Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TiO2-NP Characterization
2.3. Biocompatibility of TiO2-NPs
2.3.1. Cell Culture
2.3.2. Preparation of TiO2 Nanomaterial Suspensions
2.3.3. HFF-1 Cell Viability
2.4. Production and Purification of Serratiopeptidase
2.5. Proteolytic Activity and Total Protein Assay
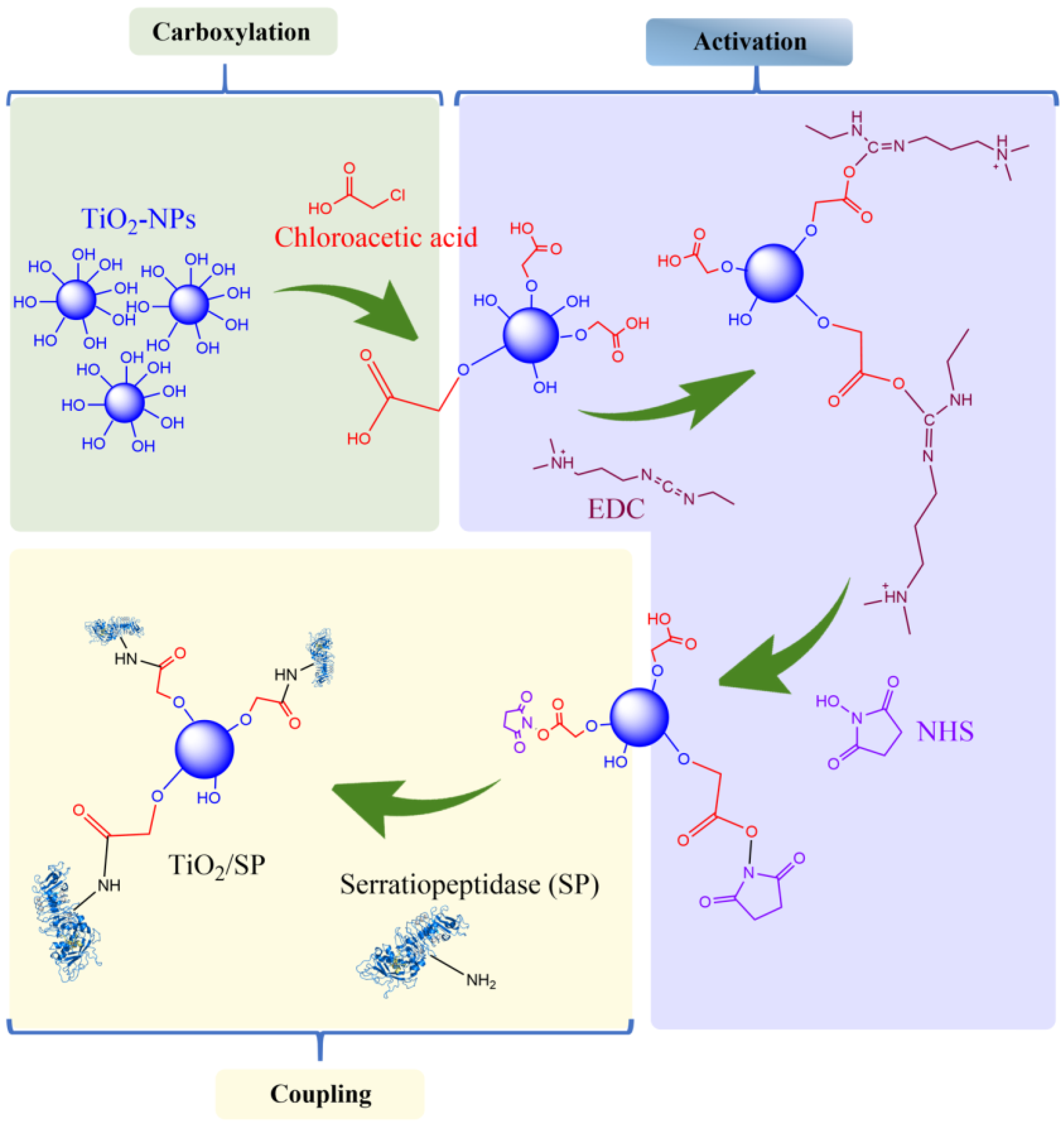
2.6. Immobilization of the Serratiopeptidase Enzyme on Titanium Oxide Nanoparticles (TiO2/SP)
2.7. Characterization of the Bioconjugate TiO2/SP
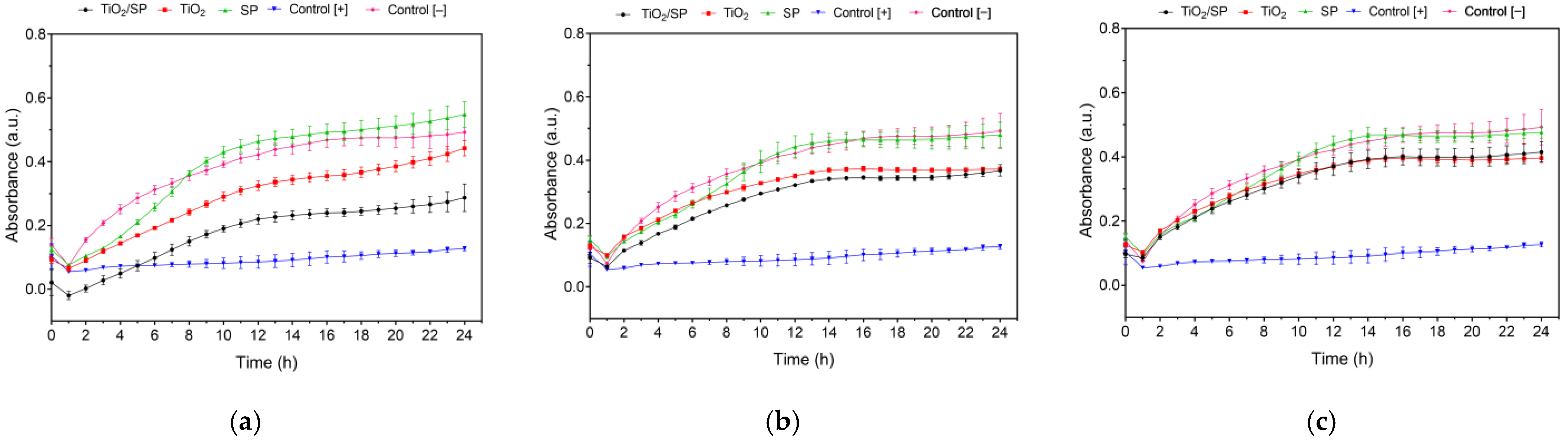
2.8. Antibacterial Activity of the Bioconjugate TiO2/SP
2.9. Statistical Analysis
3. Results and Discussion
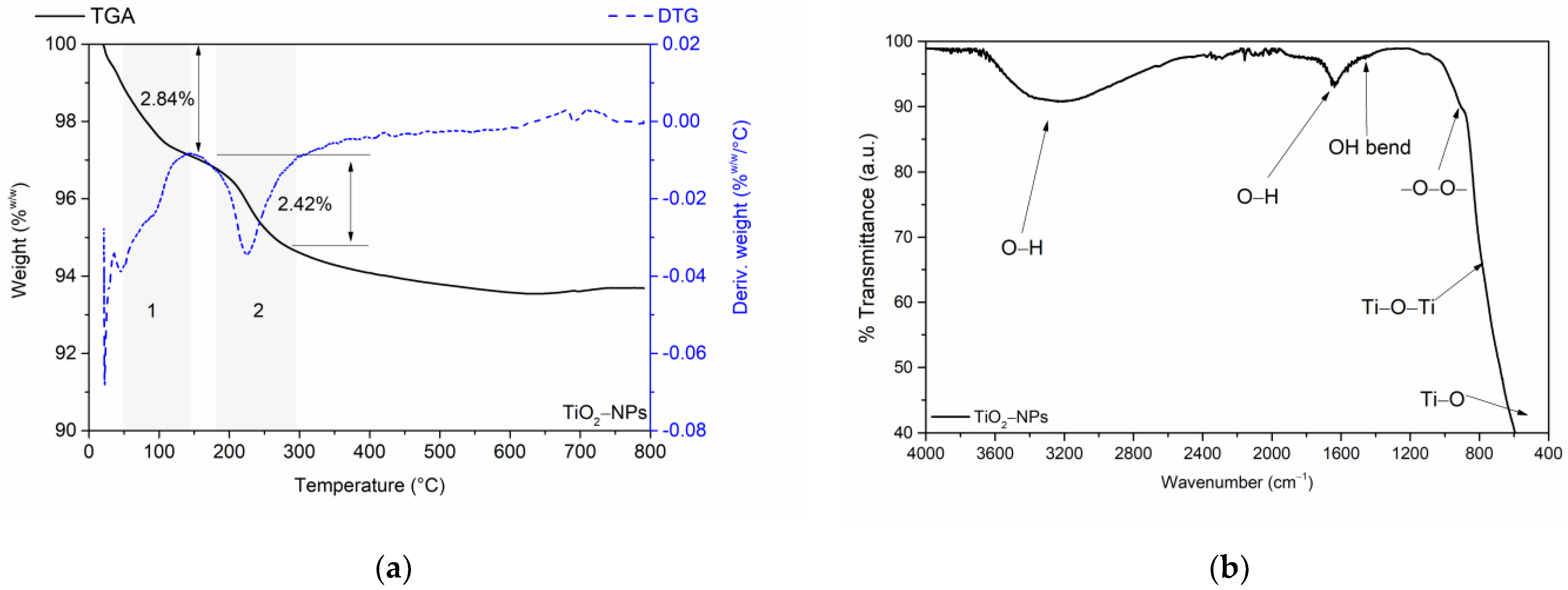
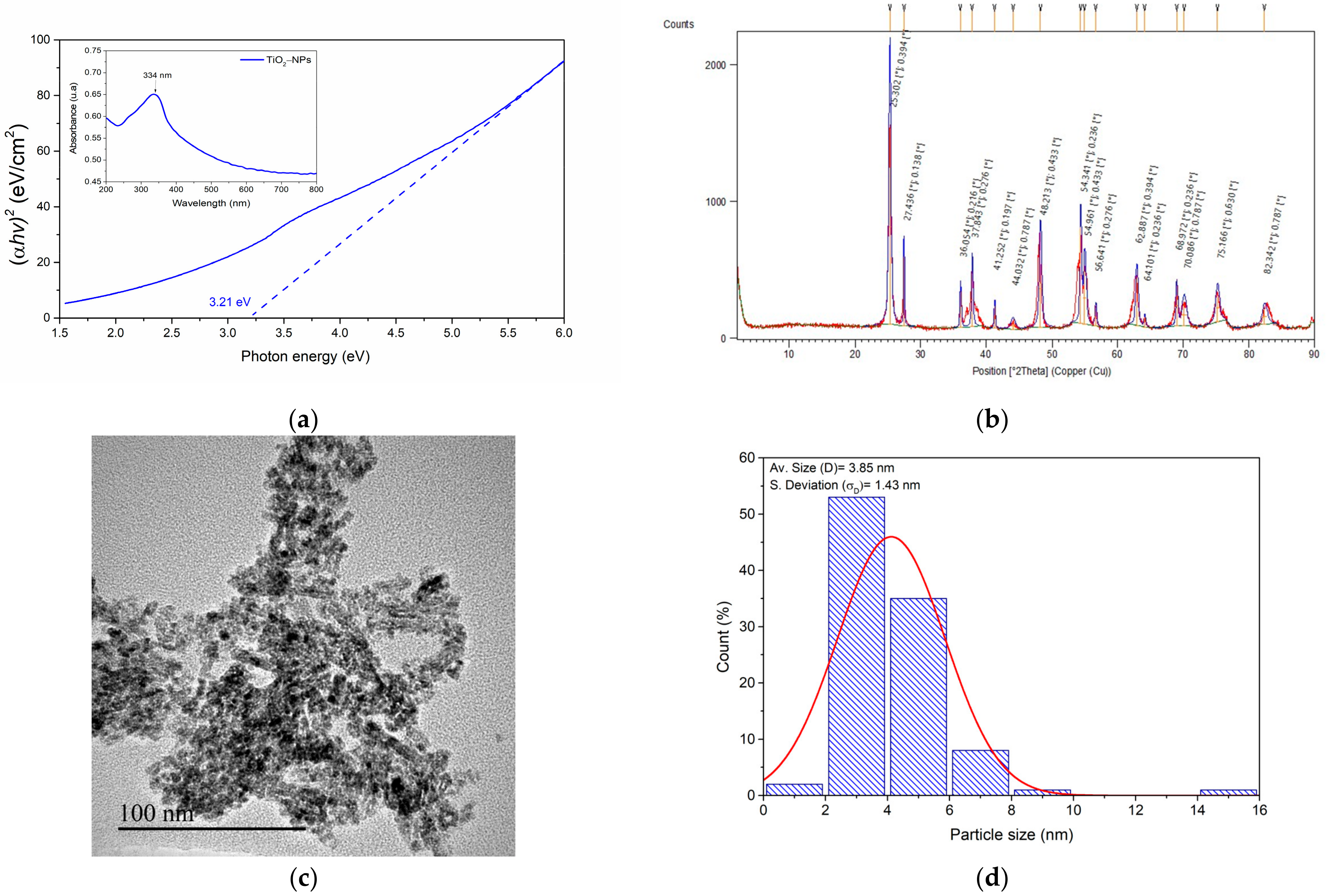
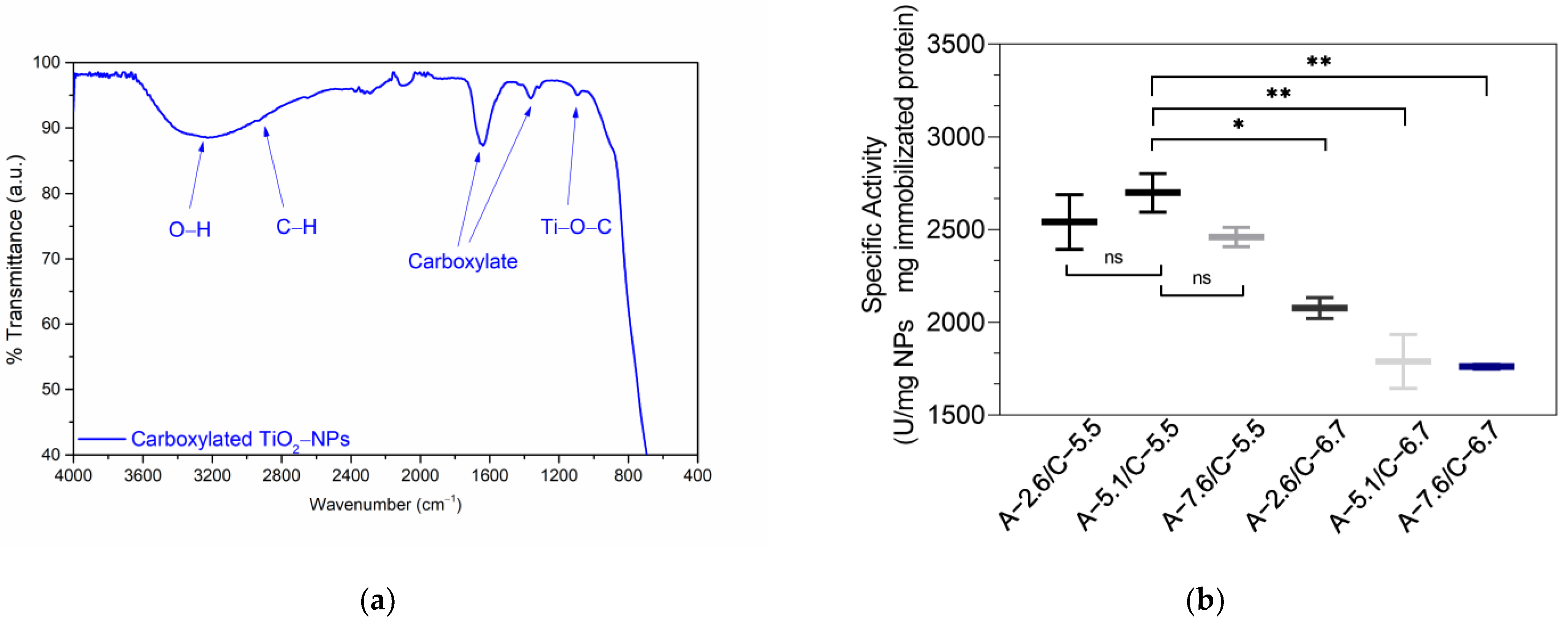
3.1. Characterization of Titanium Oxide Nanoparticles (TiO2-NPs)
3.2. Biocompatibility of Titanium Oxide Nanoparticles
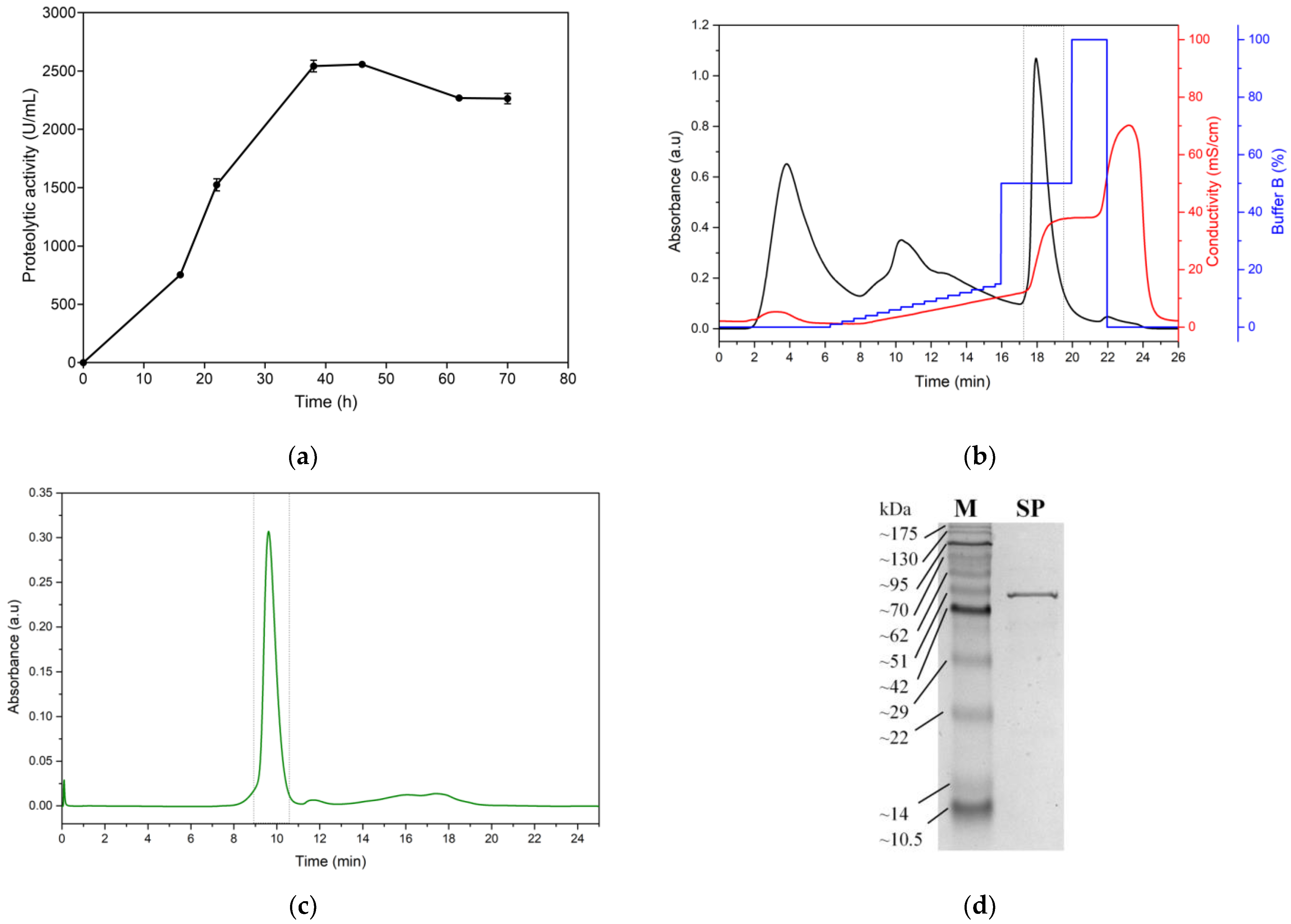
3.3. Production and Purification of Serratiopeptidase (SP)
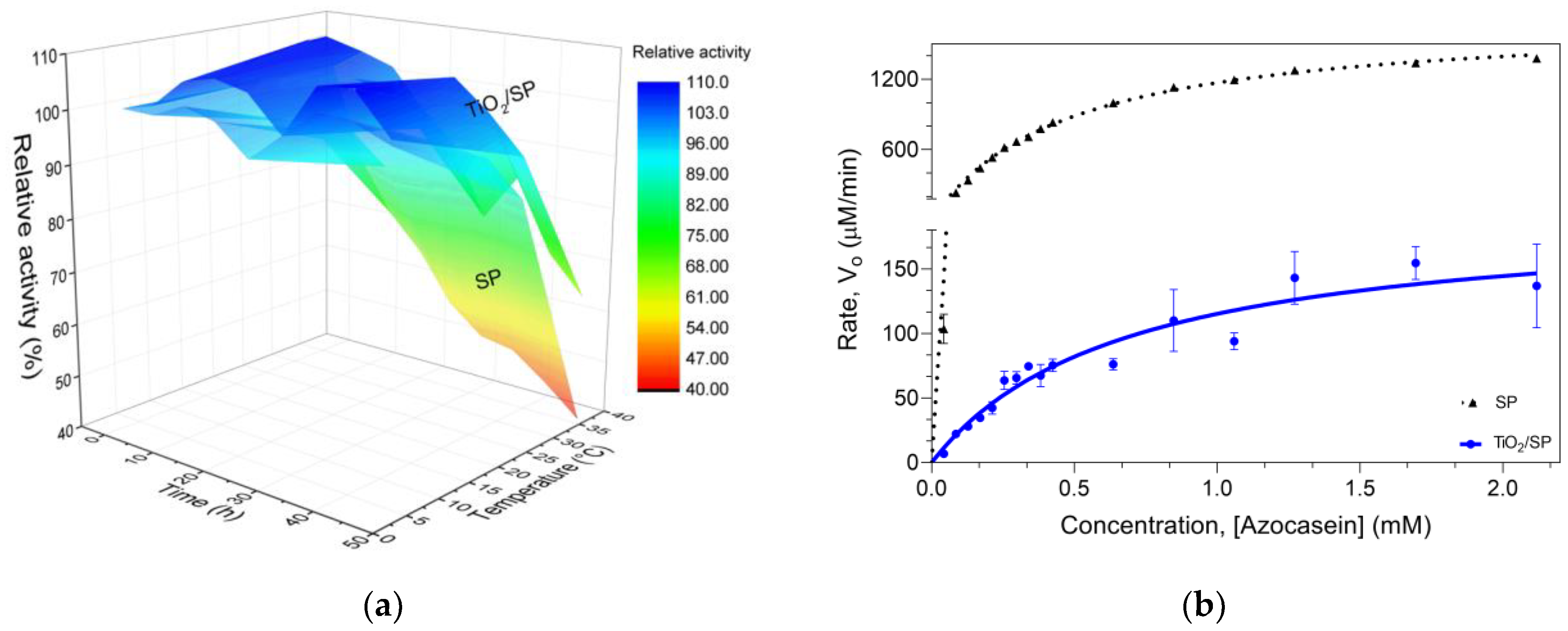
3.4. Immobilization of the Serratiopeptidase Enzyme on Titanium Oxide Nanoparticles (TiO2/SP)
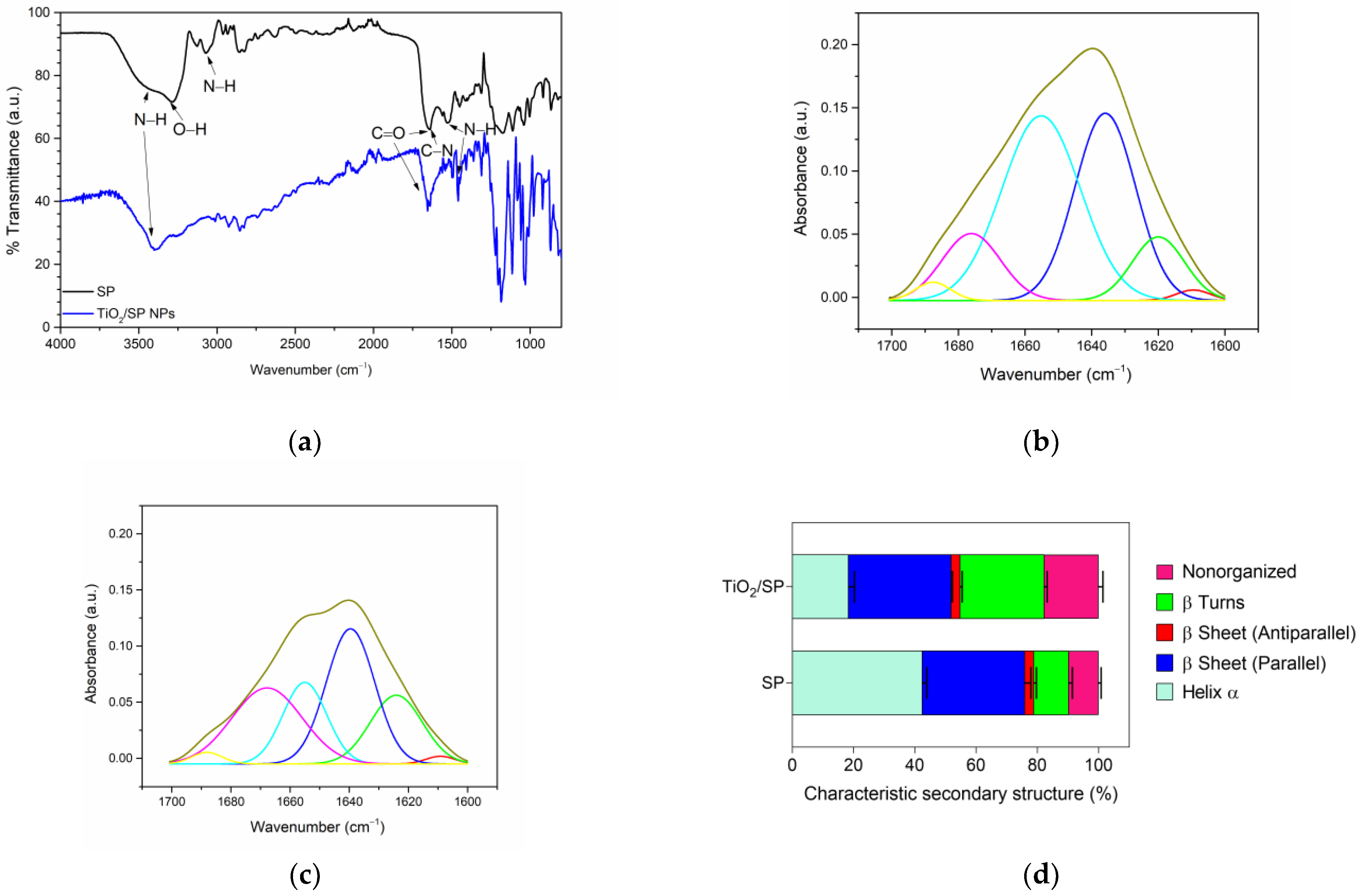
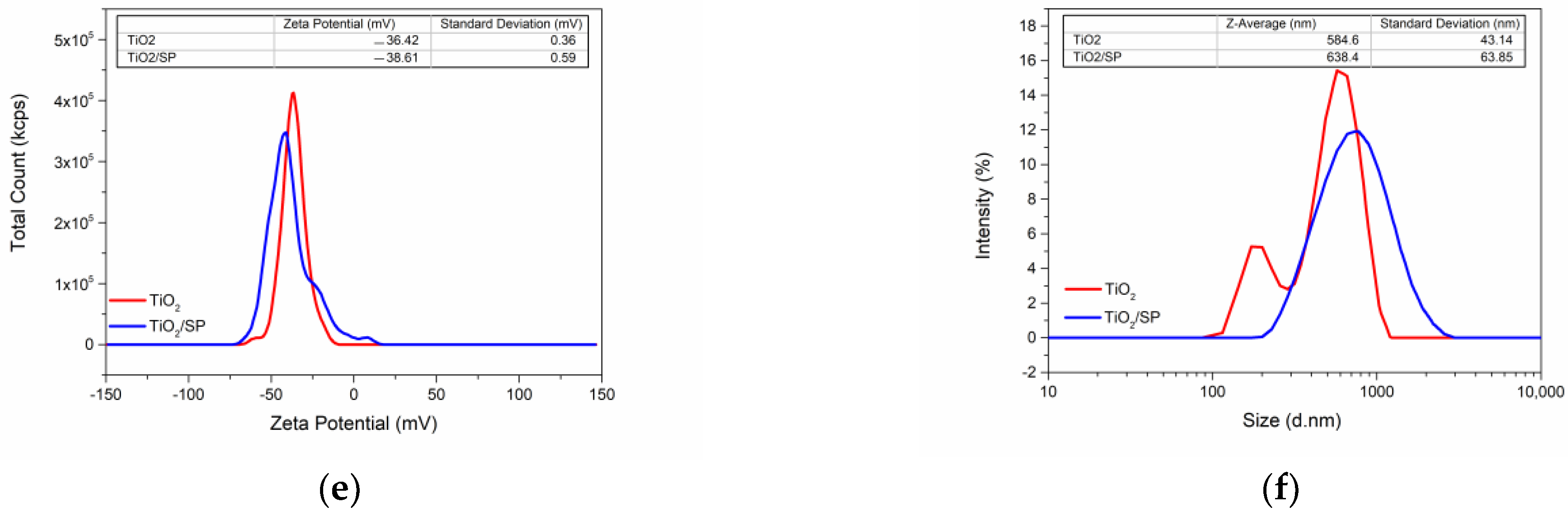
3.5. Characterization of TiO2/SP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K. Vaccines Could Avert Half a Million Deaths Associated with Anti-Microbial Resistance a Year; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Murugaiyan, J.; Halat, D.H.; El Hage, S.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic Discovery and Resistance: The Chase and the Race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef] [PubMed]
- Tonoyan, L.; Montagner, D.; Friel, R.; O’Flaherty, V. Antimicrobials Offered from Nature: Peroxidase-Catalyzed Systems and Their Mimics. Biochem. Pharmacol. 2020, 182, 114281. [Google Scholar] [CrossRef]
- Nair, S.R.; C, S.D. Serratiopeptidase: An Integrated View of Multifaceted Therapeutic Enzyme. Biomolecules 2022, 12, 1468. [Google Scholar] [CrossRef]
- Sharma, C.; Jha, N.K.; Meeran, M.F.N.; Patil, C.R.; Goyal, S.N.; Ojha, S. Serratiopeptidase, A Serine Protease Anti-Inflammatory, Fibrinolytic, and Mucolytic Drug, Can Be a Useful Adjuvant for Management in COVID-19. Front. Pharmacol. 2021, 12, 603997. [Google Scholar] [CrossRef]
