Antibacterial Activity of ZnO Nanoparticles in a Staphylococcus-aureus-Infected Galleria mellonella Model Is Tuned by Different Apple-Derived Phytocargos
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
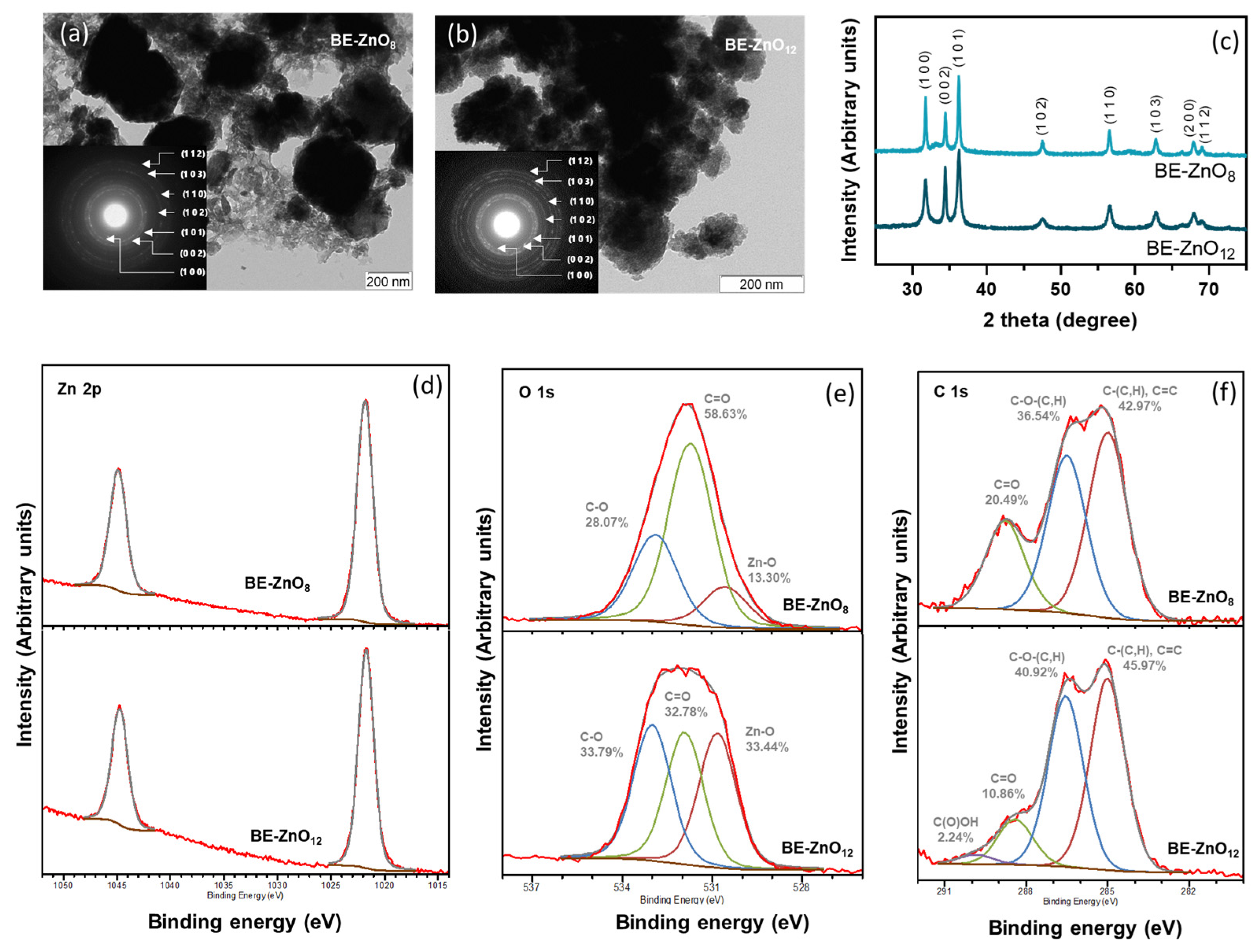
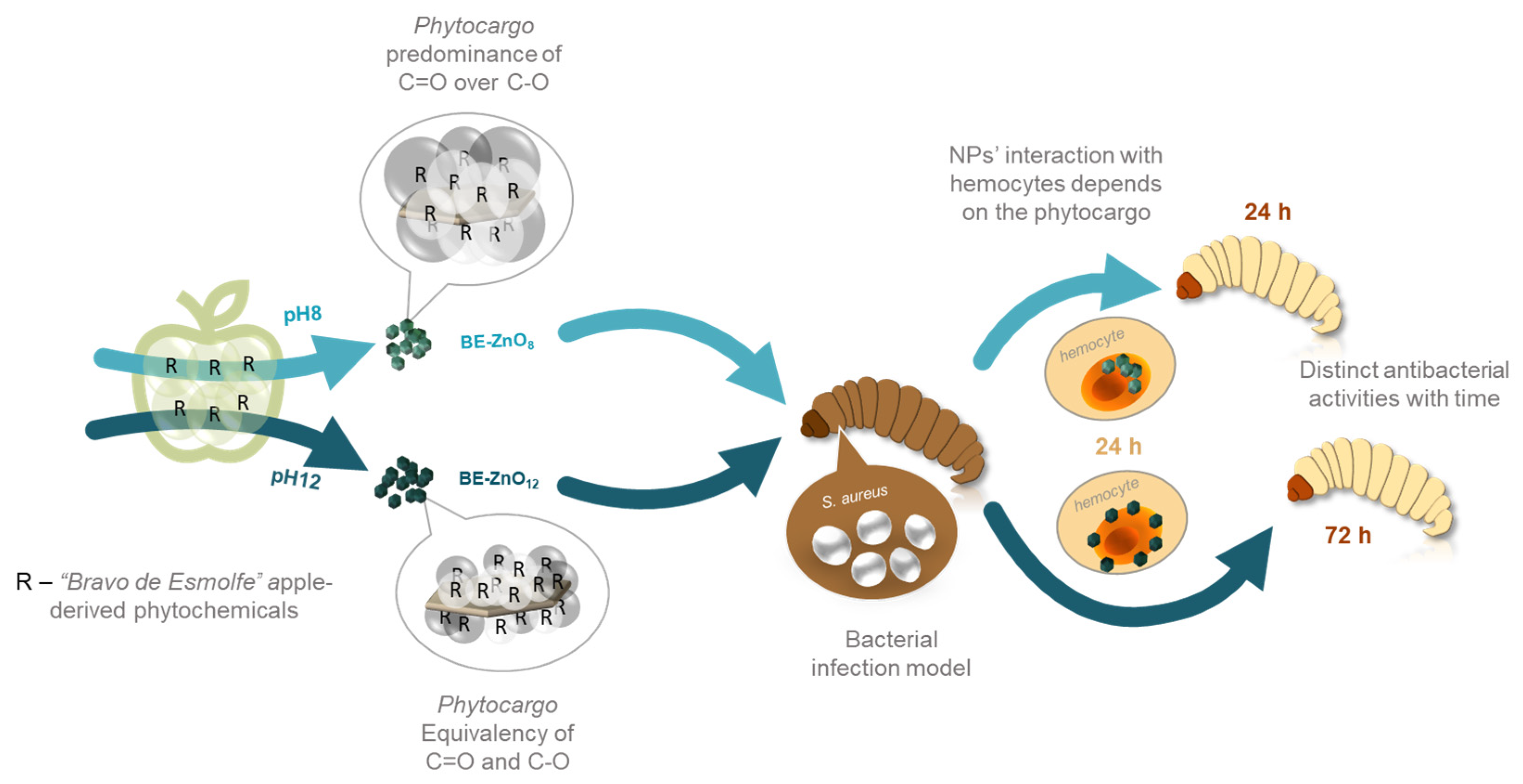
3.1. Physicochemical Properties of “Bravo de Esmolfe” Phytonanocarriers
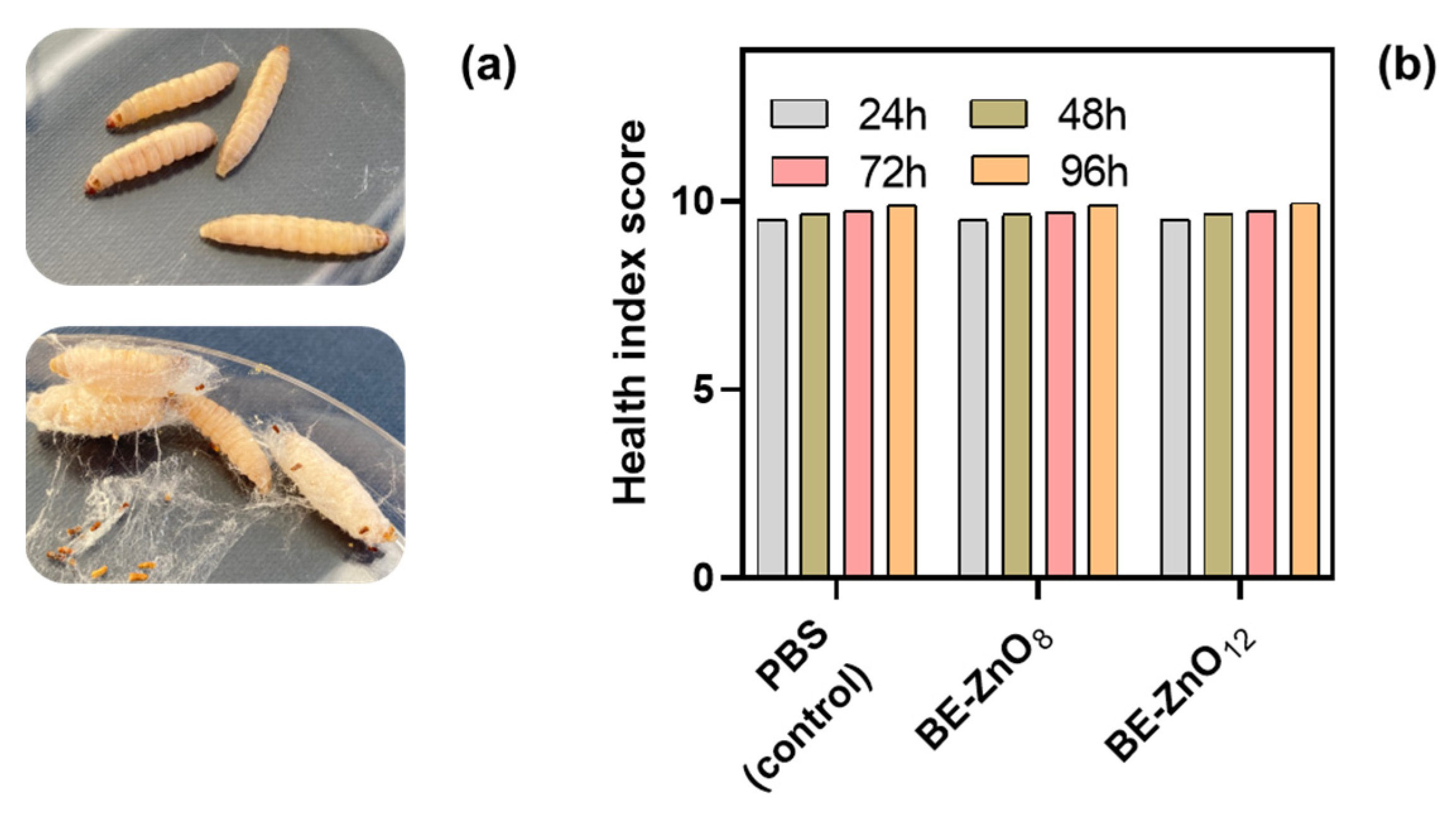
3.2. Toxicity of BE-ZnO Phytocarriers
