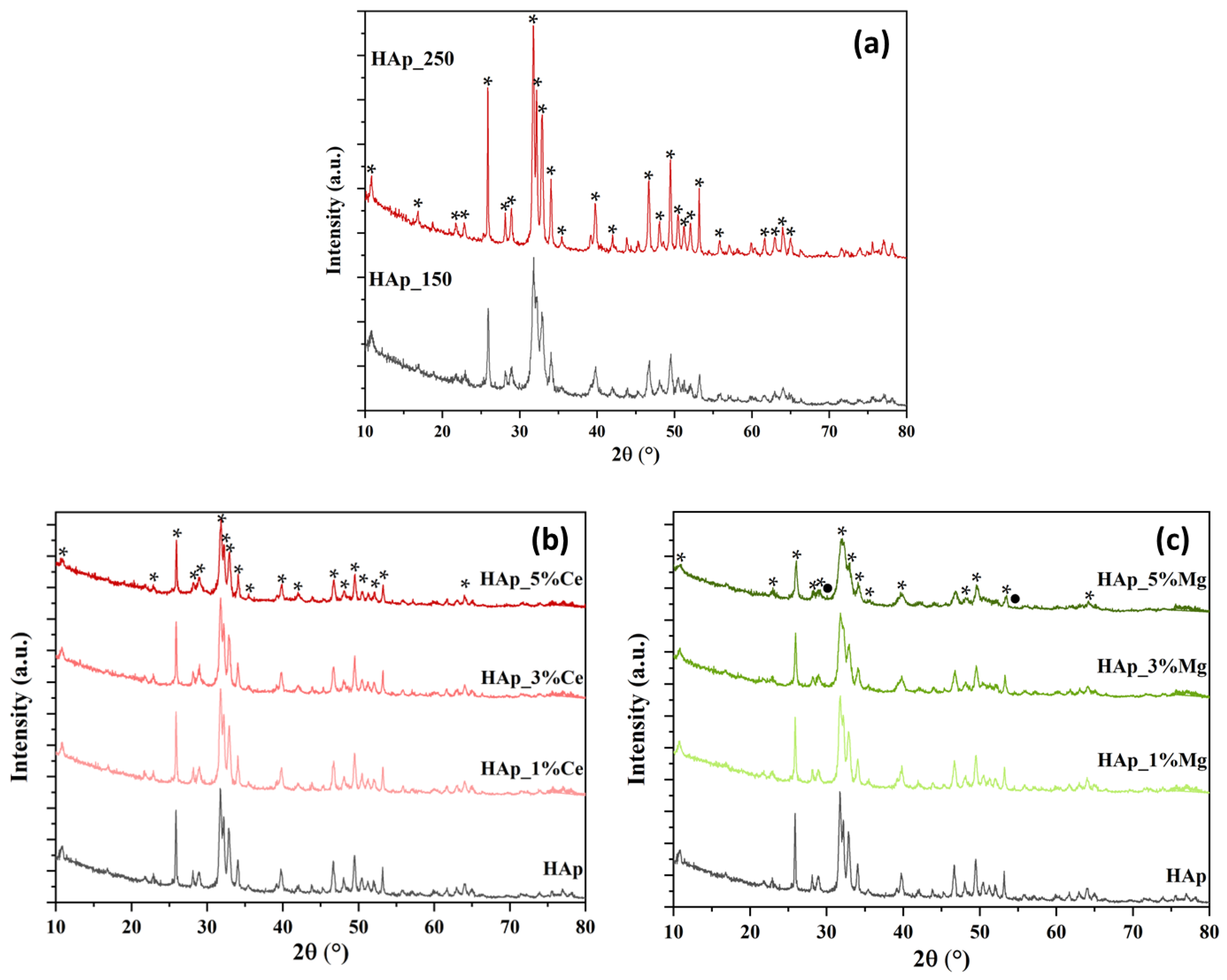
3.1. X-ray Diffraction
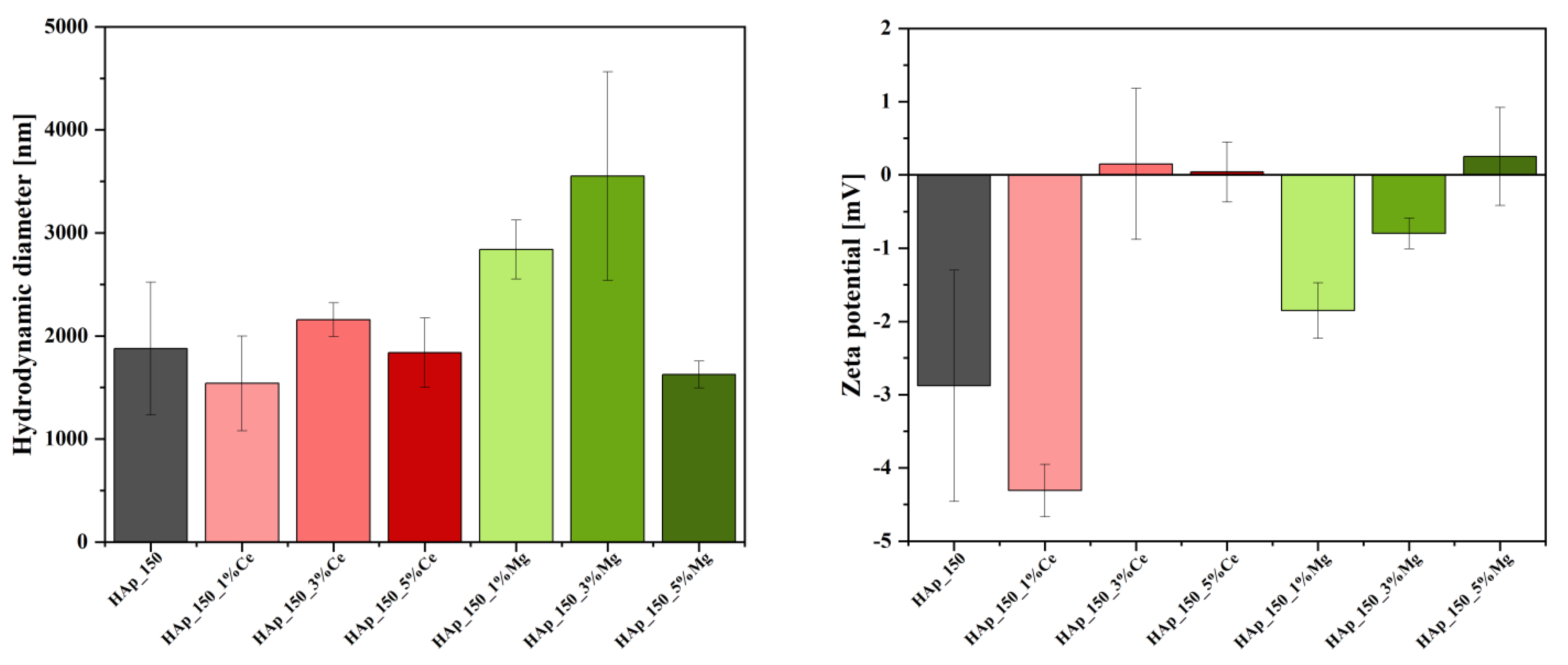
3.2. Dynamic Light Scattering (DLS) and Zeta Potential
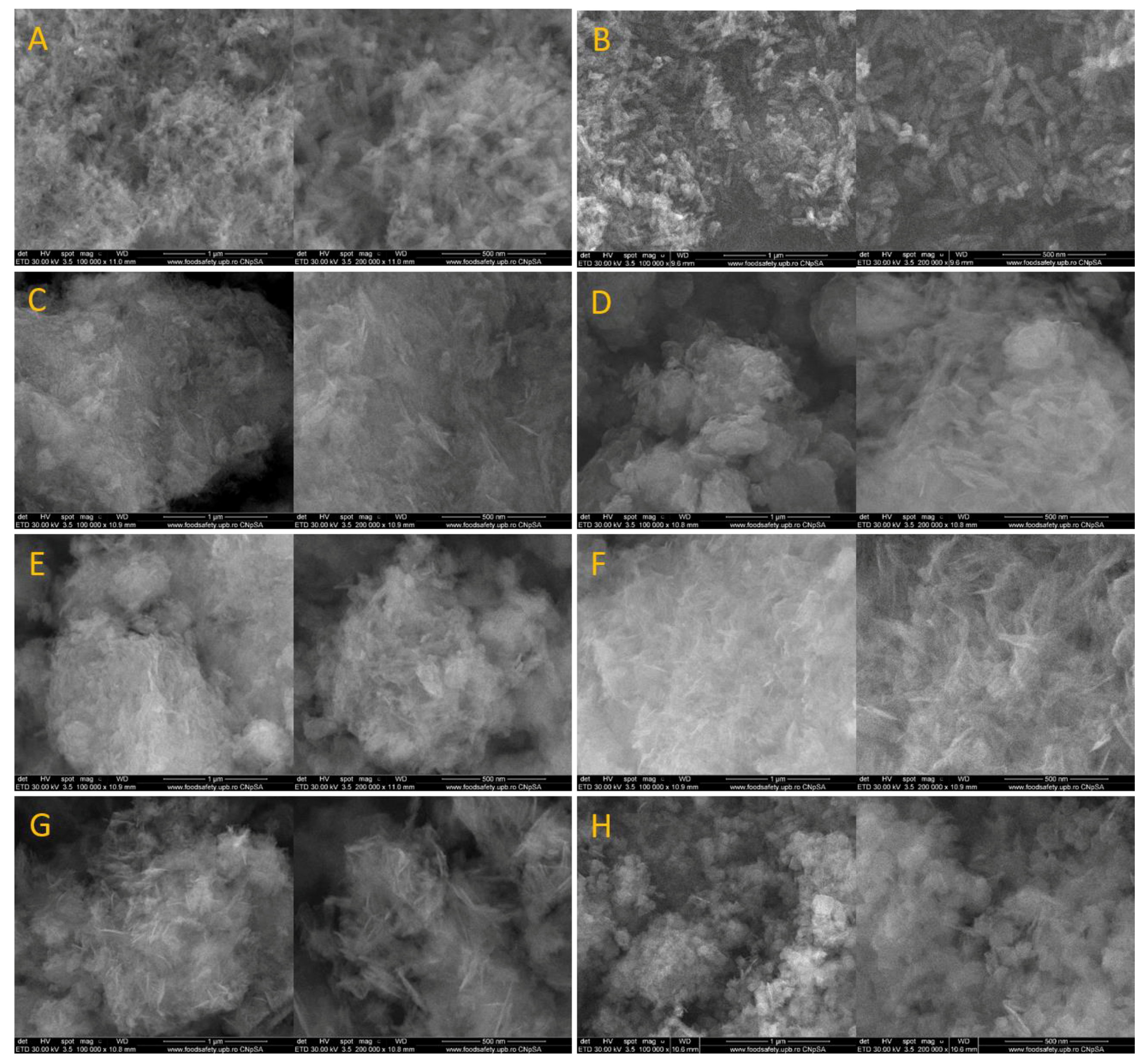
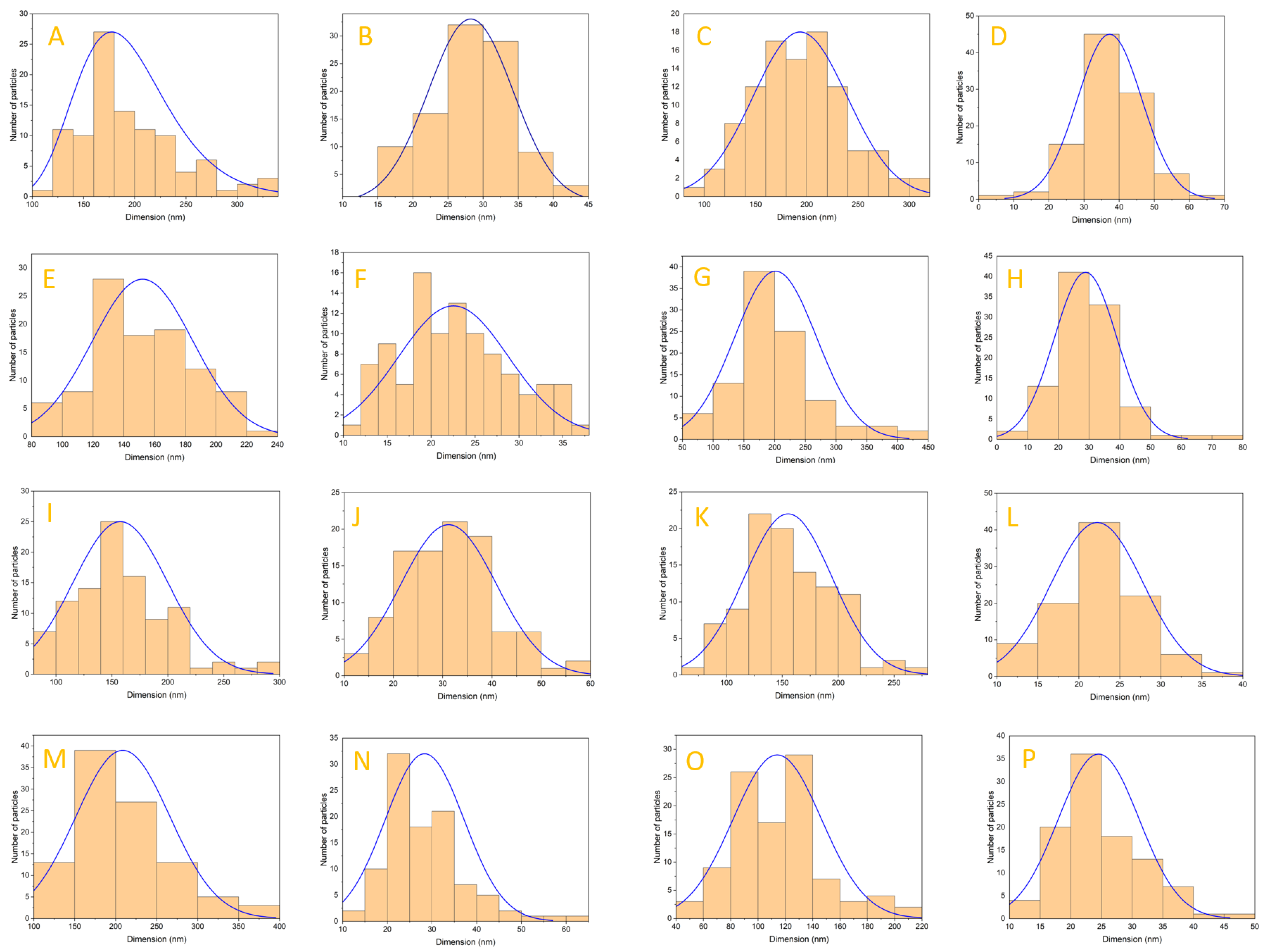
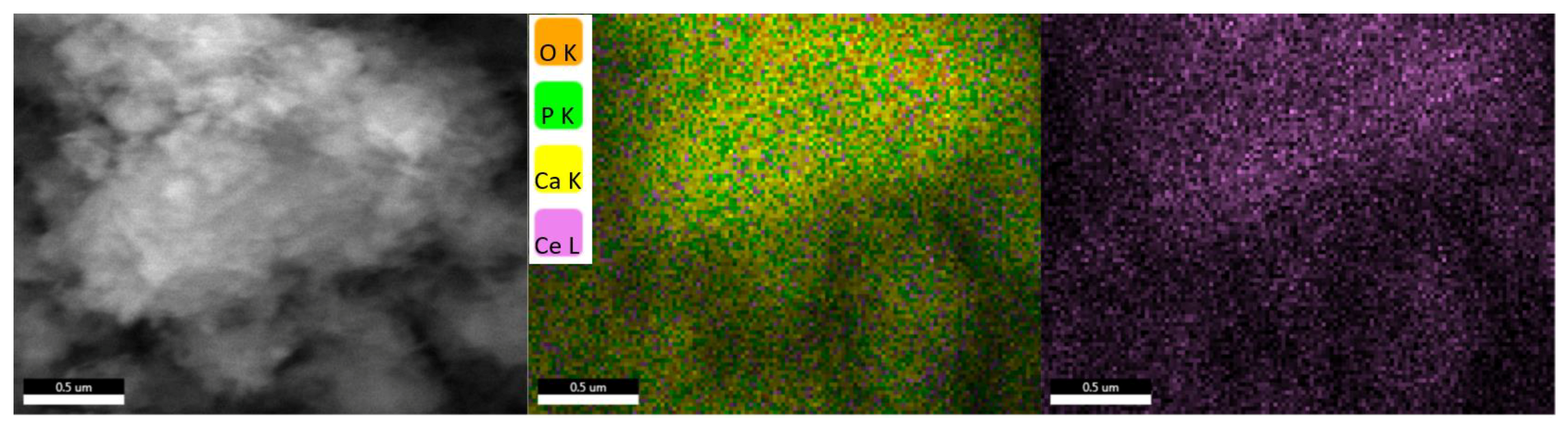
3.3. Scanning Electron Microscopy (SEM)
3.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
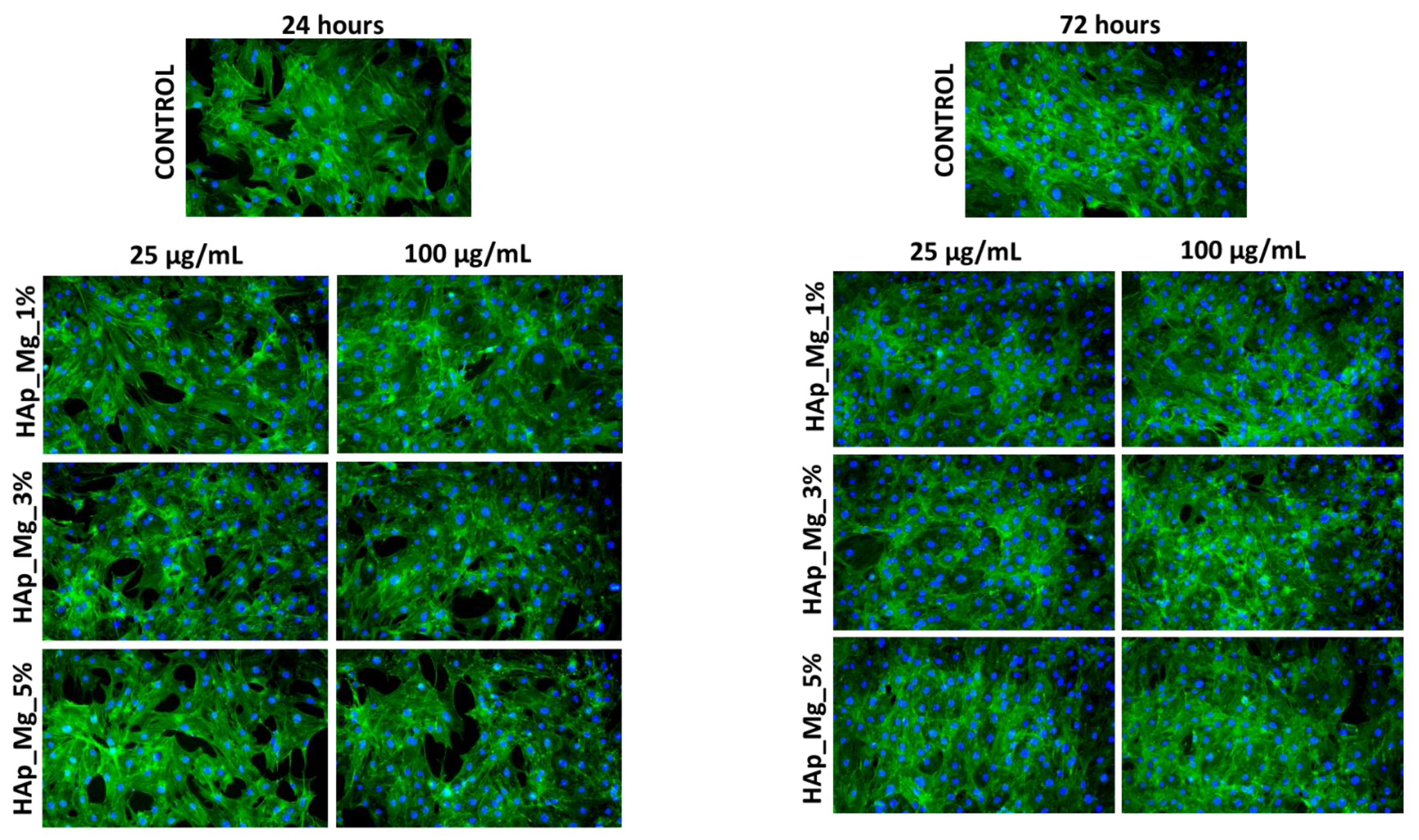
3.5. Biological Evaluation of HAp Samples
3.6. Microbiological Evaluation
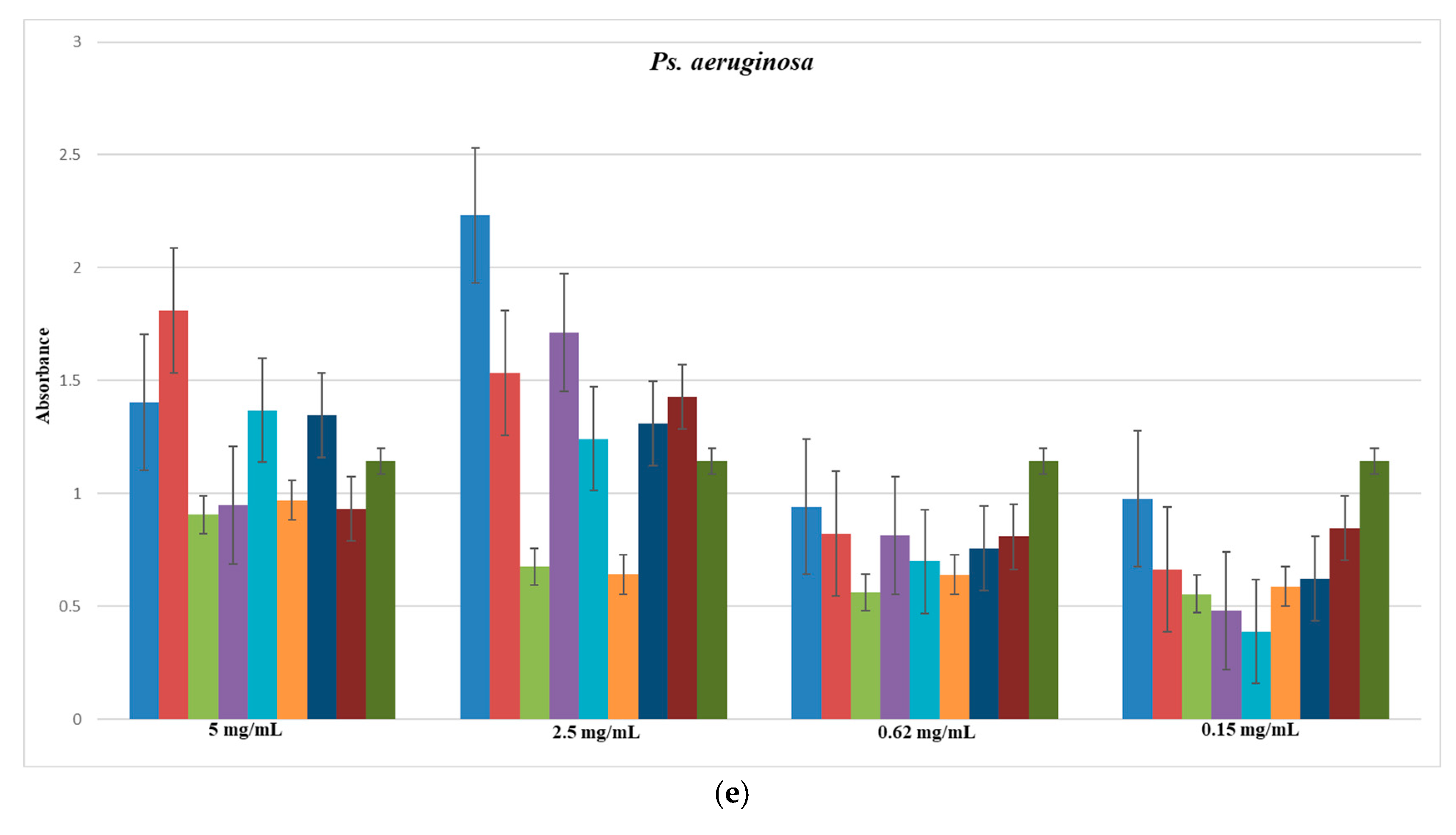
3.6.1. MIC (Minimum Inhibitory Concentration) Method
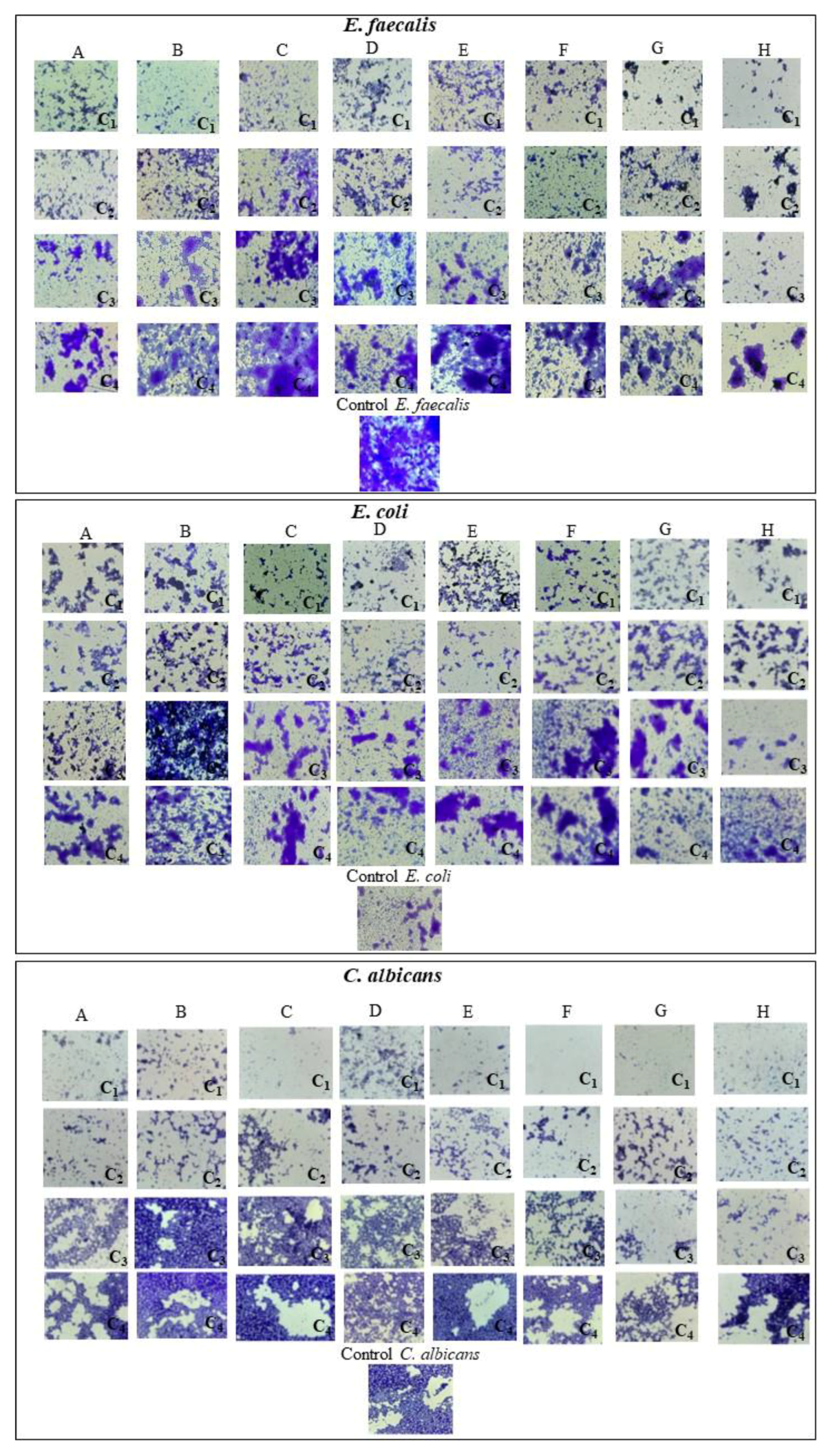
3.6.2. Development of Monospecific Biofilms
4. Discussion
4.1. X-ray Diffraction
4.2. Dynamic Light Scattering (DLS) and Zeta Potential
4.3. Scanning Electron Microscopy (SEM)
4.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
4.5. Biological Behavior Evaluation on Osteoblast Cells
4.6. Microbiological Evaluation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A review on the synthesis and properties of hydroxyapatite for biomedical applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Tălpeanu, D.; Lungu, M.V.; PĂTroi, D.; Marinescu, V.E.; Cojocaru, A. Study on porous hydroxyapatite based ceramic materials as bone substitutes for cranioplasty. Rev. Română De Mater./Rom. J. Mater. 2021, 51, 178–185. [Google Scholar]
- Ullah, I.; Hussain, Z.; Zhang, Y.; Liu, X.; Ullah, S.; Zhang, Y.; Zheng, P.; Gao, T.; Liu, Y.; Zhang, Z.; et al. Inorganic nanomaterial-reinforced hydrogel membrane as an artificial periosteum. Appl. Mater. Today 2022, 28, 101532. [Google Scholar] [CrossRef]
- Razak, A.; Isa, N.M.; Adzila, S. Synthesis of calcium phosphate extracted from eggshell waste through precipitation method. Biointerface Res. Appl. Chem 2021, 11, 15058–15067. [Google Scholar]
- Forero-Sossa, P.A.; Salazar-Martínez, J.D.; Giraldo-Betancur, A.L.; Segura-Giraldo, B.; Restrepo-Parra, E. Temperature effect in physicochemical and bioactive behavior of biogenic hydroxyapatite obtained from porcine bones. Sci. Rep. 2021, 11, 11069. [Google Scholar] [CrossRef]
- Zaman, S.U.; Irfan, M.; Irfan, M.; Zaman, M.K.U.; Muhammad, N. Overview of hydroxyapatite; composition, structure, synthesis methods and its biomedical uses. Biomed. Lett. 2020, 6, 84–99. [Google Scholar]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired By Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, I.A.; Matei, L.; Birca, A.C.; Nicoara, A.I.; Ene, V.L.; Dragu, L.D.; Ficai, A.; Bleotu, C.; Andronescu, E. Curcumin-hydroxyapatite systems used for bone cancer treatment. Rev. Romana De Mater. Rom. J. Mater. 2021, 51, 505–513. [Google Scholar]