- Artini, M.; Vrenna, G.; Trecca, M.; Tuccio Guarna Assanti, V.; Fiscarelli, E.V.; Papa, R.; Selan, L. Serratiopeptidase Affects the Physiology of Pseudomonas Aeruginosa Isolates from Cystic Fibrosis Patients. Int. J. Mol. Sci. 2022, 23, 12645. [Google Scholar] [CrossRef]
- Vélez-Gómez, J.M.; Melchor-Moncada, J.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. Purification and Characterization of a Metalloprotease Produced by the C8 Isolate of Serratia Marcescens Using Silkworm Pupae or Casein as a Protein Source. Int. J. Biol. Macromol. 2019, 135, 97–105. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Shah, N.; Rathi, A.; Rathi, V.; Rathi, A. Serratiopeptidase: Insights into the Therapeutic Applications. Biotechnol. Rep. 2020, 28, e00544. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.A.; Valizadeh, V.; Rouhani, M.; Mirkazemi, S.; Azizi, M.; Norouzian, D.; Ahangari Cohan, R. Novel Serratiopeptidase Exhibits Different Affinities to the Substrates and Inhibitors. Chem. Biol. Drug Des. 2022, 100, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Enzyme Immobilization and Co-Immobilization: Main Framework, Advances and Some Applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme Immobilization Strategies for the Design of Robust and Efficient Biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Jiang, J.; Gao, L. Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance. Antibiotics 2022, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Thallinger, B.; Prasetyo, E.N.; Nyanhongo, G.S.; Guebitz, G.M. Antimicrobial Enzymes: An Emerging Strategy to Fight Microbes and Microbial Biofilms. Biotechnol. J. 2013, 8, 97–109. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles-Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Hasanzadeh Kafshgari, M.; Goldmann, W.H. Insights into Theranostic Properties of Titanium Dioxide for Nanomedicine. Nanomicro Lett. 2020, 12, 22. [Google Scholar] [CrossRef]
- Tang, Z.; He, H.; Zhu, L.; Liu, Z.; Yang, J.; Qin, G.; Wu, J.; Tang, Y.; Zhang, D.; Chen, Q.; et al. A General Protein Unfolding-Chemical Coupling Strategy for Pure Protein Hydrogels with Mechanically Strong and Multifunctional Properties. Adv. Sci. 2022, 9, 2102557. [Google Scholar] [CrossRef]