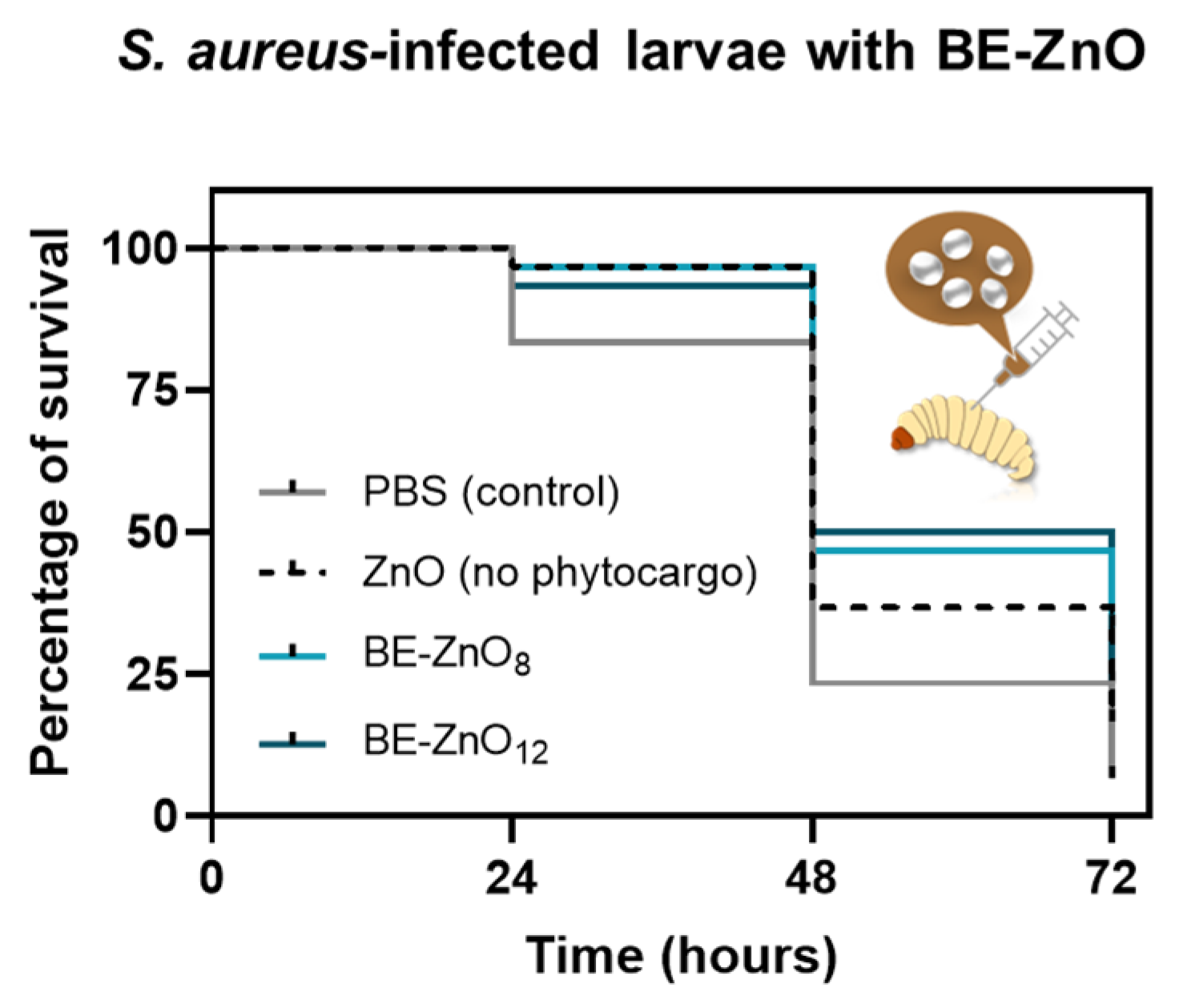
3.3. Antibacterial Activity of BE-ZnO NPs
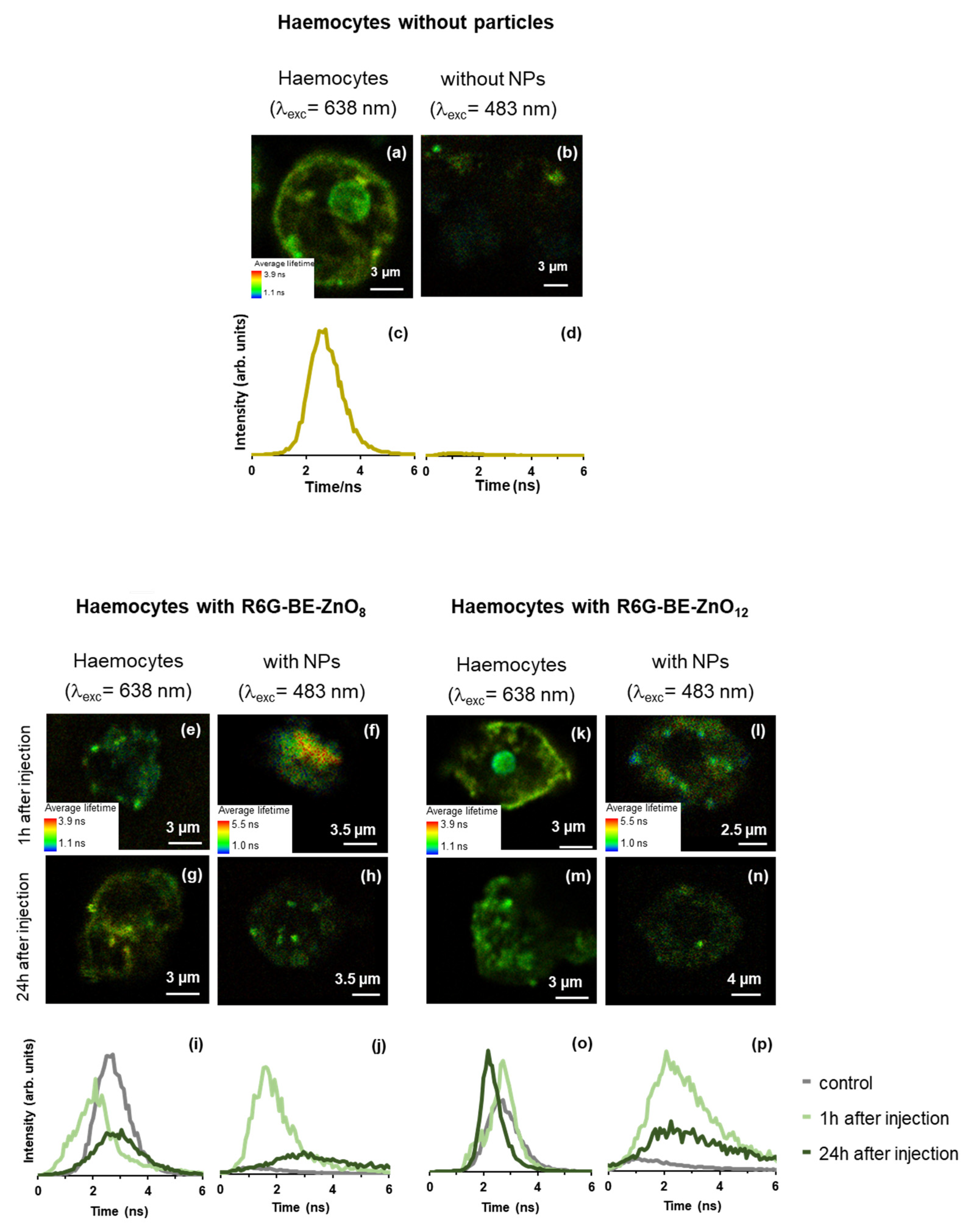
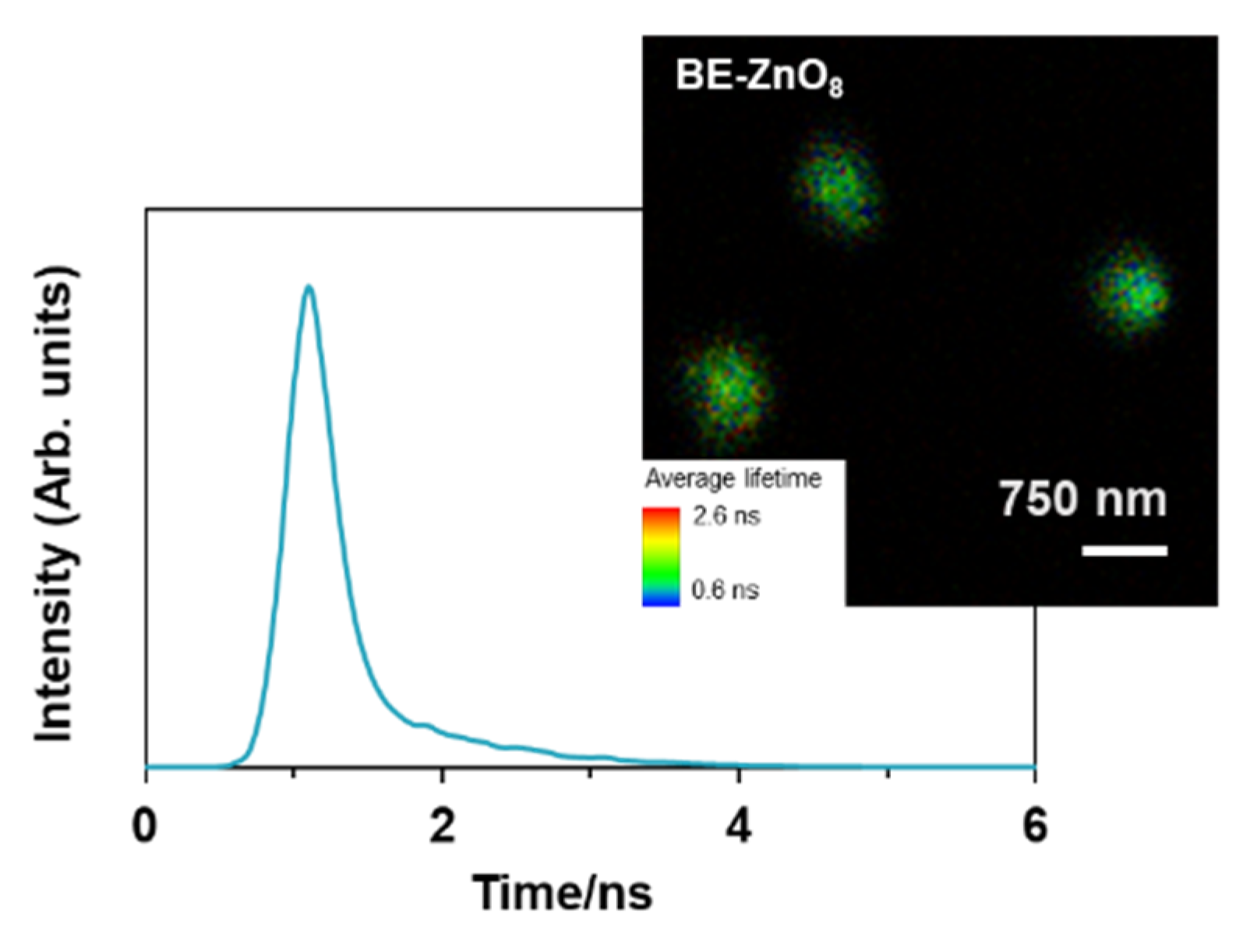
3.4. Particle Trafficking Inside Galleria mellonella
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Antimicrobial Resistance, Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- IACG; Interagency Coordination Group on Antimicrobial Resistance. No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations. 2019. Available online: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-future-from-drug-resistant-infections (accessed on 2 July 2023).
