Blending Ethnomedicine with Modern Technology—From Conventional to Tailored Products: Modulating Biopharmaceutical Properties of Berberis Extract by Solid Lipid Nanoparticles for Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Preparation of Berberis Extract
2.4. Spectrophotometric Analysis of Berberine
2.5. Preparation of Berberis Extract-Loaded Nanoformulation
2.5.1. Preparation of SLNs by High-Pressure Homogenization
2.5.2. Preparation of Berberis Extract SLNs Gel
2.5.3. Characterization of SLNs
Optical Microscopy
Field Emission Scanning Electron Microscopy (FESEM)
Particle Size and Polydispersity Index (PDI)
Total Drug Content (TDC)
Entrapment Efficiency (EE)
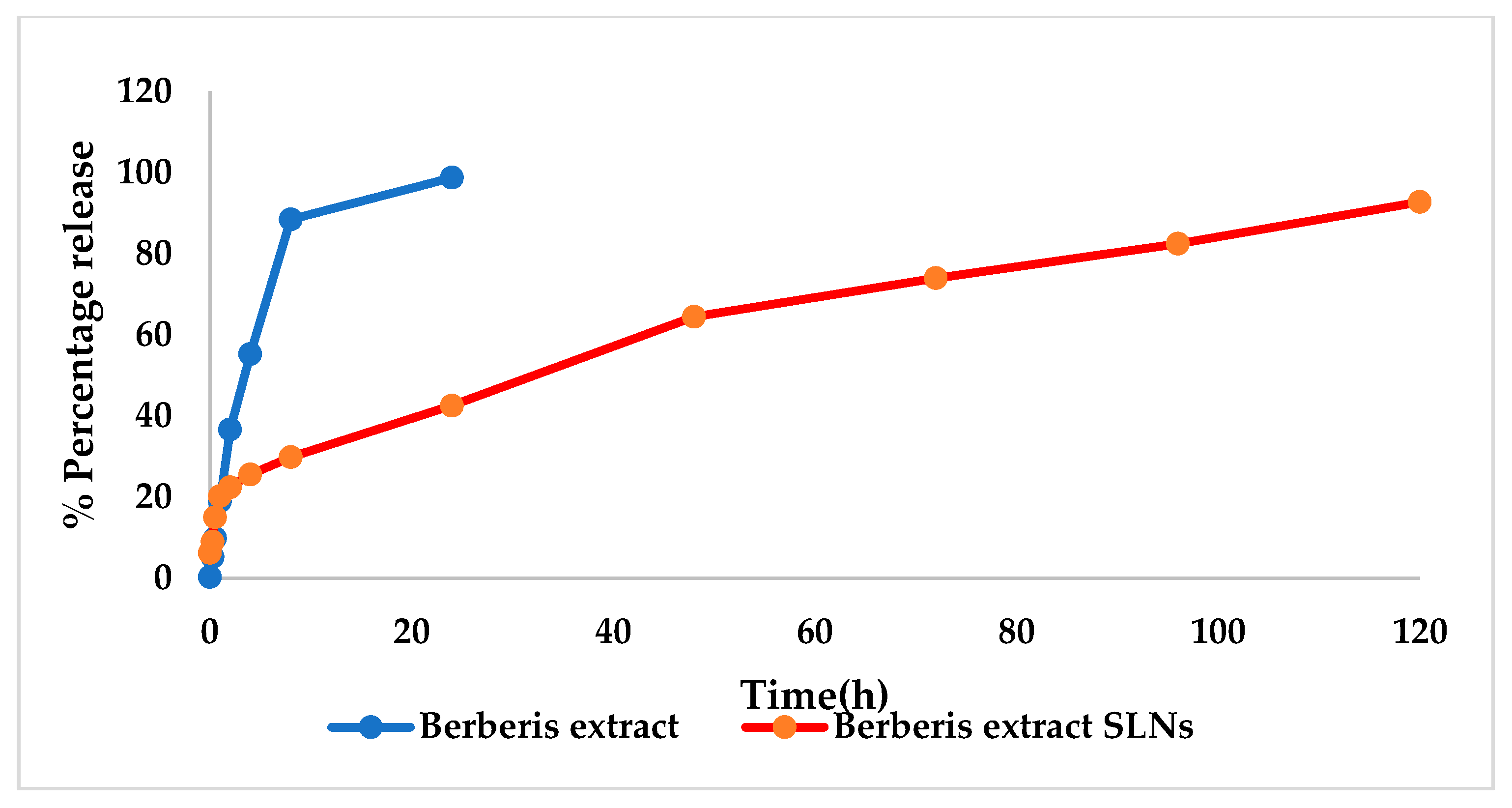
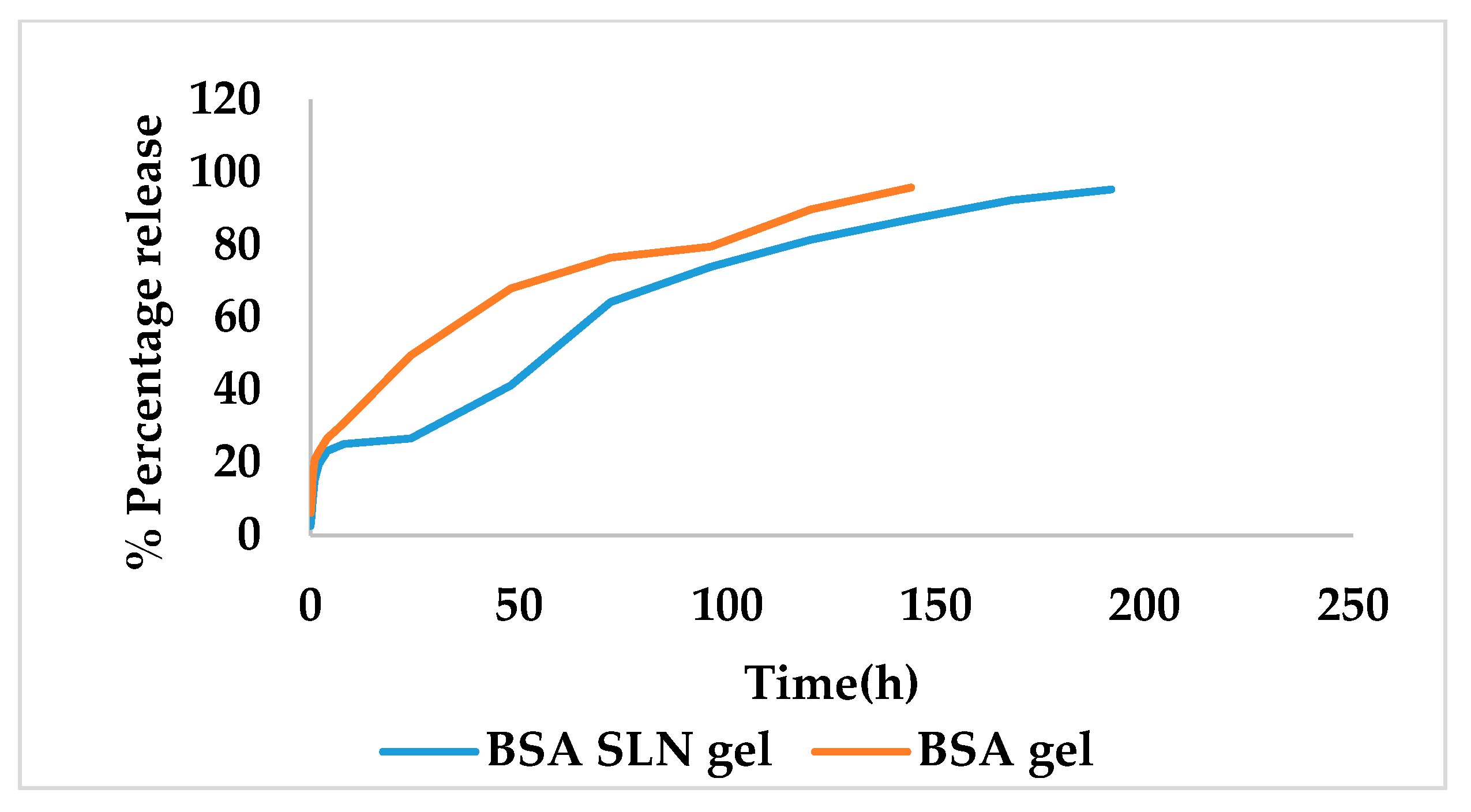
In Vitro Release Studies
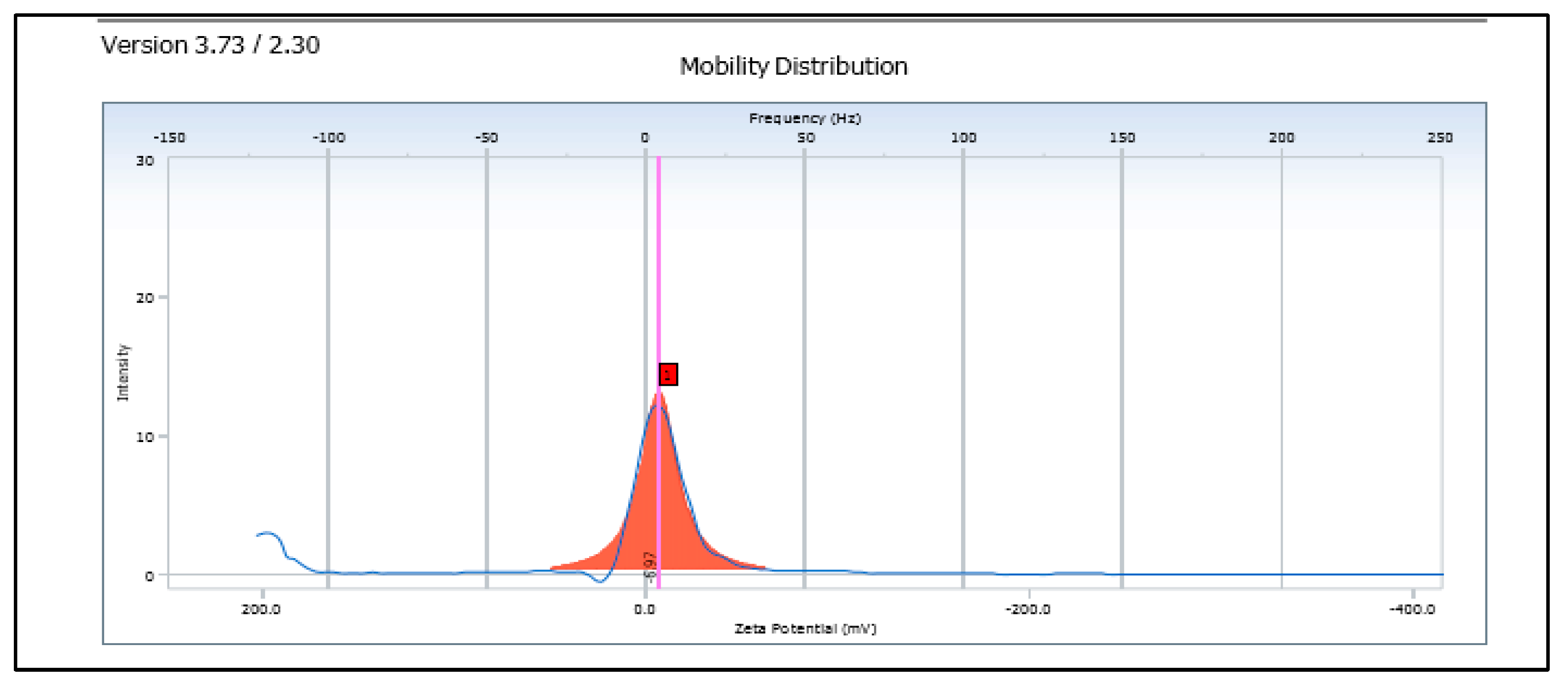
Zeta Potential
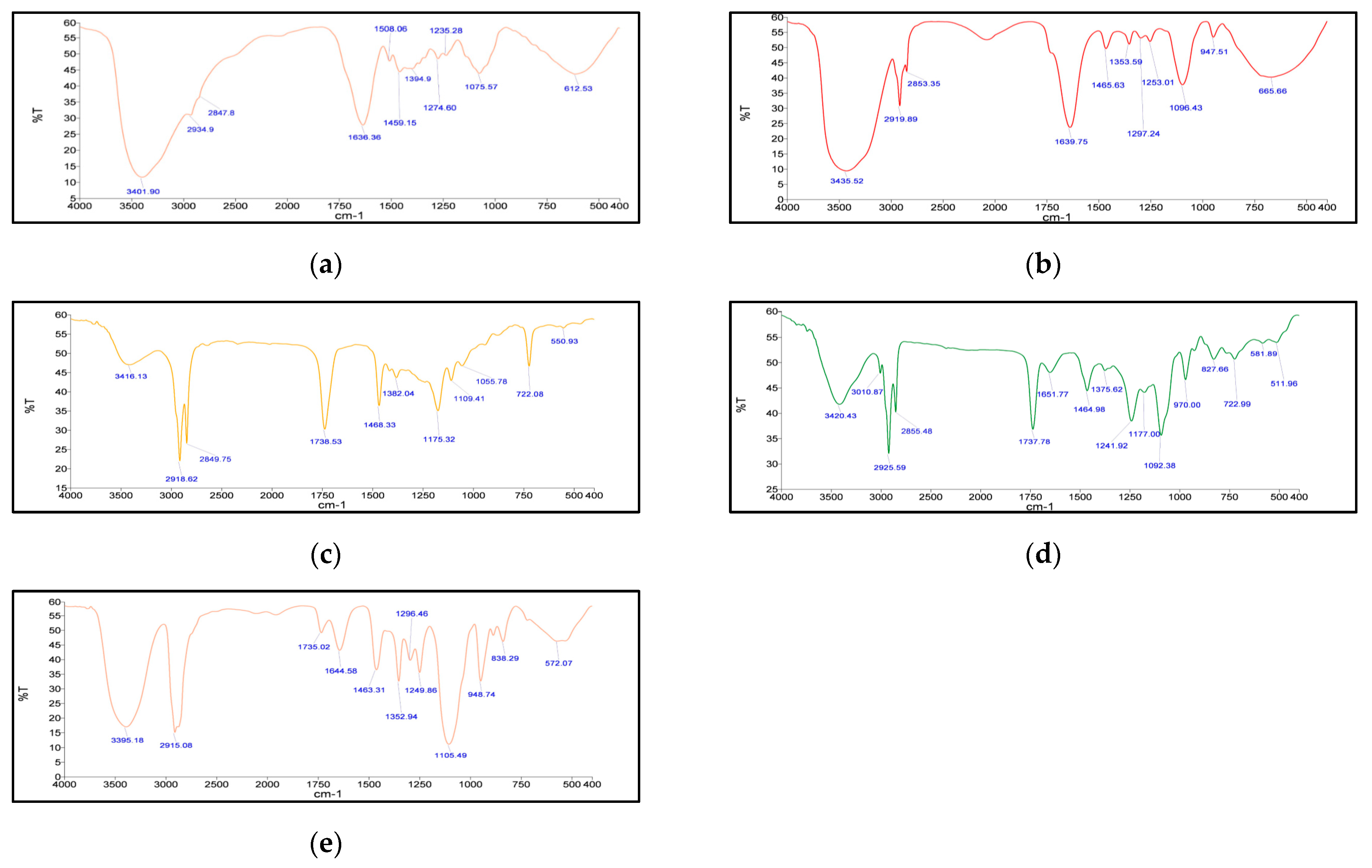
Fourier Transform Infrared Spectroscopy (FT–IR)
Differential Scanning Calorimetry (DSC)
Hot-Stage Microscopy (HSM)
Thermogravimetric Analysis (TGA)
