Chemically Pretreated Densification of Juniper Wood for Potential Use in Osteosynthesis Bone Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
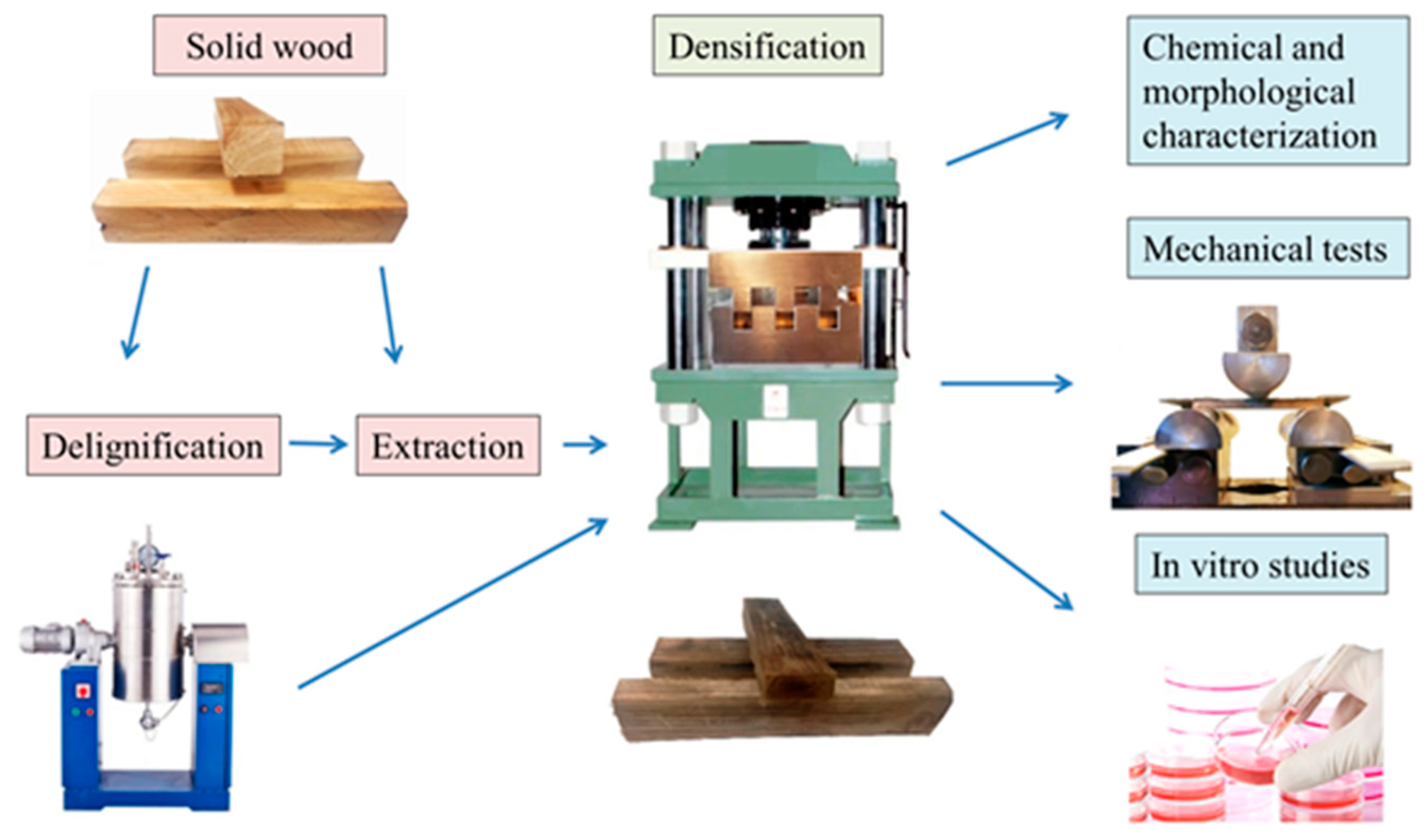
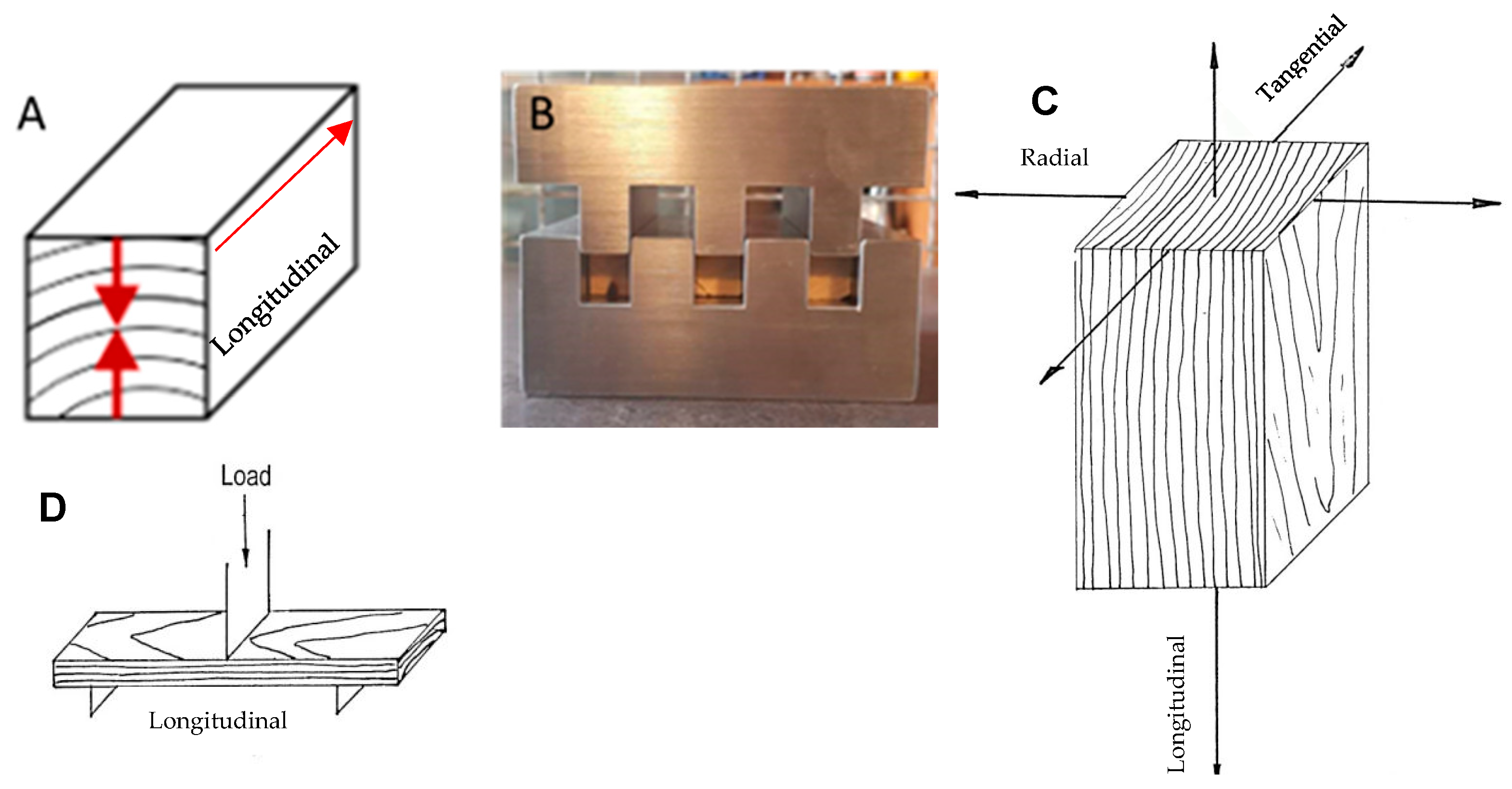
2.2. Sample Preparation
2.3. Chemical Treatment
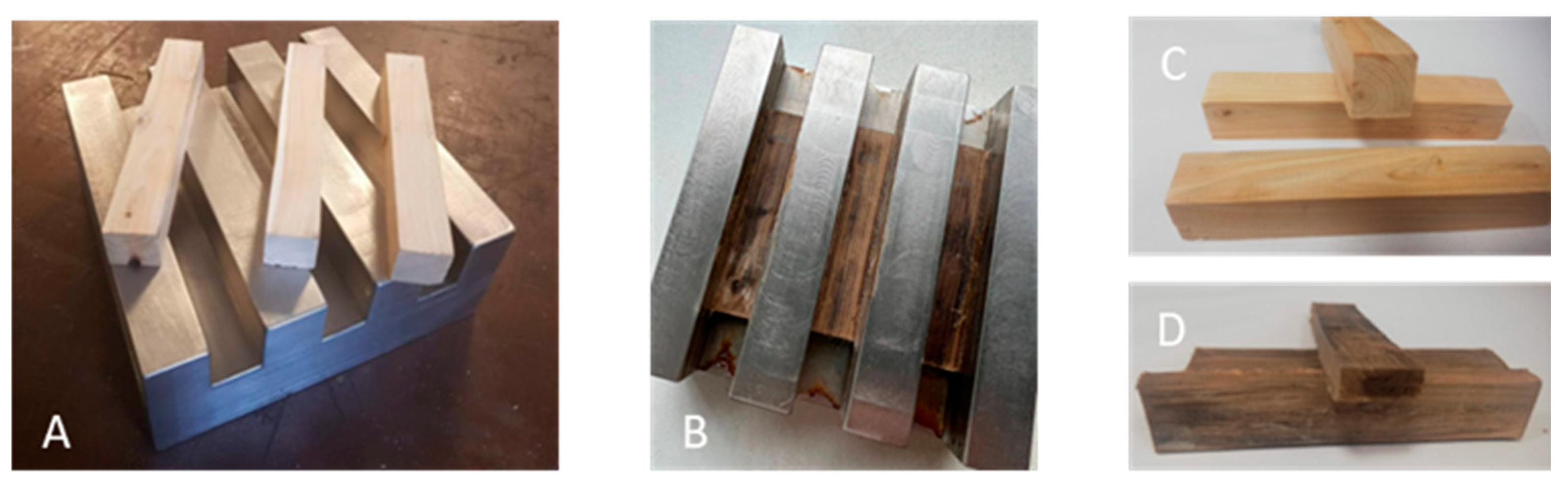
2.4. Densification
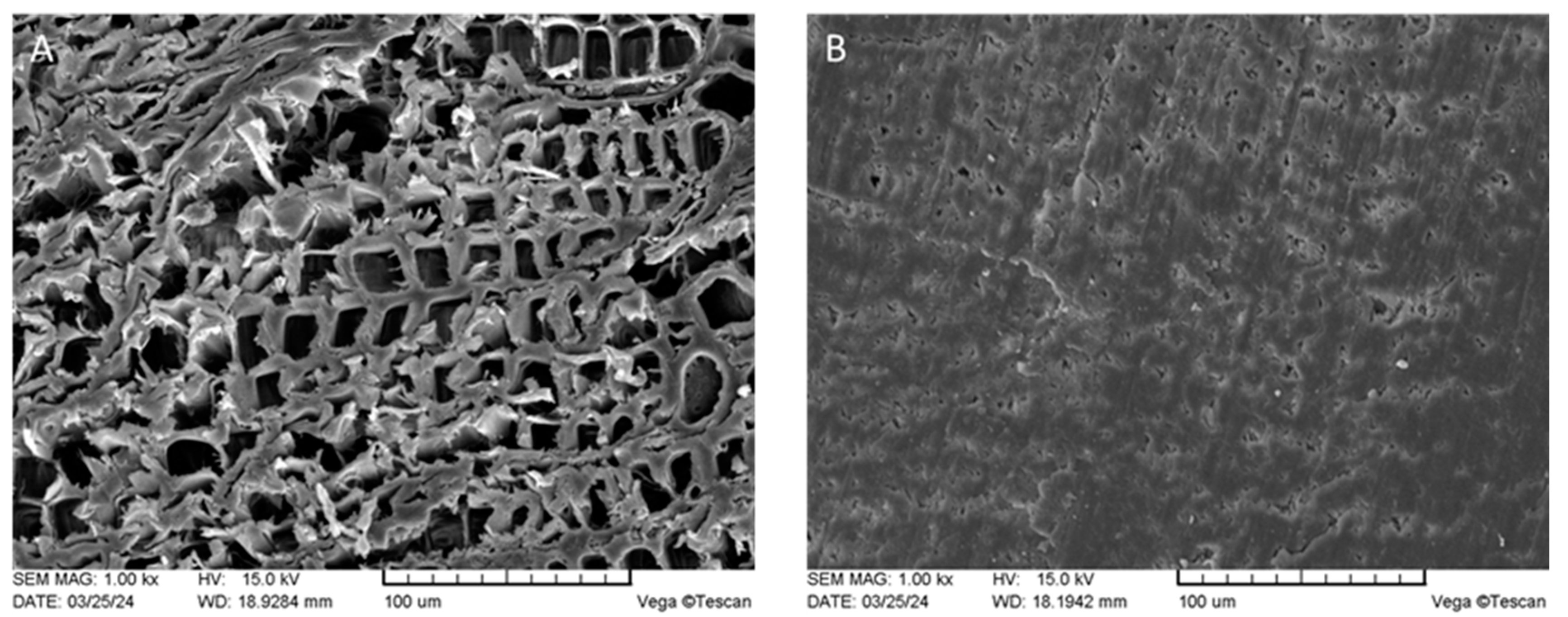
2.5. SEM
2.6. Chemical Characterization
2.6.1. Mass Loss
2.6.2. Extractives
2.6.3. Klason Lignin
2.6.4. Chemical Composition
2.6.5. FTIR
2.7. Physical-Mechanical Properties
2.7.1. Density
2.7.2. Swelling
2.7.3. Three-Point Bending
2.8. In Vitro Analysis
2.9. Statistic
3. Results and Discussion
3.1. Visual Appearance and SEM
3.2. Chemical Characterization
3.3. FTIR
3.4. Swelling