- Wickramathilaka, M.P.; Tao, B.Y. Characterization of Covalent Crosslinking Strategies for Synthesizing DNA-Based Bioconjugates. J. Biol. Eng. 2019, 13, 63. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A Facile Method for Tauc Exponent and Corresponding Electronic Transitions Determination in Semiconductors Directly from UV–Vis Spectroscopy Data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Rajaeian, B.; Heitz, A.; Tade, M.O.; Liu, S. Improved Separation and Antifouling Performance of PVA Thin Film Nanocomposite Membranes Incorporated with Carboxylated TiO2 Nanoparticles. J. Membr. Sci. 2015, 485, 48–59. [Google Scholar] [CrossRef]
- Srour, B.; Bruechert, S.; Andrade, S.L.A.; Hellwing, P. Secondary Structure Determination by Means of ATR-FTIR Spectroscopy. Methods Mol. Biol. 2017, 1635, 195–203. [Google Scholar] [PubMed]
- Zuluaga-Vélez, A.; Toro-Acevedo, C.A.; Quintero-Martinez, A.; Melchor-Moncada, J.J.; Pedraza-Ordoñez, F.; Aguilar-Fernández, E.; Sepúlveda-Arias, J.C. Performance of Colombian Silk Fibroin Hydrogels for Hyaline Cartilage Tissue Engineering. J. Funct. Biomater. 2022, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Song, J.; Jang, J. Photocatalytic Antibacterial Capabilities of TiO2−Biocidal Polymer Nanocomposites Synthesized by a Surface-Initiated Photopolymerization. Environ. Sci. Technol. 2010, 44, 5672–5676. [Google Scholar] [CrossRef]
- Kolaei, M.; Tayebi, M.; Masoumi, Z.; Tayyebi, A.; Lee, B.K. Optimal Growth of Sodium Titanate Nanoflower on TiO2 Thin Film for the Fabrication of a Novel Ti/TiO2/Na2Ti3O7 Photoanode with Excellent Stability. J. Alloys Compd. 2022, 913, 165337. [Google Scholar] [CrossRef]
- Prabhu, R.; Jeevananda, T.; Mohan, N. Spectral and Thermal Studies on Polyaniline-Titanium Dioxide Nanocomposites by Inverted Emulsion Techniques. Mater. Today Proc. 2019, 27, 2164–2168. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, L.; Peng, S.; Shi, J.; Liu, Z.; Wen, W. Wettability of Urea-Doped TiO2 Nanoparticles and Their High Electrorheological Effects. J. Solgel Sci. Technol. 2008, 47, 311–315. [Google Scholar] [CrossRef]
- Kathiravan, A.; Renganathan, R. Photosensitization of Colloidal TiO2 Nanoparticles with Phycocyanin Pigment. J. Colloid Interface Sci. 2009, 335, 196–202. [Google Scholar] [CrossRef]