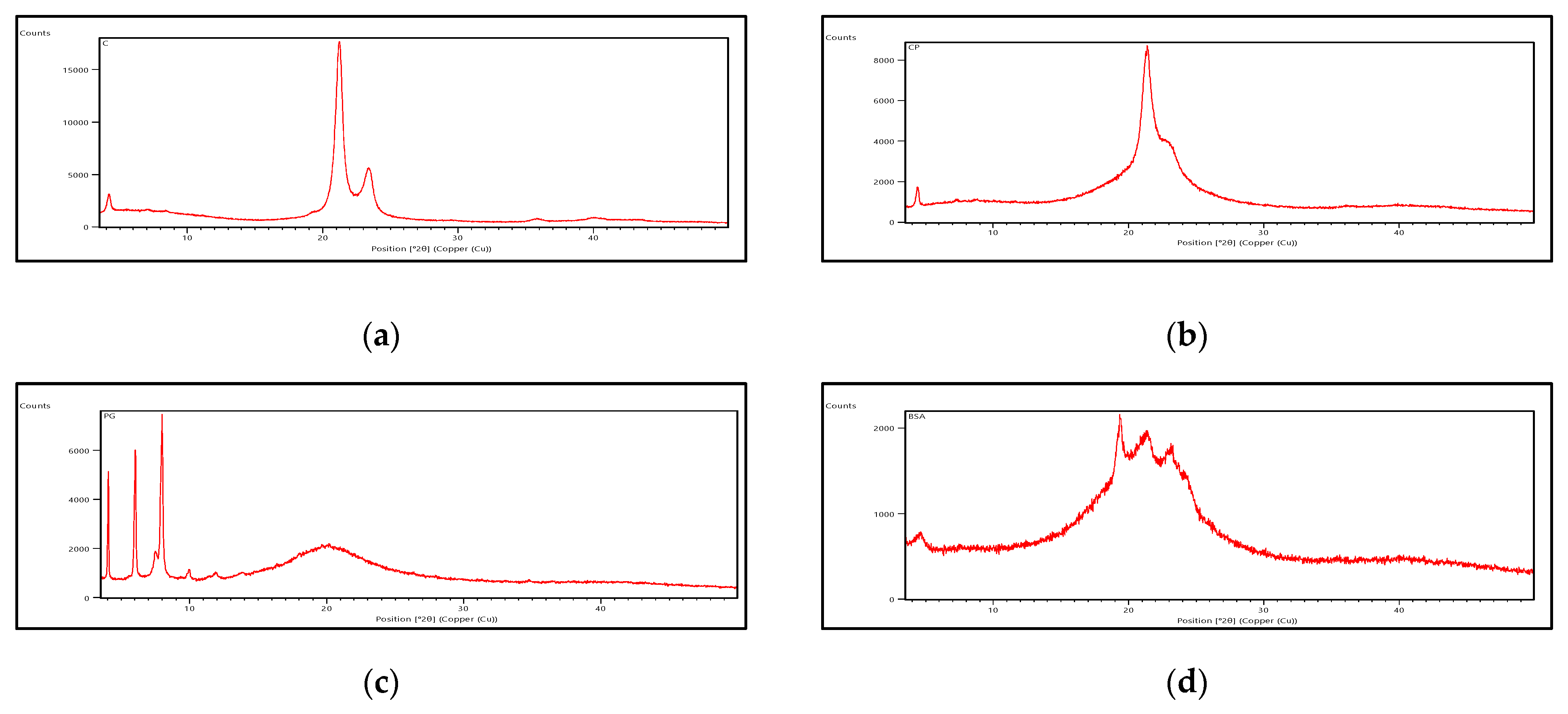
Powder X-ray Diffraction (PXRD)
Rheological Studies
pH
Texture Analysis
2.6. In Vivo Wound Healing Activity
2.6.1. Treatment
2.6.2. Biochemical Estimations
2.6.3. Histopathological Analysis
2.7. Statistical Analysis
3. Results
3.1. Preparation of Berberis Extract