- Zhou, K.; Li, C.; Chen, D.; Pan, Y.; Tao, Y.; Qu, W.; Liu, Z.; Wang, X.; Xie, S. A review on nanosystems as an effective approach against infections of Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 7333–7347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, Y.-J.; Im, G.-B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic nanoparticles applied as functional therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kumar, L.; Mukerjee, N.; Anand, U.; Dhasmana, A.; Preetam, S.; Bhaumik, S.; Sihi, S.; Pal, S.; Khare, T.; et al. The emergence of metal oxide nanoparticles (NPs) as a phytomedicine: A two-facet role in plant growth, nano-toxicity and anti-phyto-microbial activity. Biomed. Pharmacother. 2022, 155, 113658. [Google Scholar] [CrossRef] [PubMed]
- Staroń, A.; Długosz, O. Antimicrobial properties of nanoparticles in the context of advantages and potential risks of their use. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2021, 56, 680–693. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Size-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytes. J. Toxicol. Environ. Health Part A 2014, 77, 177–191. [Google Scholar] [CrossRef]
- Asghar, M.S.; Sarwar, Z.M.; Almadiy, A.A.; Shami, A.; El Hadi Mohamed, R.A.; Ahmed, N.; Waghulade, M.S.; Alam, P.; Abd Al Galil, F.M. Toxicological effects of silver and Zinc Oxide nanoparticles on the biological and life table Parameters of Helicoverpa armigera (Noctuidae: Lepidoptera). Agriculture 2022, 12, 1744. [Google Scholar] [CrossRef]
- Mekewi, M.; Shebl, A.; Imam, A.I.; Amin, M.S.; Albert, T. Screening the insecticidal efficacy of nano ZnO synthesized via in-situ polymerization of crosslinked polyacrylic acid as a template. J. Mater. Sci. Technol. 2012, 28, 961–968. [Google Scholar] [CrossRef]
- Eskin, A.; Nurullahoğlu, Z.U. Effects of zinc oxide nanoparticles (ZnO NPs) on the biology of Galleria mellonella L. (Lepidoptera: Pyralidae). J. Basic Appl. Zool. 2022, 83, 54. [Google Scholar] [CrossRef]
- Xu, M.-N.; Li, L.; Pan, W.; Zheng, H.-X.; Wang, M.-L.; Peng, X.-M.; Dai, S.-Q.; Tang, Y.-M.; Zeng, K.; Huang, X.-W. Zinc oxide nanoparticles prime a protective immune response in Galleria mellonella to defend against Candida albicans. Front. Microbiol. 2021, 12, 766138. [Google Scholar] [CrossRef]
- Eskin, A.; Öztürk, Ş.; körükçü, M. Determination of the acute toxic effects of zinc oxide nanoparticles (ZnO NPs) in total hemocytes counts of Galleria mellonella (Lepidoptera: Pyralidae) with two different methods. Ecotoxicology 2019, 28, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; He, J.; Yu, T.; Sun, C.; Shi, D.; Jiang, Y.; Xianyu, Y.; Shao, Y. Dietary exposure of copper and zinc oxides nanoparticles affect the fitness, enzyme activity, and microbial community of the model insect, silkworm Bombyx mori. Sci. Total Environ. 2022, 813, 152608. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Thakral, F.; Bhatia, G.K.; Tuli, H.S.; Sharma, A.K.; Sood, S. Zinc oxide nanoparticles: From biosynthesis, characterization, and optimization to synergistic antibacterial potential. Curr. Pharmacol. Rep. 2021, 7, 15–25. [Google Scholar] [CrossRef]
- Alberto, M.R.; Canavosio, M.A.R.; Nadra, M.C.M.d. Antimicrobial effect of polyphenols from apple skins on human bacterial pathogens. Electron. J. Biotechnol. 2006, 9. Available online: http://www.ejbiotechnology.info/index.php/ejbiotechnology/article/view/v9n3-1/268 (accessed on 2 July 2023). [CrossRef]
- Serra, A.T.; Matias, A.A.; Frade, R.F.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Figueira, M.E.; de Carvalho, A.; Duarte, C.M.M. Characterization of traditional and exotic apple varieties from Portugal. Part 2—Antioxidant and antiproliferative activities. J. Funct. Foods 2010, 2, 46–53. [Google Scholar] [CrossRef]