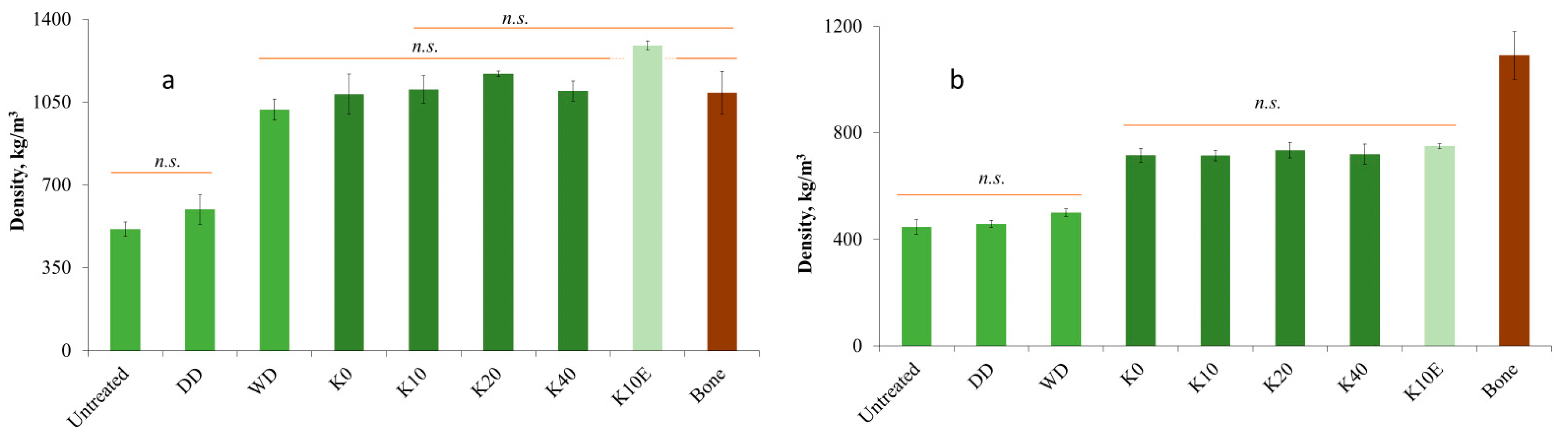
3.5. Density
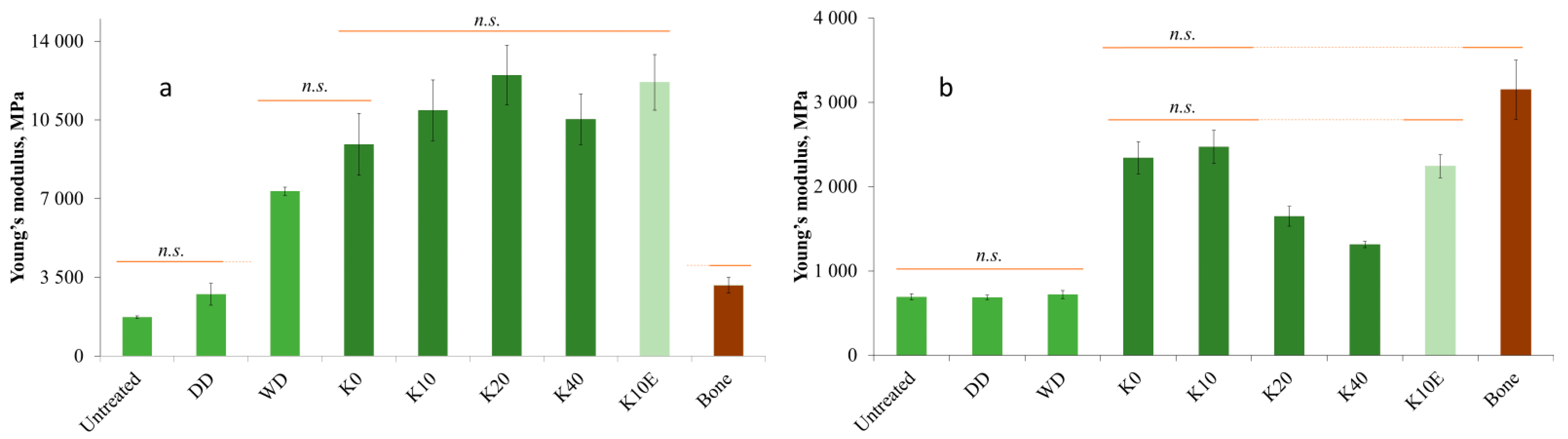
3.6. Mechanical Properties
3.7. In Vitro Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Hou, X.; Cheng, X.; Zhang, T. Unilateral External Fixator Combined with Lateral Auxiliary Frame for Ultimate Treatment of Tibia and Fibula Shaft Fractures with Poor Soft Tissue Conditions. Biomed. Res. Int. 2022, 2022, 9990744. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Pagani, D.; Melato, M. The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Cadosch, D.; Al-Mushaiqri, M.S.; Gautschi, O.P.; Meagher, J.; Simmen, H.P.; Filgueira, L. Biocorrosion and Uptake of Titanium by Human Osteoclasts. J. Biomed. Mater. Res.