3.2. Development of Nanoformulation of Berberis Extract
Preparation of Freeze-Dried Berberis Extract-Loaded SLNs
3.3. Characterization of SLNs
3.4. Characterization of Gel Formulation Incorporating SLN Dispersion
3.5. Acute Dermal Irritation Studies
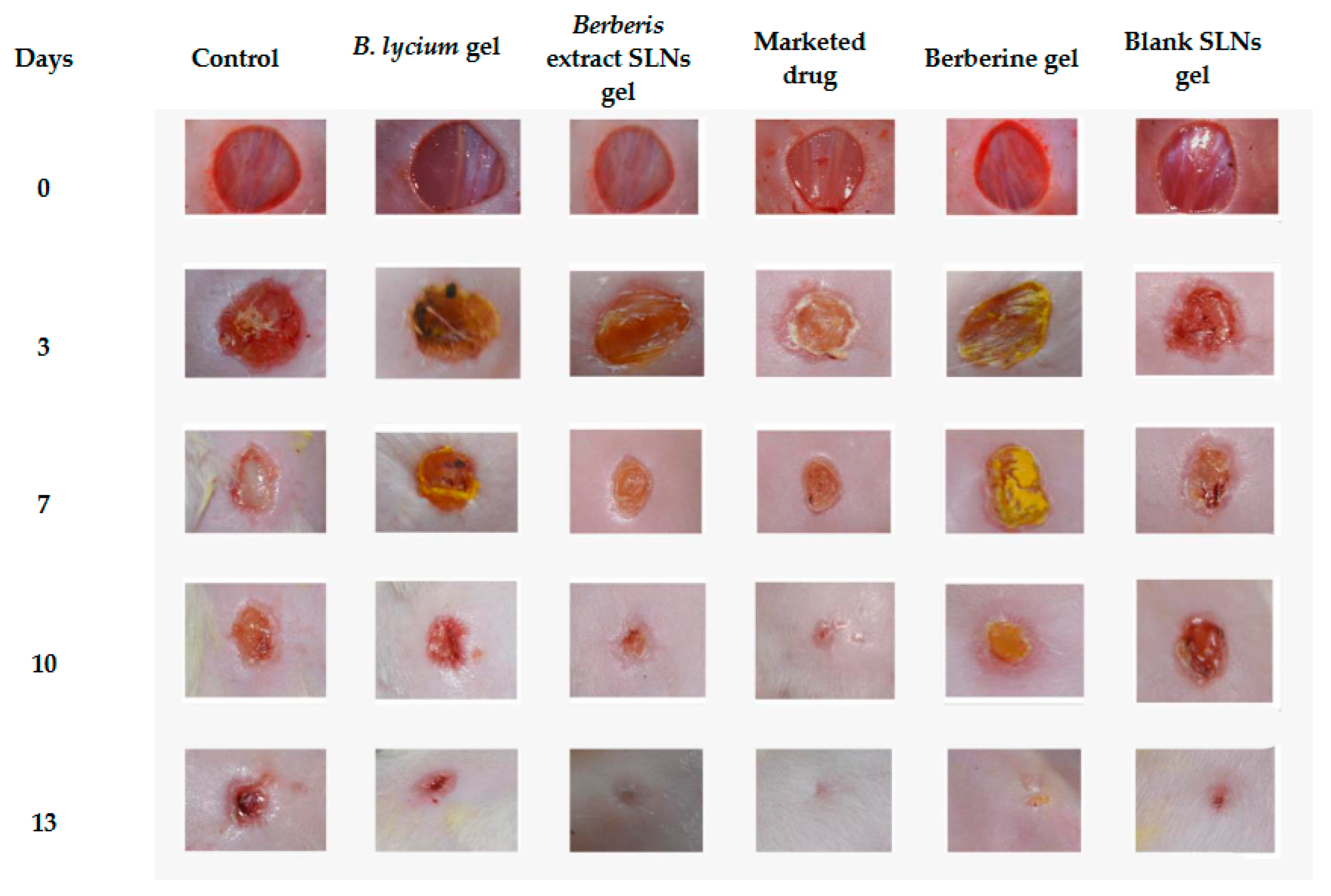
3.6. Excision-Wound Model
3.7. Biochemical Investigations
- a.