- Ojo, O.A.; Olayide, I.I.; Akalabu, M.C.; Ajiboye, B.O.; Ojo, A.B.; Oyinloye, B.E.; Ramalingam, M. Nanoparticles and their biomedical applications. Biointerface Res. Appl. Chem. 2021, 11, 8431–8445. [Google Scholar]
- Ucar, S.; Bjørnøy, S.H.; Bassett, D.C.; Strand, B.L.; Sikorski, P.; Andreassen, J.-P. Formation of Hydroxyapatite via Transformation of Amorphous Calcium Phosphate in the Presence of Alginate Additives. Cryst. Growth Des. 2019, 19, 7077–7087. [Google Scholar] [CrossRef]
- Oni, O.P.; Hu, Y.; Tang, S.; Yan, H.; Zeng, H.; Wang, H.; Ma, L.; Yang, C.; Ran, J. Syntheses and applications of mesoporous hydroxyapatite: A review. Mater. Chem. Front. 2023, 7, 9–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Qiang, L.; Liu, Y.; Fan, M.; Si, X.; Zheng, P. Biomaterial-assisted tumor therapy: A brief review of hydroxyapatite nanoparticles and its composites used in bone tumors therapy. Front. Bioeng. Biotechnol. 2023, 11, 1167474. [Google Scholar] [CrossRef]
- Kelly, R.R.; Sidles, S.J.; LaRue, A.C. Effects of Neurological Disorders on Bone Health. Front. Psychol. 2020, 11, 612366. [Google Scholar] [CrossRef]
- Bhat, S.; Uthappa, U.T.; Altalhi, T.; Jung, H.Y.; Kurkuri, M.D. Functionalized Porous Hydroxyapatite Scaffolds for Tissue Engineering Applications: A Focused Review. ACS Biomater. Sci. Eng. 2022, 8, 4039–4076. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, P.C.S.; Srinivas, K.; Akhil, S.; Bollikolla, H.B.; Chandu, B. A Comprehensive Review on Novel Graphene-Hydroxyapatite Nanocomposites For Potential Bioimplant Applications. ChemistrySelect 2023, 8, e202204585. [Google Scholar] [CrossRef]
- Kumar Yadav, M.; Hiren Shukla, R.; Prashanth, K.G. A comprehensive review on development of waste derived hydroxyapatite (HAp) for tissue engineering application. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Lertcumfu, N.; Jarupoom, P.; Pengpat, K.; Tunkasiri, T.; Rujijanagul, G. Effect of Metal Oxide Nanoparticles Addition on Physical Properties of Hydroxyapatite. Adv. Mater. Res. 2012, 506, 234–237. [Google Scholar] [CrossRef]
- He, M.; Chen, L.; Yin, M.; Xu, S.; Liang, Z. Review on magnesium and magnesium-based alloys as biomaterials for bone immobilization. J. Mater. Res. Technol. 2023, 23, 4396–4419. [Google Scholar] [CrossRef]