-Part A 2010, 95, 1004–1010. [Google Scholar] [CrossRef]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Simmen, H.P.; Filgueira, L. Bio-Corrosion of Stainless Steel by Osteoclasts-in Vitro Evidence. J. Orthop. Res. 2009, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, B.A.; Worters, P.W.; Pauly, K.B.; Pauly, J.M.; Koch, K.M.; Gold, G.E. Metal-Induced Artifacts in MRI. Am. J. Roentgenol. 2011, 197, 547–555. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Xing, L. Metal Artifact Reduction in X-Ray Computed Tomography (CT) by Constrained Optimization. Med. Phys. 2011, 38, 701–711. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Boden, S.D. Biologic Enhancement of Spinal Fusion. Orthop. Clin. N. Am. 1998, 29, 621–631. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological Properties of Calcium Phosphate Biomaterials for Bone Repair: A Review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Caroprese, M.; Gianfreda, F.; Melloni, F.; de Santis, D. Exploring the Potential of Calcium-Based Biomaterials for Bone Regeneration in Dentistry: A Systematic Review. Minerva Dent. Oral Sci. 2023, 73, 169–180. [Google Scholar] [CrossRef]
- Dinesh, K.R.; Hatti, G. Polymers Used as Implant Biomaterials: A Review. 2018. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.researchgate.net/profile/Gururaj-Hatti/publication/330753275_Polymers_used_as_implant_Biomaterials_A_review/links/5c52b272299bf12be3eff424/Polymers-used-as-implant-Biomaterials-A-review.pdf&ved=2ahUKEwj4stSPsdqIAxU2oK8BHWdWOC0QFnoECBYQAQ&usg=AOvVaw3eDFzudZPHd-Nmm849_qN0 (accessed on 18 September 2024).