- Mobeen Amanulla, A.; Sundaram, R. Green Synthesis of TiO2 Nanoparticles Using Orange Peel Extract for Antibacterial, Cytotoxicity and Humidity Sensor Applications. Mater. Today Proc. 2019, 8, 323–331. [Google Scholar] [CrossRef]
- Valsaraj, P.V. Divyarthana. Structural, Optical and Antimicrobial Properties of Green Synthesized Cerium Oxide Nanoparticles. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; Volume 2162. [Google Scholar]
- Mendonça, C.D.; Khan, S.U.; Rahemi, V.; Verbruggen, S.W.; Machado, S.A.S.; De Wael, K. Surface Plasmon Resonance-Induced Visible Light Photocatalytic TiO2 Modified with AuNPs for the Quantification of Hydroquinone. Electrochim. Acta 2021, 389, 138734. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Del Campo, A.; Fernández, J.F. Enhancement of UV Absorption Behavior in ZnO-TiO2 composites. Bol. Soc. Esp. Ceram. Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.H. Size Dependency of Nanocrystalline TiO2 on Its Optical Property and Photocatalytic Reactivity Exemplified by 2-Chlorophenol. Appl. Catal. B 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Reconsideration of Intrinsic Band Alignments within Anatase and Rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Dhivya Pushpa, M.; Sanclemente Crespo, M.; Cristopher, M.M.; Karthick, P.; Sridharan, M.; Sanjeeviraja, C.; Jeyadheepan, K. Influence of Pyrolytic Temperature on Optoelectronic Properties and the Energy Harvesting Applications of High Pressure TiO2 Thin Films. Vacuum 2019, 161, 81–91. [Google Scholar] [CrossRef]
- Ko, K.C.; Bromley, S.T.; Lee, J.Y.; Illas, F. Size-Dependent Level Alignment between Rutile and Anatase TiO2 Nanoparticles: Implications for Photocatalysis. J. Phys. Chem. Lett. 2017, 8, 5593–5598. [Google Scholar] [CrossRef]
- Leynen, N.; Tytgat, J.S.; Bijnens, K.; Jaenen, V.; Verleysen, E.; Artois, T.; Van Belleghem, F.; Saenen, N.D.; Smeets, K. Assessing the in Vivo Toxicity of Titanium Dioxide Nanoparticles in Schmidtea Mediterranea: Uptake Pathways and (Neuro)Developmental Outcomes. Aquat. Toxicol. 2024, 270, 106895. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.A.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of Zinc Oxide Nanoparticles by Cell-Biomass and Supernatant of Lactobacillus Plantarum TA4 and Its Antibacterial and Biocompatibility Properties. Sci. Rep. 2020, 10, 19996. [Google Scholar] [CrossRef]