- Protein estimation
- b.
- Measurement of free radical and antioxidant level
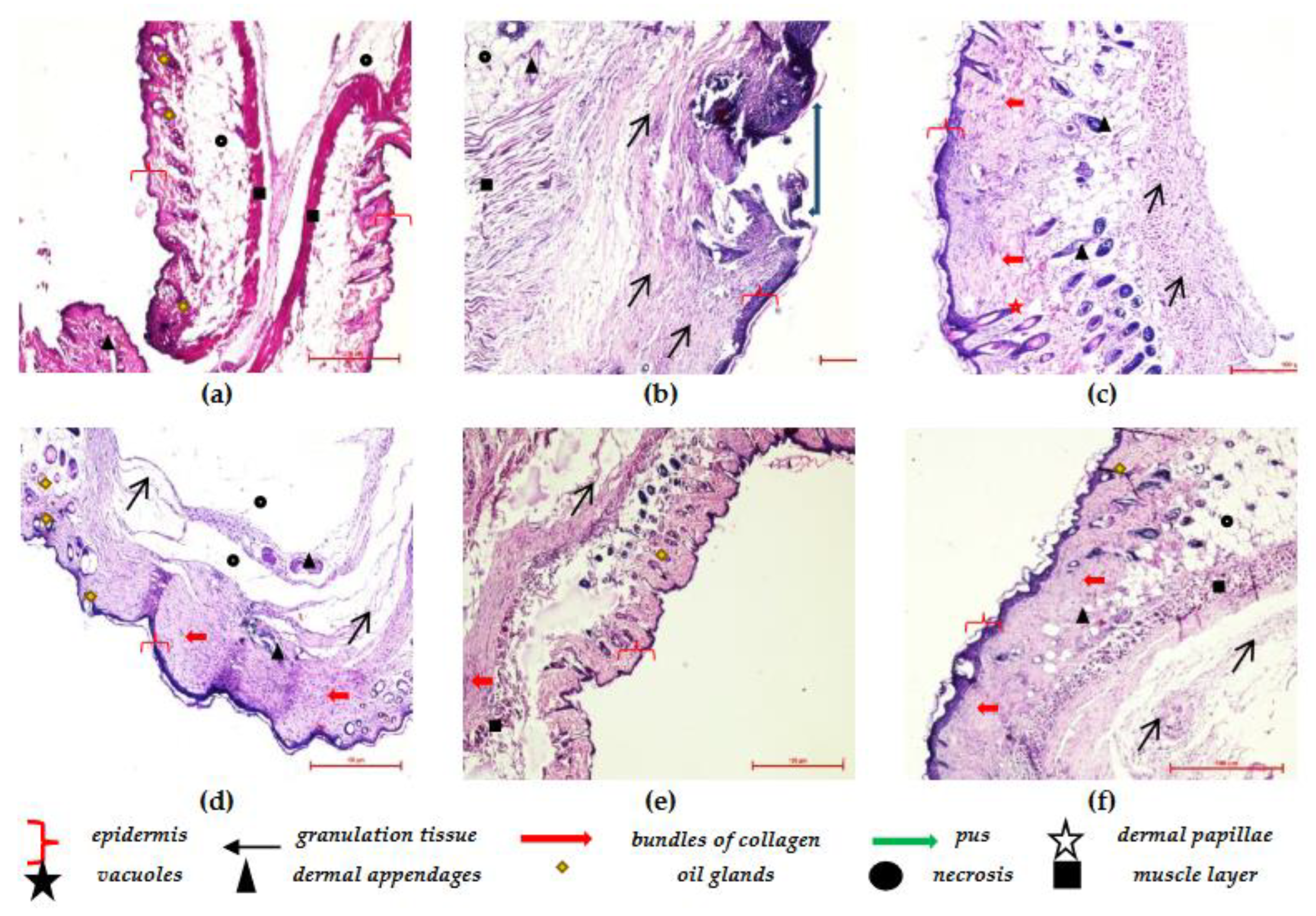
3.8. Histopathological Studies
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shende, P.; Narvenker, R. Herbal nanotherapy: A new paradigm over conventional obesity treatment. J. Drug Deliv. Sci. Technol. 2020, 61, 102291. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Carmona, F.; Pereira, A.M.S. Herbal medicines: Old and new concepts, truths and misunderstandings. Rev. Bras. Farm. 2013, 23, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Mishra, Y.; Amin, H.I.M.; Mishra, V.; Vyas, M.; Prabhakar, P.K.; Gupta, M.; Kanday, R.; Sudhakar, K.; Saini, S.; Hromić-Jahjefendić, A.; et al. Application of nanotechnology to herbal antioxidants as improved phytomedicine: An expanding horizon. Biomed. Pharmacother. 2022, 153, 113413. [Google Scholar] [CrossRef]
- Ajazuddin; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Potdar, D.; Hirwani, R.; Dhulap, S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef]
- Jabeen, N.; Saleem, A.; Anwaar, S.; Hussain, Z. Berberis lycium Royle (Royle, 1837): A Threatened Medicinal Plant and Its Biological Activities. 2015. Available online: https://www.semanticscholar.org/paper/Berberis-lycium-Royle-(-Royle-%2C-1837-)-%3A-A-Plant-Jabeen-Saleem/8c0d5c9ccdcbf23d9d8b4050e7a7f484cb9410b5 (accessed on 12 March 2023).