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, D.H.; Pashneh-Tala, S.; Bains, A.K.; Moorehead, R.D.; Kassos, N.; Kelly, A.L.; Paterson, T.E.; Orozco-Diaz, C.A.; Gill, A.A.; Asencio, I.O. Demonstrating the Potential of Using Bio-Based Sustainable Polyester Blends for Bone Tissue Engineering Applications. Bioengineering 2022, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- De Bortoli, L.S.; Schabbach, L.M.; Fredel, M.C.; Hotza, D.; Henriques, B. Ecological Footprint of Biomaterials for Implant Dentistry: Is the Metal-Free Practice an Eco-Friendly Shift? J. Clean. Prod. 2019, 213, 723–732. [Google Scholar] [CrossRef]
- Trimeche, M. Biomaterials for Bone Regeneration: An Overview. Biomater. Tissue Technol. Rev. 2017, 1, 1–5. [Google Scholar]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Olawade, D.B. Recent Advances in Biopolymeric Composite Materials: Future Sustainability of Bone-Implant. Renew. Sustain. Energy Rev. 2021, 150, 111505. [Google Scholar] [CrossRef]
- Yadav, D.; Garg, R.K.; Ahlawat, A.; Chhabra, D. 3D Printable Biomaterials for Orthopedic Implants: Solution for Sustainable and Circular Economy. Resour. Policy 2020, 68, 101767. [Google Scholar] [CrossRef]
- Nefjodovs, V.; Andze, L.; Andzs, M.; Filipova, I.; Tupciauskas, R.; Vecbiskena, L.; Kapickis, M. Wood as Possible Renewable Material for Bone Implants—Literature Review. J. Funct. Biomater. 2023, 14, 266. [Google Scholar] [CrossRef]
- Kristen, H.; Bosch, P.; Bednar, H.; Plenk, H. Biocompatibility of Wood in Bone Tissue. Arch. Orthop. Trauma Surg. 1977, 89, 1–14. [Google Scholar]
- Kristen, H.; Bösch, P.; Bednar, H.; Plenk, H. The Effects of Dynamic Loading on Intracalcaneal Wood Implants and on the Tissues Surrounding Them. Arch. Orthop. Trauma. Surg. 1979, 93, 287–292. [Google Scholar] [CrossRef]
- Bösch, P.; Kristen, H.; Braun, F.; Kovac, W. Reaction of Connective Tissue and Striated Muscle Tissue to Implanted Ashwood. Wien Med. Wochenschr. 1979, 15, 419–423. [Google Scholar]
- Aho, A.J.; Rekola, J.; Matinlinna, J.; Gunn, J.; Tirri, T.; Viitaniemi, P.; Vallittu, P. Natural Composite of Wood as Replacement Material for Ostechondral Bone Defects. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2007, 83, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Horský, I.; Huraj, E.; Paukovic, J. Utilization of Wood in the Manufacture of Orthopedic Implants. Acta Chir. Orthop. Traumatol. Cech. 1987, 1, 13. [Google Scholar]
- Gross, K.A.; Ezerietis, E. Juniper Wood as a Possible Implant Material. J. Biomed. Mater. Res.-Part A 2003, 64, 672–683. [Google Scholar] [CrossRef]
- Rekola, J.; Lassila, L.V.; Hirvonen, J.; Lahdenperä, M.; Grenman, R.; Aho, A.J.; Vallittu, P.K. Effects of Heat Treatment of Wood on Hydroxylapatite Type Mineral Precipitation and Biomechanical Properties in Vitro. J. Mater. Sci. Mater. Med. 2010, 21, 2345–2354. [Google Scholar] [CrossRef]
- Rekola, J.; Lassila, L.V.J.; Nganga, S.; Ylä-Soininmäki, A.; Fleming, G.J.P.; Grenman, R.; Aho, A.J.; Vallittu, P.K. Effect of Heat Treatment of Wood on the Morphology, Surface Roughness and Penetration of Simulated and Human Blood. Biomed. Mater. Eng. 2014, 24, 1595–1607. [Google Scholar] [CrossRef]
- Andze, L.; Andzs, M.; Skute, M.; Nefjodovs, V.; Kapickis, M.; Tupciauskas, R. Preliminary Study of Chemically Pretreated Densification of Juniper Wood for Use in Bone Implants. Mater. Sci. Forum 2022, 1071, 101–108. [Google Scholar] [CrossRef]