- Romoser, A.A.; Figueroa, D.E.; Sooresh, A.; Scribner, K.; Chen, P.L.; Porter, W.; Criscitiello, M.F.; Sayes, C.M. Distinct Immunomodulatory Effects of a Panel of Nanomaterials in Human Dermal Fibroblasts. Toxicol. Lett. 2012, 210, 293–301. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, S.; Lou, X. Controlling Silica Coating Thickness on TiO2 Nanoparticles for Effective Photodynamic Therapy. Colloids Surf. B Biointerfaces 2013, 107, 220–226. [Google Scholar] [CrossRef]
- Franchi, L.P.; Manshian, B.B.; de Souza, T.A.J.; Soenen, S.J.; Matsubara, E.Y.; Rosolen, J.M.; Takahashi, C.S. Cyto- and Genotoxic Effects of Metallic Nanoparticles in Untransformed Human Fibroblast. Toxicology In Vitro 2015, 29, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Senapati, V.A.; Singh, D.; Dubey, K.; Maurya, R.; Pandey, A.K. Impact of Anatase Titanium Dioxide Nanoparticles on Mutagenic and Genotoxic Response in Chinese Hamster Lung Fibroblast Cells (V-79): The Role of Cellular Uptake. Food Chem. Toxicol. 2017, 105, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Kovrlija, I.; Pańczyszyn, E.; Demir, O.; Laizane, M.; Corazzari, M.; Locs, J.; Loca, D. Doxorubicin Loaded Octacalcium Phosphate Particles as Controlled Release Drug Delivery Systems: Physico-Chemical Characterization, in Vitro Drug Release and Evaluation of Cell Death Pathway. Int. J. Pharm. 2024, 653, 123932. [Google Scholar] [CrossRef] [PubMed]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozzi, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.D.; Armato, C.; Rossi, A.M.; Schilirò, T. Shape-Engineered Titanium Dioxide Nanoparticles (TiO 2 -NPs): Cytotoxicity and Genotoxicity in Bronchial Epithelial Cells. Food Chem. Toxicol. 2019, 127, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Bhargavi, P.L.; Prakasham, R.S. Agro-Industrial Wastes Utilization for the Generation of Fibrinolytic Metalloprotease by Serratia Marcescens RSPB11. Biocatal. Agric. Biotechnol. 2017, 9, 201–208. [Google Scholar] [CrossRef]
- Kim, H.S.; Golyshin, P.N.; Timmis, K.N. Characterization and Role of a Metalloprotease Induced by Chitin in Serratia sp. KCK. J. Ind. Microbiol. Biotechnol. 2007, 34, 715–721. [Google Scholar] [CrossRef]