- Melinescu, A.; Ionita, G.; Trusca, R.; Preda, M. Ceramic porous materials obtained by the geopolymer route. Rev. Romana De Mater.-Rom. J. Mater. 2020, 50, 146–150. [Google Scholar]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu Păltânea, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Blašković, M.; Butorac Prpić, I.; Blašković, D.; Rider, P.; Tomas, M.; Čandrlić, S.; Botond Hangyasi, D.; Čandrlić, M.; Perić Kačarević, Ž. Guided Bone Regeneration Using a Novel Magnesium Membrane: A Literature Review and a Report of Two Cases in Humans. J. Funct. Biomater. 2023, 14, 307. [Google Scholar] [CrossRef]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef]
- Esfahanizadeh, N.; Montazeri, M.; Nourani, M.R.; Harandi, M. Use of bioactive glass doped with magnesium or strontium for bone regeneration: A rabbit critical-size calvarial defects study. Dent. Res. J. 2022, 19, 18. [Google Scholar] [CrossRef]
- Fu, Z.; Cui, J.; Zhao, B.; Shen, S.G.F.; Lin, K. An overview of polyester/hydroxyapatite composites for bone tissue repairing. J. Orthop. Transl. 2021, 28, 118–130. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, W.; Ngai, T. Polymer coatings on magnesium-based implants for orthopedic applications. J. Polym. Sci. 2022, 60, 32–51. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Tiomno, O.; Coelho, F.; Pellizaro, T.; Chanfrau, R.; Capote, T.; Basmaji, P.; Veranes Pantoja, Y.; Carlos, G. Preparation of Scaffolds of Amorphous Calcium Phosphate and Bacterial Cellulose for Use in Tissue Regeneration by Freeze-Drying Process. Biointerface Res. Appl. Chem. 2021, 11, 7357–7367. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.M.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D₃. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, E.M.; Zoccolotti, J.D.; Tiomnova, O.T.; Tolaba, A.G.; Chanfrau, J.E.R.; Jorge, J.H.; Basmaji, P.; Guastaldi, A.C. Sterilization of scaffolds of calcium phosphates and bacterial cellulose for their use in tissue regeneration. Biointerface Res. Appl. Chem. 2021, 11, 10089–10098. [Google Scholar]
- Hernández-Montes, V.; Buitrago-Sierra, R.; Echeverry-Rendón, M.; Santa-Marín, J.F. Ceria-based coatings on magnesium alloys for biomedical applications: A literature review. RSC Adv. 2023, 13, 1422–1433. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Mao, X. Magnesium Enhances Osteogenesis of BMSCs by Tuning Osteoimmunomodulation. BioMed Res. Int. 2019, 2019, 7908205. [Google Scholar] [CrossRef]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone 2022, 154, 116233. [Google Scholar] [CrossRef]