- Rai, R.S.; Girish, J.P.; Bajpai, V.; Khan, M.I.; Elboughdiri, N.; Shanableh, A.; Luque, R. An eco-friendly approach on green synthesis, bio-engineering applications, and future outlook of ZnO nanomaterial: A critical review. Environ. Res. 2023, 221, 114807. [Google Scholar] [CrossRef]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- Alves, M.M.; Andrade, S.M.; Grenho, L.; Fernandes, M.H.; Santos, C.; Montemor, M.F. Influence of apple phytochemicals in ZnO nanoparticles formation, photoluminescence and biocompatibility for biomedical applications. Mater. Sci. Eng. C 2019, 101, 76–87. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Ribeiro, I.A.C.; Rodrigues, C.; Coelhoso, I.; Fernando, A.L. Biodegradable chitosan films with ZnO nanoparticles synthesized using food industry by-products—Production and characterization. Coatings 2021, 11, 646. [Google Scholar] [CrossRef]
- Soliman, M.M.A.; Alegria, E.C.B.A.; Ribeiro, A.P.C.; Alves, M.M.; Saraiva, M.S.; Fátima Montemor, M.; Pombeiro, A.J.L. Green synthesis of zinc oxide particles with apple-derived compounds and their application as catalysts in the transesterification of methyl benzoates. Dalton Trans. 2020, 49, 6488–6494. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.M.; Vaz Serra, V.; Bueno-Alejo, C.J.; Garcia, A.R.; Carvalho, M.F.N.N.; Ilharco, L.M.; Neves, M.G.P.M.S.; Costa, S.M.B. Covalent and noncovalent hybrids of di-amino porphyrin functionalized graphene oxide and their interaction with gold nanoparticles. J. Lumin. 2022, 250, 119097. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Fialho, A.M. A BCAM0223 mutant of Burkholderia cenocepacia is deficient in hemagglutination, serum resistance, adhesion to epithelial cells and virulence. PLoS ONE 2012, 7, e41747. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.M.S.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard. M07, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guidelines. M26-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Kennedy, A.D.; Otto, M.; Braughton, K.R.; Whitney, A.R.; Chen, L.; Mathema, B.; Mediavilla, J.R.; Byrne, K.A.; Parkins, L.D.; Tenover, F.C.; et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: Recent clonal expansion and diversification. Proc. Natl. Acad. Sci. USA 2008, 105, 1327–1332. [Google Scholar] [CrossRef]
- Silva, L.O.; Nobre, L.S.; Mil-Homens, D.; Fialho, A.; Saraiva, L.M. Repair of Iron Centers RIC protein contributes to the virulence of Staphylococcus aureus. Virulence 2018, 9, 312–317. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Valente, S.; Pimenta, C.; Pires, J.R.A.; Alves, M.M.; Santos, C.F.; Coelhoso, I.M.; Fernando, A.L. Eco-Friendly ZnO/Chitosan bionanocomposites films for packaging of fresh poultry meat. Coatings 2020, 10, 110. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, F. Understanding the Correlation of Crystal Atoms with Photochemistry Property: Zn5(OH)6(CO3)2 vs. ZnCO3. Chem. Sel. 2018, 3, 8886–8894. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Hsieh, P.T.; Chen, Y.C.; Kao, K.S.; Wang, C.M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Basnet, P.; Samanta, D.; Chanu, T.I.; Mukherjee, J.; Chatterjee, S. Tea-phytochemicals functionalized Ag modified ZnO nanocomposites for visible light driven photocatalytic removal of organic water pollutants. Mater. Res. Express 2019, 6, 085095. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Major, G.H.; Linford, M.R.; Easton, C.D. Practical guides for x-ray photoelectron spectroscopy (XPS): Interpreting the carbon 1s spectrum. J. Vac. Sci. Technol. A 2021, 39, 013204. [Google Scholar] [CrossRef]
- Abdol Aziz, R.A.; Abd Karim, S.F.; Rosli, N.A. The Effect of pH on Zinc Oxide Nanoparticles Characteristics Synthesized from Banana Peel Extract. Key Eng. Mater. 2019, 797, 271–279. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.-Y. Galleria mellonella as a suitable model of bacterial infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef]