- Bach, E.; Sant’Anna, V.; Daroit, D.J.; Corrêa, A.P.F.; Segalin, J.; Brandelli, A. Production, One-Step Purification, and Characterization of a Keratinolytic Protease from Serratia Marcescens P3. Process Biochem. 2012, 47, 2455–2462. [Google Scholar] [CrossRef][Green Version]
- Badhe, R.V.; Nanda, R.K.; Kulkarni, M.B.; Bhujbal, M.N.; Patil, P.S.; Badhe, S.R. Media Optimization Studies for Serratiopeptidase Production from Serratia Marcescens ATCC 13880. Hindustan Antibiot. Bull. 2009, 51, 17–23. [Google Scholar]
- Koul, D.; Chander, D.; Manhas, R.S.; Hossain, M.M.; Dar, M.J.; Chaubey, A. Purification, Functional Characterization and Enhanced Production of Serratiopeptidase from Serratia Marcescens MES-4: An Endophyte Isolated from Morus Rubra. J. Biotechnol. 2024, 387, 58–68. [Google Scholar] [CrossRef]
- Melchor-Moncada, J.J.; García-Barco, A.; Zuluaga-Vélez, A.; Veloza, L.A.; Sepúlveda-Arias, J.C. Scale-Up of the Fermentation Process for the Production and Purification of Serratiopeptidase Using Silkworm Pupae as a Substrate. Methods Protoc. 2024, 7, 19. [Google Scholar] [CrossRef]
- Bohdziewicz, J. Ultrafiltration of Technical Amylolytic Enyzymes. Process Biochem. 1996, 31, 185–191. [Google Scholar] [CrossRef]
- Schratter, P. Purification and Concentration by Ultrafiltration. In Protein Purification Protocols; Humana Press: Totowa, NJ, USA, 2004; Volume 244, pp. 101–116. [Google Scholar]
- Doshi, P.; Dantroliya, S.; Modi, A.; Shukla, A.; Patel, D.; Joshi, C.; Joshi, M. Enhanced Production Process of Recombinant Mature Serratiopeptidase in Escherichia Coli Using Fed-Batch Culture by Self-Proteolytic Activity of Fusion Protein. Fermentation 2022, 8, 307. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S. Advances in the Application of Immobilized Enzyme for the Remediation of Hazardous Pollutant: A Review. Chemosphere 2022, 299, 134390. [Google Scholar] [CrossRef] [PubMed]
- Raghav, R.; Srivastava, S. Immobilization Strategy for Enhancing Sensitivity of Immunosensors: L-Asparagine-AuNPs as a Promising Alternative of EDC-NHS Activated Citrate-AuNPs for Antibody Immobilization. Biosens. Bioelectron. 2016, 78, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Xing, Y.; Wang, G.; Feng, Q.; Chen, Q.; Feng, H.; Sun, X.; Liu, L. Probing of EDC/NHSS-Mediated Covalent Coupling Reaction by the Immobilization of Electrochemically Active Biomolecules. Int. J. Electrochem. Sci. 2013, 8, 2459–2467. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Wagner, H.; Rapp, B.E.; Franzreb, M. Optimization of Enzyme Immobilization on Magnetic Microparticles Using 1-Ethyl-3-(3-Dimethylaminopropyl)Carbodiimide (EDC) as a Crosslinking Agent. Anal. Methods 2015, 7, 10291–10298. [Google Scholar] [CrossRef]
- Miyata, K.; Maejima, K.; Tomoda, K.; Isono, M. Serratia Protease: Part I. Purification and General Properties of the Enzyme. Agric. Biol. Chem. 1970, 34, 310–318. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzym. Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef]
- Kumar, S.; Jana, A.K.; Dhamija, I.; Singla, Y.; Maiti, M. Preparation, Characterization and Targeted Delivery of Serratiopeptidase Immobilized on Amino-Functionalized Magnetic Nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 413–426. [Google Scholar] [CrossRef]
- Guo, H.; Lei, B.; Yu, J.; Chen, Y.; Qian, J. Immobilization of Lipase by Dialdehyde Cellulose Crosslinked Magnetic Nanoparticles. Int. J. Biol. Macromol. 2021, 185, 287–296. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Fang, H.; Jiang, S.; Hou, H. Mechanically Strong Sulfonated Polybenzimidazole PEMs with Enhanced Proton Conductivity. Mater. Lett. 2019, 234, 354–356. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Wu, D.; Ran, T.; Wang, W.; Xu, D. Structure of a thermostable serralysin from Serratia sp. FS14 at 1.1 Å resolution. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 10–15. [Google Scholar] [CrossRef] [PubMed]
- López-Gallego, F.; Guisán, J.M.; Betancor, L. Stabilization of Enzymes by Multipoint Covalent Immobilization on Supports Activated with Glyoxyl Groups. In Immobilizaion of Enzymes and Cells; Guisan, J.M., Ed.; Springer: London, UK, 2013; Volume 1051, pp. 59–71. ISBN 9781627035491. [Google Scholar]
- Rodriguez-Loya, J.; Lerma, M.; Gardea-Torresdey, J.L. Dynamic Light Scattering and Its Application to Control Nanoparticle Aggregation in Colloidal Systems: A Review. Micromachines 2024, 15, 24. [Google Scholar] [CrossRef]