- Uppal, G.; Thakur, A.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloys 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Vieira, E.; Silva, M.; Maia-Filho, A.; Ferreira, D.; Figuerêdo-Silva, J.; Rovaris, K.; Fialho, A.C.; Leite-Oliveira, A.; Menezes de Oliveira, A.L.; da Fonseca, M.G.; et al. Effect of Cerium-Containing Hydroxyapatite in Bone Repair in Female Rats with Osteoporosis Induced by Ovariectomy. Minerals 2021, 11, 377. [Google Scholar] [CrossRef]
- Allu, I.; Kumar Sahi, A.; Kumari, P.; Sakhile, K.; Sionkowska, A.; Gundu, S. A Brief Review on Cerium Oxide (CeO2NPs)-Based Scaffolds: Recent Advances in Wound Healing Applications. Micromachines 2023, 14, 865. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.; Shepherd, J.; Asencio, I.O. The Use of Cerium Compounds as Antimicrobials for Biomedical Applications. Molecules 2022, 27, 2678. [Google Scholar] [CrossRef] [PubMed]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhai, X.; Ma, T.; Huang, Y.; Jin, M.; Yang, H.; Fu, H.; Zhang, S.; Sun, T.; Jin, X.; et al. Sequential Therapy for Bone Regeneration by Cerium Oxide-Reinforced 3D-Printed Bioactive Glass Scaffolds. ACS Nano 2023, 17, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Stephen Inbaraj, B.; Chen, B.-H. An overview on recent in vivo biological application of cerium oxide nanoparticles. Asian J. Pharm. Sci. 2020, 15, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of Cerium oxide nanoparticles—A Review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and Its Oxidant-Based Nanomaterials for Antibacterial Applications: A State-of-the-Art Review. Front. Mater. 2020, 7, 213. [Google Scholar] [CrossRef]
- Pop, O.L.; Mesaros, A.; Vodnar, D.C.; Suharoschi, R.; Tăbăran, F.; Magerușan, L.; Tódor, I.S.; Diaconeasa, Z.; Balint, A.; Ciontea, L.; et al. Cerium Oxide Nanoparticles and Their Efficient Antibacterial Application In Vitro against Gram-Positive and Gram-Negative Pathogens. Nanomaterials 2020, 10, 1614. [Google Scholar] [CrossRef]
- Hosseini, M.; Mozafari, M. Cerium Oxide Nanoparticles: Recent Advances in Tissue Engineering. Materials 2020, 13, 3072. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Al Bakri Abdullah, M.M.; Abd Rahim, S.Z.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabiałek, M. Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 2022, 48, 14959–14979. [Google Scholar] [CrossRef]
- Schmidt, R.; Prado-Gonjal, J.; Morán, E. Microwaves: Microwave Assisted Hydrothermal Synthesis of Nanoparticles; CRC Press: Boca Raton, FL, USA, 2015; pp. 561–572. [Google Scholar]