- Malhotra, B.; Kulkarni, G.T.; Dhiman, N.; Joshi, D.; Chander, S.; Kharkwal, A.; Sharma, A.K.; Kharkwal, H. Recent advances on Berberis aristata emphasizing berberine alkaloid including phytochemistry, pharmacology and drug delivery system. J. Herb. Med. 2021, 27, 100433. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, V.; Kumar, S.; Arya, K.R.; Sharma, K.R.; Kumar, B. Quantitative determination of isoquinoline alkaloids and chlorogenic acid in Berberis species using ultra high performance liquid chromatography with hybrid triple quadrupole linear ion trap mass spectrometry. J. Sep. Sci. 2015, 38, 2007–2013. [Google Scholar] [CrossRef]
- Ikram, M. A review on the chemical and pharmacological aspects of genus Berberis. Planta Medica 1975, 28, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- Cui, H.-X.; Hu, Y.-N.; Li, J.-W.; Yuan, K.; Guo, Y. Preparation and Evaluation of Antidiabetic Agents of Berberine Organic Acid Salts for Enhancing the Bioavailability. Molecules 2018, 24, 103. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kwon, M.; Han, J.; Kim, J.; Ahn, J.; Jeong, E. Nano-Particles Containing Water-Soluble Extracts from Berberis Koreana Entrapped into Gelatin, a Manufacturing Method Thereof and a Decreasing Method of Cytotoxicity Using the Same. KR100998534B1, 7 December 2010. Available online: https://patents.google.com/patent/KR100998534B1/en?oq=KR100998534B1 (accessed on 26 March 2023).
- Tamarkin, D.; Eini, M.; Friedman, D.; Besonov, A.; Schuz, D.; Berman, T.; Danziger, J.; Keynan, R.; Zlatkis, E. Hydrophilic, Non-Aqueous Pharmaceutical Carriers and Compositions and Uses. Google Patents US8486374B2, 16 July 2013. Available online: https://patents.google.com/patent/US8486374B2/en (accessed on 26 March 2023).
- Kwon, T.K.; Lee, H.Y.; Kim, J.D.; Shin, W.C.; Park, S.K.; Kim, J.-C. In vitro skin permeation of cubosomes containing water soluble extracts of Korean barberry. Colloid J. 2010, 72, 205–210. [Google Scholar] [CrossRef]
- Mehmood, A.; Murtaza, G.; Bhatti, T.M.; Kausar, R.; Ahmed, M.J. Biosynthesis, characterization and antimicrobial action of silver nanoparticles from root bark extract of Berberis lycium Royle. Pak. J. Pharm. Sci. 2016, 29, 131–137. [Google Scholar]
- Karan, M.; Vasisht, K.; Sharma, N.; Kaur, I.P.; Gautam, V.; Sandhu, S.K.; Kaur, J. Berberis Extract Nano-Formulation and Process of Preparation Thereof. WO2022168124A1, 11 August 2022. Available online: https://patents.google.com/patent/WO2022168124A1/en?q=(freeze+dried+berberis)&oq=freeze+dried+berberis (accessed on 26 March 2023).