- Kutnar, A.; Šernek, M. Densification of Wood. Zb. Gozdarstva Lesar. 2007, 82, 53–62. [Google Scholar]
- Cabral, J.P.; Kafle, B.; Subhani, M.; Reiner, J.; Ashraf, M. Densification of Timber: A Review on the Process, Material Properties, and Application. J. Wood Sci. 2022, 68, 20. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing Bulk Natural Wood into a High-Performance Structural Material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Shi, J.; Peng, J.; Huang, Q.; Cai, L.; Shi, S.Q. Fabrication of Densified Wood via Synergy of Chemical Pretreatment, Hot-Pressing and Post Mechanical Fixation. J. Wood Sci. 2020, 66, 5. [Google Scholar] [CrossRef]
- Mania, P.; Wróblewski, M.; Wójciak, A.; Roszyk, E.; Moliński, W. Hardness of Densified Wood in Relation to Changed Chemical Composition. Forests 2020, 11, 506. [Google Scholar] [CrossRef]
- Raman, V.; Liew, K.C. Density of Densified Paraserianthes Falcataria Wood Pre-Treated with Alkali. Proc. IOP Conf. Ser. Earth Environ. Sci. 2020, 549, 012030. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sable, I.; Grinfelds, U.; Vikele, L.; Rozenberga, L.; Lazdina, D.; Zeps, M.; Jansons, A. Chemical Composition and Fi Ber Properties of Fast-Growing Species in Latvia and Its Potential for Forest Bioindustry. For. Stud. 2017, 66, 27–32. [Google Scholar] [CrossRef]
- Sable, I.; Grinfelds, U.; Vikele, L.; Rozenberga, L.; Zeps, M.; Neimane, U.; Jansons, A. Effect of Refining on the Properties of Fibres from Young Scots (Pinus Sylvestris) and Lodgepole Pines (Pinus Contorta). Balt. For. 2017, 23, 529–533. [Google Scholar]
- Shi, J.; Lu, Y.; Zhang, Y.; Cai, L.; Shi, S.Q. Effect of Thermal Treatment with Water, H2SO4 and NaOH Aqueous Solution on Color, Cell Wall and Chemical Structure of Poplar Wood. Sci. Rep. 2018, 8, 17735. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fishwild, S.J.; Begel, M.; Zhu, J.Y. Properties of Densified Poplar Wood through Partial Delignification with Alkali and Acid Pretreatment. J. Mater. Sci. 2020, 55, 14664–14676. [Google Scholar] [CrossRef]
- Ran, Y.; Lu, D.; Jiang, J.; Huang, Y.; Wang, W.; Jinzhen, C. Deep Eutectic Solvents-Assisted Wood Densification: A Promising Strategy for Shape-Fixation. Chem. Eng. J. 2023, 471, 144476. [Google Scholar] [CrossRef]
- Maturana, J.C.; Guindos, P.; Lagos, J.; Arroyave, C.; Echeverría, F.; Correa, E. Two-Step Hot Isostatic Pressing Densification Achieved Non-Porous Fully-Densified Wood with Enhanced Physical and Mechanical Properties. Sci. Rep. 2023, 13, 14324. [Google Scholar] [CrossRef]
- Bao, M.; Huang, X.; Jiang, M.; Yu, W.; Yu, Y. Effect of Thermo-Hydro-Mechanical Densification on Microstructure and Properties of Poplar Wood (Populus tomentosa). J. Wood Sci. 2017, 63, 591–605. [Google Scholar] [CrossRef]
- Vila, C.; Romero, J.; Francisco, J.L.; Santos, V.; Parají, J.C. On the Recovery of Hemicellulose before Kraft Pulping. BioResources 2012, 7, 4179–4189. [Google Scholar] [CrossRef]
- Lipeh, S.; Schimleck, L.R.; Mankowski, M.E.; McDonald, A.G.; Morrell, J.J. Relationship between Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy of Western Juniper and Natural Resistance to Fungal and Termite Attack. Holzforschung 2020, 74, 246–259. [Google Scholar] [CrossRef]