- Ebrahimi, S.; Stephen Sipaut@ Mohd Nasri, C.; Bin Arshad, S.E. Hydrothermal synthesis of hydroxyapatite powders using Response Surface Methodology (RSM). PLoS ONE 2021, 16, e0251009. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Liaqat, U.; Hussain, Z.; Sohail, M. Synthesis, Characterization and Process Optimization of Bone Whitlockite. Nanomaterials 2020, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A. Magnesium whitlockite—Omnipresent in pathological mineralisation of soft tissues but not a significant inorganic constituent of bone. Acta Biomater. 2021, 125, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.M.; Portela, T.O.; Correa, G.S.; Oliveira, M.M.; Rangel, J.H.G.; Rodrigues, S.F.; Mercury, J.M.R. Synthesis of hydroxyapatite by hydrothermal and microwave irradiation methods from biogenic calcium source varying pH and synthesis time. Boletín Soc. Española Cerámica Y Vidr. 2022, 61, 35–41. [Google Scholar] [CrossRef]
- Paduraru, A.V.; Musuc, A.M.; Oprea, O.C.; Trusca, R.; Iordache, F.; Vasile, B.S.; Andronescu, E. Synthesis and Characterization of Photoluminescent Ce(III) and Ce(IV) Substituted Hydroxyapatite Nanomaterials by Co-Precipitation Method: Cytotoxicity and Biocompatibility Evaluation. Nanomaterials 2021, 11, 1911. [Google Scholar] [CrossRef]
- Jang, H.L.; Lee, H.K.; Jin, K.; Ahn, H.-Y.; Lee, H.-E.; Nam, K.T. Phase transformation from hydroxyapatite to the secondary bone mineral, whitlockite. J. Mater. Chem. B 2015, 3, 1342–1349. [Google Scholar] [CrossRef]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef]
- Lagier, R.; Baud, C.A. Magnesium Whitlockite, a Calcium Phosphate Crystal of Special Interest in Pathology. Pathol. Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef]
- McComiskey, K.P.M.; Tajber, L. Comparison of particle size methodology and assessment of nanoparticle tracking analysis (NTA) as a tool for live monitoring of crystallisation pathways. Eur. J. Pharm. Biopharm. 2018, 130, 314–326. [Google Scholar] [CrossRef]
- Singh, G.; Jolly, S.S.; Singh, R.P. Cerium substituted hydroxyapatite mesoporous nanorods: Synthesis and characterization for drug delivery applications. Mater. Today: Proc. 2020, 28, 1460–1466. [Google Scholar] [CrossRef]
- Cegla, R.-N.; Macha, I.; Ben-Nissan, B.; Grossin, D.; Heness, G.; Chung, R.-J. Comparative Study of Conversion of Coral with Ammonium Dihydrogen Phosphate and Orthophosphoric Acid to Produce Calcium Phosphates. J. Aust. Ceram. Soc. 2014, 50, 154–161. [Google Scholar]
- Nigar, F.; Johnston, A.-L.; Smith, J.; Oakley, W.; Islam, T.; Felfel, R.; Grant, D.; Lester, E.; Ahmed, I. Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived Products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy. Materials 2023, 16, 2138. [Google Scholar] [CrossRef] [PubMed]