- Kaur, A.; Goindi, S.; Katare, O.P. Formulation, characterisation and in vivo evaluation of lipid-based nanocarrier for topical delivery of diflunisal. J. Microencapsul. 2016, 33, 475–486. [Google Scholar] [CrossRef]
- Amorim, J.L.; Figueiredo, J.d.B.; Amaral, A.C.F.; Barros, E.G.d.O.; Palmero, C.; Mpalantinos, M.A.; Ramos, A.d.S.; Ferreira, J.L.P.; Silva, J.R.d.A.; Benjamim, C.F.; et al. Wound healing properties of Copaifera paupera in diabetic mice. PLoS ONE 2017, 12, e0187380. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.; Gautam, M.K.; Goel, S.; Purohit, V.; Sharma, H.; Goel, R.K. Evaluation of In Vivo Wound Healing Activity of Bacopa monniera on Different Wound Model in Rats. BioMed Res. Int. 2013, 2013, 972028. [Google Scholar] [CrossRef] [Green Version]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [PubMed]
- Wills, E. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966, 99, 667–676. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.A. Handbook Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Kent, K.; Harper, W.; Bomser, J. Effect of whey protein isolate on intracellular glutathione and oxidant-induced cell death in human prostate epithelial cells. Toxicol. Vitr. 2002, 17, 27–33. [Google Scholar] [CrossRef]
- Ilyas, S.; Tabasum, R.; Iftikhar, A.; Nazir, M.; Hussain, A.; Hussain, A.; Ali, M.S.; Saleem, F.; Saleem, U.; Froeyen, M.; et al. Effect of Berberis vulgaris L. root extract on ifosfamide-induced in vivo toxicity and in vitro cytotoxicity. Sci. Rep. 2021, 11, 1708. [Google Scholar] [CrossRef]
- Khoshandam, A.; Imenshahidi, M.; Hosseinzadeh, H. Pharmacokinetic of berberine, the main constituent of Berberis vulgaris L.: A comprehensive review. Phytother. Res. 2022, 36, 4063–4079. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D. Nanostructured delivery systems in food: Latest developments and potential future directions. Curr. Opin. Food Sci. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the Antitumor Activity of Berberine Hydrochloride by Solid Lipid Nanoparticle Encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Setterstrom, J.A.; Tice, T.R.; Myers, W.E. Development of Encapsulated Antibiotics for Topical Administration to Wounds. In Recent Advances in Drug Delivery Systems; Anderson, J.M., Kim, S.W., Eds.; Springer US: Boston, MA, USA, 1984; pp. 185–198. [Google Scholar] [CrossRef]
- Deuschle, V.C.K.N.; Deuschle, R.A.N.; Bortoluzzi, M.R.; Athayde, M.L. Physical chemistry evaluation of stability, spreadability, in vitro antioxidant, and photo-protective capacities of topical formulations containing Calendula officinalis L. leaf extract. Braz. J. Pharm. Sci. 2015, 51, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Vandita, K.; Shashi, B.; Santosh, K.G.; Pal, K.I. Enhanced Apoptotic Effect of Curcumin Loaded Solid Lipid Nanoparticles. Mol. Pharm. 2012, 9, 3411–3421. [Google Scholar] [CrossRef]
- Boskabadi, M.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Hashemi, S.M.H.; Babaei, A. Topical Gel of Vitamin A Solid Lipid Nanoparticles: A Hopeful Promise as a Dermal Delivery System. Adv. Pharm. Bull. 2020, 11, 663–674. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Bennison, L.R.; Miller, C.N.; Summers, R.J.; Minnis, A.M.B.; Sussman, G.; McGuiness, W. The pH of wounds during healing and infection: A descriptive literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2017, 25, 63–69. [Google Scholar]
- Maver, T.; Kurečič, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Plant-Derived Medicines with Potential Use in Wound Treatment; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/58513 (accessed on 29 April 2023).








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Vasisht, K.; Kaur, J.; Sandhu, S.K.; Dey, K.; Hameed, B.A.; Bajaj, R.; Kaur, I.P.; Karan, M. Blending Ethnomedicine with Modern Technology—From Conventional to Tailored Products: Modulating Biopharmaceutical Properties of Berberis Extract by Solid Lipid Nanoparticles for Wound Healing. J. Funct. Biomater. 2023, 14, 418. https://doi.org/10.3390/jfb14080418
Sharma N, Vasisht K, Kaur J, Sandhu SK, Dey K, Hameed BA, Bajaj R, Kaur IP, Karan M. Blending Ethnomedicine with Modern Technology—From Conventional to Tailored Products: Modulating Biopharmaceutical Properties of Berberis Extract by Solid Lipid Nanoparticles for Wound Healing. Journal of Functional Biomaterials. 2023; 14(8):418. https://doi.org/10.3390/jfb14080418
Chicago/Turabian StyleSharma, Neetika, Karan Vasisht, Jasmine Kaur, Simarjot Kaur Sandhu, Kaustav Dey, Bakr Ahmed Hameed, Rakesh Bajaj, Indu Pal Kaur, and Maninder Karan. 2023. "Blending Ethnomedicine with Modern Technology—From Conventional to Tailored Products: Modulating Biopharmaceutical Properties of Berberis Extract by Solid Lipid Nanoparticles for Wound Healing" Journal of Functional Biomaterials 14, no. 8: 418. https://doi.org/10.3390/jfb14080418
APA StyleSharma, N., Vasisht, K., Kaur, J., Sandhu, S. K., Dey, K., Hameed, B. A., Bajaj, R., Kaur, I. P., & Karan, M. (2023). Blending Ethnomedicine with Modern Technology—From Conventional to Tailored Products: Modulating Biopharmaceutical Properties of Berberis Extract by Solid Lipid Nanoparticles for Wound Healing. Journal of Functional Biomaterials, 14(8), 418. https://doi.org/10.3390/jfb14080418