- Emmanuel, V.; Odile, B.; Céline, R. FTIR Spectroscopy of Woods: A New Approach to Study the Weathering of the Carving Face of a Sculpture. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 136, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, I.; Serra-Prat, M.; Carlos Yébenes, J. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Mantanis, G.I.; Young, R.A.; Rowell, R.M. Swelling of Wood Part III. Effect of Temperature and Extractives on Rate and Maximum Swelling. Holzforschung 1995, 49, 239–248. [Google Scholar] [CrossRef]
- Welzbacher, C.R.; Wehsener, J.; Rapp, A.O.; Haller, P. Thermo-Mechanische Verdichtung Und Thermische Modifikation von Fichtenholz (Picea Abies Karst) Im Industriellen Maßstab—Betrachtung Der Dimensionsstabilität Und Dauerhaftigkeit. Holz Als Roh-Und Werkst. 2008, 66, 39–49. [Google Scholar] [CrossRef]
- Laine, K.; Belt, T.; Rautkari, L.; Ramsay, J.; Hill, C.A.S.; Hughes, M. Measuring the Thickness Swelling and Set-Recovery of Densified and Thermally Modified Scots Pine Solid Wood. J. Mater. Sci. 2013, 48, 8530–8538. [Google Scholar] [CrossRef]
- Xu, B.H.; Wang, B.L.; Yu, K.B.; Bouchaïr, A. An Optional Connection Material in Timber Structures: Densified Poplar. J. Mater. Sci. 2021, 56, 14114–14125. [Google Scholar] [CrossRef]
- Grönquist, P.; Schnider, T.; Thoma, A.; Gramazio, F.; Kohler, M.; Burgert, I.; Rüggeberg, M. Investigations on Densified Beech Wood for Application as a Swelling Dowel in Timber Joints. Holzforschung 2019, 73, 559–568. [Google Scholar] [CrossRef]
- Fang, C.H.; Mariotti, N.; Cloutier, A.; Koubaa, A.; Blanchet, P. Densification of Wood Veneers by Compression Combined with Heat and Steam. Eur. J. Wood Wood Prod. 2012, 70, 155–163. [Google Scholar] [CrossRef]
- Singh, D.; Rana, A.; Jhajhria, S.K.; Garg, B.; Pandey, P.M.; Kalyanasundaram, D. Experimental Assessment of Biomechanical Properties in Human Male Elbow Bone Subjected to Bending and Compression Loads. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018793816. [Google Scholar] [CrossRef]
- Meema, H.E.; Meema, S. Compact Bone Mineral Density of the Normal Human Radius. Acta Oncol. 1978, 17, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Björklund, J.; Fonti, M.V.; Fonti, P.; Van den Bulcke, J.; von Arx, G. Cell Wall Dimensions Reign Supreme: Cell Wall Composition Is Irrelevant for the Temperature Signal of Latewood Density/Blue Intensity in Scots Pine. Dendrochronologia 2021, 65, 125785. [Google Scholar] [CrossRef]
- Bockel, S.; Harling, S.; Grönquist, P.; Niemz, P.; Pichelin, F.; Weiland, G.; Konnerth, J. Characterization of Wood-Adhesive Bonds in Wet Conditions by Means of Nanoindentation and Tensile Shear Strength. Eur. J. Wood Wood Prod. 2020, 78, 449–459. [Google Scholar] [CrossRef]
- Bader, M.; Nemeth, R. Moisture-dependent Mechanical Properties of Longitudinally Compressed Wood. Eur. J. Wood Wood Prod. 2019, 77, 1009–1019. [Google Scholar] [CrossRef]
- Kuai, B.; Wang, Z.; Gao, J.; Tong, J.; Zhan, T.; Zhang, Y.; Lu, J.; Cai, L. Development of Densified Wood with High Strength and Excellent Dimensional Stability by Impregnating Delignified Poplar by Sodium Silicate. Constr. Build. Mater. 2022, 344, 128282. [Google Scholar] [CrossRef]
- Bogolitsyn, K.G.; Gusakova, M.A.; Khviyuzov, S.S.; Zubov, I.N. Physicochemical Properties of Conifer Lignins Using Juniperus Communis as an Example. Chem. Nat. Compd. 2014, 50, 337–341. [Google Scholar] [CrossRef]