- Chanfrau, R. Evaluation of the influence of microwaves radiation on a biomaterial composed of three phases of calcium phosphates. Biointerface Res. Appl. Chem. 2020, 10, 5141–5144. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Negut, I.; Dumitrescu, M.F.; Stan, M.S.; Nica, I.C.; Holban, A.M.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857. [Google Scholar] [CrossRef]
- El Makhloufy, S.; Oubouaza, R.; Ouasri, A.; Belaaouad, S. X-Ray diffraction and infrared spectroscopy data review analyses of the Calcium phosphates. Biointerface Res. Appl. Chem. 2022, 12, 732–755. [Google Scholar]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi, M.; Abu Osman, N.A. Magnesium incorporated hydroxyapatite: Synthesis and structural properties characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef] [Green Version]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Koski, C.; Vu, A.A.; Bose, S. Effects of chitosan-loaded hydroxyapatite on osteoblasts and osteosarcoma for chemopreventative applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111041. [Google Scholar] [CrossRef]
- Omidi, M.; Ahmad Agha, N.; Müller, A.; Feyerabend, F.; Helmholz, H.; Willumeit-Römer, R.; Schlüter, H.; Luthringer-Feyerabend, B.J.C. Investigation of the impact of magnesium versus titanium implants on protein composition in osteoblast by label free quantification†. Metallomics 2020, 12, 916–934. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahrabalee, S.Q.; Jaber, H.A. Bioinorganic Preparation of Hydroxyapatite and Rare Earth Substituted Hydroxyapatite for Biomaterials Applications. Bioinorg. Chem. Appl. 2023, 2023, 7856300. [Google Scholar] [CrossRef] [PubMed]
- Farazin, A.; Aghadavoudi, F.; Motififard, M.; Saber-Samandari, S.; Khandan, A. Nanostructure, Molecular Dynamics Simulation and Mechanical Performance of PCL Membranes Reinforced with Antibacterial Nanoparticles. J. Appl. Comput. Mech. 2021, 7, 1907–1915. [Google Scholar] [CrossRef]
- Baskaran, P.; Udduttula, A.; Uthirapathy, V. Development and characterisation of novel Ce-doped hydroxyapatite–Fe3 O4 nanocomposites and their in vitro biological evaluations for biomedical applications. IET Nanobiotechnol. 2018, 12, 138–146. [Google Scholar] [CrossRef]
- Gonzalez-Moreno, M.; Morovic, P.; Tkhilaishvili, T.; Trampuz, A. Bacteriophages for Treatment of Biofilm Infections. In Bone and Joint Infections; Wiley: Hoboken, NJ, USA, 2021; pp. 65–80. [Google Scholar]
- Wang, H.; Xiong, C.; Yu, Z.; Zhang, J.; Huang, Y.; Zhou, X. Research Progress on Antibacterial Coatings for Preventing Implant-Related Infection in Fractures: A Literature Review. Coatings 2022, 12, 1921. [Google Scholar] [CrossRef]