- Kavitha, A.; Doss, A.; Praveen Pole, R.P.; Pushpa Rani, T.P.K.; Prasad, R.; Satheesh, S. A mini review on plant-mediated zinc oxide nanoparticles and their antibacterial potency. Biocatal. Agric. Biotechnol. 2023, 48, 102654. [Google Scholar] [CrossRef]
- Doan Thi, T.U.; Nguyen, T.T.; Thi, Y.D.; Ta Thi, K.H.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Mir, A.H.; Qamar, A.; Qadir, I.; Naqvi, A.H.; Begum, R. Accumulation and trafficking of zinc oxide nanoparticles in an invertebrate model, Bombyx mori, with insights on their effects on immuno-competent cells. Sci. Rep. 2020, 10, 1617. [Google Scholar] [CrossRef]
- Pérez-Bueno, J.J.; Vasquez-García, S.R.; García-González, L.; Vorobiev, Y.V.; Luna-Bárcenas, G.; González-Hernández, J. Optical processes in PMMA, SiO2, and hybrid organic−inorganic sol−gel films colored with rhodamine 6GDN. J. Phys. Chem. B 2002, 106, 1550–1556. [Google Scholar] [CrossRef]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Xiong, H.-M. Photoluminescent ZnO Nanoparticles and Their Biological Applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Allonsius, C.N.; Van Beeck, W.; De Boeck, I.; Wittouck, S.; Lebeer, S. The microbiome of the invertebrate model host Galleria mellonella is dominated by Enterococcus. Anim. Microbiome 2019, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Yasur, J.; Usha Rani, P. Lepidopteran insect susceptibility to silver nanoparticles and measurement of changes in their growth, development and physiology. Chemosphere 2015, 124, 92–102. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, W.; Ma, L.; Cui, X.; Lynch, I.; Wu, G. Acute toxicity of Zinc Oxide nanoparticles to silkworm (Bombyx mori L.). Chemosphere 2020, 259, 127481. [Google Scholar] [CrossRef]
- Andrade, S.M.; Bueno-Alejo, C.J.; Serra, V.V.; Rodrigues, J.M.M.; Neves, M.G.P.M.S.; Viana, A.S.; Costa, S.M.B. Anchoring of Gold Nanoparticles on Graphene Oxide and Noncovalent Interactions with Porphyrinoids. ChemNanoMat 2015, 1, 502–510. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Barahona, S.; Moreira, R.N.; Silva, I.J.; Pinto, S.N.; Fialho, A.M.; Arraiano, C.M. Stress response protein BolA influences fitness and promotes Salmonella enterica Serovar Typhimurium virulence. Appl. Environ. Microbiol. 2018, 84, e02850-17. [Google Scholar] [CrossRef]
- Dustmann, J.H.; von der Ohe, K. Scanning electron microscopic studies on pollen from honey. IV. Surface pattern of pollen of Sapium sebiferum and Euphorbia spp (Euphorbiaceae). Apidologie 1993, 24, 59–66. [Google Scholar] [CrossRef]
- Andrade, S.M.; Raja, P.; Saini, V.K.; Viana, A.S.; Serp, P.; Costa, S.M.B. Polyelectrolyte-Assisted Noncovalent Functionalization of Carbon Nanotubes with Ordered Self-Assemblies of a Water-Soluble Porphyrin. ChemPhysChem 2012, 13, 3622–3631. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.F.; Andrade, S.M.; Mil-Homens, D.; Montemor, M.F.; Alves, M.M. Antibacterial Activity of ZnO Nanoparticles in a Staphylococcus-aureus-Infected Galleria mellonella Model Is Tuned by Different Apple-Derived Phytocargos. J. Funct. Biomater. 2023, 14, 463. https://doi.org/10.3390/jfb14090463
Santos CF, Andrade SM, Mil-Homens D, Montemor MF, Alves MM. Antibacterial Activity of ZnO Nanoparticles in a Staphylococcus-aureus-Infected Galleria mellonella Model Is Tuned by Different Apple-Derived Phytocargos. Journal of Functional Biomaterials. 2023; 14(9):463. https://doi.org/10.3390/jfb14090463
Chicago/Turabian StyleSantos, Catarina F., Suzana M. Andrade, Dalila Mil-Homens, M. Fátima Montemor, and Marta M. Alves. 2023. "Antibacterial Activity of ZnO Nanoparticles in a Staphylococcus-aureus-Infected Galleria mellonella Model Is Tuned by Different Apple-Derived Phytocargos" Journal of Functional Biomaterials 14, no. 9: 463. https://doi.org/10.3390/jfb14090463