- Hänninen, T.; Tukiainen, P.; Svedström, K.; Serimaa, R.; Saranpää, P.; Kontturi, E.; Hughes, M.; Vuorinen, T. Ultrastructural Evaluation of Compression Wood-like Properties of Common Juniper (Juniperus communis L.). Holzforschung 2012, 66, 389–395. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Li, S.H.; Liu, Q.; De Wijn, J.R.; Zhou, B.L.; De Groot, K. In Vitro Calcium Phosphate Formation on a Natural Composite Material, Bamboo. Biomaterials 1997, 18, 389–395. [Google Scholar] [CrossRef]



| Sample Abbreviation | Untreated | DD | WD | K0 | K10 | K20 | K40 | K10E |
|---|---|---|---|---|---|---|---|---|
| Cooking time (min) after reaching 165 °C | - | - | - | 0 | 10 | 20 | 40 | 10 |
| Treatment before densification | - | Dry | Soaked in water (wet) | Washed and soaked in water | Extracted by ethanol and water | |||
| Sample | Untreated | DD | WD | K0 | K10 | K20 | K40 | K10E |
|---|---|---|---|---|---|---|---|---|
| thickness, mm (dry) | 14.93 ± 0.07 | 13.89 ± 0.06 | 8.05 ± 0.03 | 4.83 ± 0.02 | 4.81 ± 0.07 | 4.26 ± 0.04 | 3.82 ± 0.05 | 3.99 ± 0.04 |
| thickness, mm (wet) | 15.27 ± 0.11 | 15.20 ± 0.10 | 15.10 ± 0.11 | 8.07 ± 0.12 | 7.5 ± 0.3 | 8.07 ± 0.08 | 6.43 ± 0.13 | 5.70 ± 0.10 |
| Weight Loss, % | Cellulose, % | Hemicelluloses, % | Lignin, % | Extracts, % | Inorganic Compounds, % | Other Components, % | |
|---|---|---|---|---|---|---|---|
| Untreated | 0 | 38.0 ± 2.1 | 21.1 ± 1.9 | 33.1 ± 0.5 | 4.8 ± 0.3 | 0.060 ± 0.002 | 2.94 |
| 0 | 21.7 ± 1.8 | 38.6 ± 0.5 | 14.4 ± 0.5 | 20.6 ± 0.1 | 3.3 ± 0.5 | 0.050 ± 0.003 | 1.35 |
| K10 | 25.9 ± 2.0 | 38.3 ± 0.9 | 12.7 ± 1.2 | 18.2 ± 0.2 | 3.4 ± 0.2 | 0.041 ± 0.001 | 1.459 |
| K20 | 27.2 ± 0.8 | 38.1 ± 0.9 | 12.2 ± 0.5 | 17.8 ± 0.5 | 3.2 ± 0.3 | 0.042 ± 0.005 | 1.458 |
| K40 | 32.2 ± 1.2 | 36.4 ± 1.2 | 9.1 ± 1.3 | 17.3 ± 0.7 | 3.5 ± 0.7 | 0.040 ± 0.007 | 1.46 |
| Sample Swelling | Untreated | DD | WD | K0 | K10 | K20 | K40 | K10E |
|---|---|---|---|---|---|---|---|---|
| Volume, % | 13 ± 6 | 22 ± 6 | 52 ± 3 | 34 ± 8 | 32 ± 4 | 44 ± 2 | 38 ± 4 | 44 ± 1 |
| Longitudial, % | 0.08 ± 0.02 | 0.11 ± 0.03 | 0.13 ± 0.09 | 0.09 ± 0.04 | 0.2 ± 0.1 | 0.10 ± 0.04 | 0.17 ± 0.01 | 0.19 ± 0.11 |
| Tangential (width), % | 5.5 ± 0.8 | 4.0 ± 0.6 | 4.9 ± 0.3 | 4.1 ± 0.7 | 4.8 ± 0.9 | 3.7 ± 0.1 | 4.0 ± 0.7 | 3.8 ± 0.5 |
| Radial (thickness), % | 20 ± 3 | 34 ± 5 | 67 ± 4 | 49 ± 5 | 57 ± 5 | 52 ± 2 | 60 ± 4 | 55 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andze, L.; Nefjodovs, V.; Andzs, M.; Skute, M.; Zoldners, J.; Kapickis, M.; Dubnika, A.; Locs, J.; Vetra, J. Chemically Pretreated Densification of Juniper Wood for Potential Use in Osteosynthesis Bone Implants. J. Funct. Biomater. 2024, 15, 287. https://doi.org/10.3390/jfb15100287
Andze L, Nefjodovs V, Andzs M, Skute M, Zoldners J, Kapickis M, Dubnika A, Locs J, Vetra J. Chemically Pretreated Densification of Juniper Wood for Potential Use in Osteosynthesis Bone Implants. Journal of Functional Biomaterials. 2024; 15(10):287. https://doi.org/10.3390/jfb15100287
Chicago/Turabian StyleAndze, Laura, Vadims Nefjodovs, Martins Andzs, Marite Skute, Juris Zoldners, Martins Kapickis, Arita Dubnika, Janis Locs, and Janis Vetra. 2024. "Chemically Pretreated Densification of Juniper Wood for Potential Use in Osteosynthesis Bone Implants" Journal of Functional Biomaterials 15, no. 10: 287. https://doi.org/10.3390/jfb15100287
APA StyleAndze, L., Nefjodovs, V., Andzs, M., Skute, M., Zoldners, J., Kapickis, M., Dubnika, A., Locs, J., & Vetra, J. (2024). Chemically Pretreated Densification of Juniper Wood for Potential Use in Osteosynthesis Bone Implants. Journal of Functional Biomaterials, 15(10), 287. https://doi.org/10.3390/jfb15100287