- Silva-Holguín, P.N.; Reyes-López, S.Y. Synthesis of Hydroxyapatite-Ag Composite as Antimicrobial Agent. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820951342. [Google Scholar] [CrossRef]
- Oshima, S.; Sato, T.; Honda, M.; Suetsugu, Y.; Ozeki, K.; Kikuchi, M. Fabrication of Gentamicin-Loaded Hydroxyapatite/Collagen Bone-Like Nanocomposite for Anti-Infection Bone Void Fillers. Int. J. Mol. Sci. 2020, 21, 551. [Google Scholar] [CrossRef] [Green Version]
- Bee, S.L.; Bustami, Y.; Ul-Hamid, A.; Lim, K.; Abdul Hamid, Z.A. Synthesis of silver nanoparticle-decorated hydroxyapatite nanocomposite with combined bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2021, 32, 106. [Google Scholar] [CrossRef]
- Coulon, A.; Laurencin, D.; Grandjean, A.; Cau Dit Coumes, C.; Rossignol, S.; Campayo, L. Immobilization of iodine into a hydroxyapatite structure prepared by cementation. J. Mater. Chem. A 2014, 2, 20923–20932. [Google Scholar] [CrossRef]
- Surmeneva, M.; Sharonova, A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.; Shulepov, I.; Epple, M.; Surmenev, R. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surf. B Biointerfaces 2017, 156, 104–113. [Google Scholar] [CrossRef]
- Guangjian, D.A.I.; Aili, Y.U.; Xiang, C.A.I.; Qingshan, S.H.I.; Ouyang, Y.; Shaozao, T.A.N. Synthesis, characterization and antimicrobial activity of zinc and cerium co-doped α-zirconium phosphate. J. Rare Earths 2012, 30, 820–825. [Google Scholar]
- Carmen, C.; Popa, C.; Predoi, D. Cerium doped hydroxyapatite nanoparticles synthesized by coprecipitation method. J. Serbian Chem. Soc. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Zheng, J.X.; Li, H.; Pu, Z.Y.; Wang, H.Y.; Deng, X.B.; Liu, X.J.; Deng, Q.W.; Yu, Z.J. Bloodstream infections caused by Enterococcus spp: A 10-year retrospective analysis at a tertiary hospital in China. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Braz, V.S.; Melchior, K.; Moreira, C.G. Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Front. Cell. Infect. Microbiol. 2020, 10, 548492. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [Green Version]
- Coelho, C.C.; Araújo, R.; Quadros, P.A.; Sousa, S.R.; Monteiro, F.J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Mater. Sci. Eng. C 2019, 97, 529–538. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Yamamoto, O.; Ohira, T.; Alvarez, K.; Fukuda, M. Antibacterial characteristics of CaCO3–MgO composites. Mater. Sci. Eng. B 2010, 173, 208–212. [Google Scholar] [CrossRef]
- Chou, Y.-F.; Chiou, W.-A.; Xu, Y.; Dunn, J.C.Y.; Wu, B.M. The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials 2004, 25, 5323–5331. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Padrão, T.; Costa, L.; Pinto, M.T.; Costa, P.C.; Domingues, V.F.; Quadros, P.A.; Monteiro, F.J.; Sousa, S.R. The antibacterial and angiogenic effect of magnesium oxide in a hydroxyapatite bone substitute. Sci. Rep. 2020, 10, 19098. [Google Scholar] [CrossRef] [PubMed]






| Sample | Unit Cell Parameters | Average Crystallite Size [nm] | Crystallinity [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a [Å] | b [Å] | c [Å] | α [°] | β [°] | γ [°] | ||||
| HAp_150 | 9.431 | 9.431 | 6.880 | 90 | 90 | 120 | 18.65 | 27.45 | |
| HAp_250 | 9.425 | 9.425 | 6.880 | 90 | 90 | 120 | 46.13 | 40.49 | |
| HAp_Ce_1% | 9.444 | 9.444 | 6.877 | 90 | 90 | 120 | 16.15 | 22.99 | |
| HAp_Ce_3% | 9.449 | 9.449 | 6.873 | 90 | 90 | 120 | 12.16 | 21.13 | |
| HAp_Ce_5% | 9.455 | 9.455 | 6.865 | 90 | 90 | 120 | 10.42 | 18.31 | |
| HAp_Mg_1% | 9.446 | 9.446 | 6.878 | 90 | 90 | 120 | 12.81 | 25.50 | |
| HAp_Mg_3% | 9.446 | 9.446 | 6.876 | 90 | 90 | 120 | 12.79 | 26.54 | |
| HAp_Mg_5% | |||||||||
| * | HAp 50.1% | 9.450 | 9.450 | 6.878 | 90 | 90 | 120 | 12.23 | 30.99 |
| ● | Whitlockit 49.9% | 10.419 | 10.419 | 37.292 | 90 | 90 | 120 | 49.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burdusel, A.-C.; Neacsu, I.A.; Birca, A.C.; Chircov, C.; Grumezescu, A.-M.; Holban, A.M.; Curutiu, C.; Ditu, L.M.; Stan, M.; Andronescu, E. Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration. J. Funct. Biomater. 2023, 14, 378. https://doi.org/10.3390/jfb14070378
Burdusel A-C, Neacsu IA, Birca AC, Chircov C, Grumezescu A-M, Holban AM, Curutiu C, Ditu LM, Stan M, Andronescu E. Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration. Journal of Functional Biomaterials. 2023; 14(7):378. https://doi.org/10.3390/jfb14070378
Chicago/Turabian StyleBurdusel, Alexandra-Cristina, Ionela Andreea Neacsu, Alexandra Catalina Birca, Cristina Chircov, Alexandru-Mihai Grumezescu, Alina Maria Holban, Carmen Curutiu, Lia Mara Ditu, Miruna Stan, and Ecaterina Andronescu. 2023. "Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration" Journal of Functional Biomaterials 14, no. 7: 378. https://doi.org/10.3390/jfb14070378
APA StyleBurdusel, A.-C., Neacsu, I. A., Birca, A. C., Chircov, C., Grumezescu, A.-M., Holban, A. M., Curutiu, C., Ditu, L. M., Stan, M., & Andronescu, E. (2023). Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration. Journal of Functional Biomaterials, 14(7), 378. https://doi.org/10.3390/jfb14070378