- Marucco, A.; Prono, M.; Beal, D.; Alasonati, E.; Fisicaro, P.; Bergamaschi, E.; Carriere, M.; Fenoglio, I. Biotransformation of Food-Grade and Nanometric TiO2 in the Oral-Gastro-Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials 2020, 10, 2132. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting Zeta Potential, the Key Feature of Interfacial Phenomena, with Applications and Recent Advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Keleştemur, S.; Altunbek, M.; Culha, M. Influence of EDC/NHS Coupling Chemistry on Stability and Cytotoxicity of ZnO Nanoparticles Modified with Proteins. Appl. Surf. Sci. 2017, 403, 455–463. [Google Scholar] [CrossRef]
- Yang, J.Y.; Bae, J.; Jung, A.; Park, S.; Chung, S.; Seok, J.; Roh, H.; Han, Y.; Oh, J.M.; Sohn, S.; et al. Surface Functionalization-Specific Binding of Coagulation Factors by Zinc Oxide Nanoparticles Delays Coagulation Time and Reduces Thrombin Generation Potential in Vitro. PLoS ONE 2017, 12, e0181634. [Google Scholar] [CrossRef]
- Khan, M.F.; Kundu, D.; Hazra, C.; Patra, S. A Strategic Approach of Enzyme Engineering by Attribute Ranking and Enzyme Immobilization on Zinc Oxide Nanoparticles to Attain Thermostability in Mesophilic Bacillus Subtilis Lipase for Detergent Formulation. Int. J. Biol. Macromol. 2019, 136, 66–82. [Google Scholar] [CrossRef]
- Selan, L.; Berlutti, F.; Passariello, C.; Comodi-Ballanti, M.R.; Thaller, M.C. Proteolytic Enzymes: A New Treatment Strategy for Prosthetic Infections? Antimicrob. Agents Chemother. 1993, 37, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Klem, H.; Alegre-Requena, J.V.; Paton, R.S. Catalytic Effects of Active Site Conformational Change in the Allosteric Activation of Imidazole Glycerol Phosphate Synthase. ACS Catal. 2023, 13, 16249–16257. [Google Scholar] [CrossRef] [PubMed]
- Solanki, L.A.; Dinesh, S.P.S.; Jain, R.K.; Balasubramaniam, A. Effects of Titanium Oxide Coating on the Antimicrobial Properties, Surface Characteristics, and Cytotoxicity of Orthodontic Brackets–A Systematic Review and Meta Analysis of in-Vitro Studies. J. Oral Biol. Craniofacial Res. 2023, 13, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.V.; Nirwane, A.M.; Belubbi, T.; Nagarsenker, M.S. Pulmonary Delivery of Synergistic Combination of Fluoroquinolone Antibiotic Complemented with Proteolytic Enzyme: A Novel Antimicrobial and Antibiofilm Strategy. Nanomedicine 2017, 13, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.; Valizadeh, V.; Bakhshandeh, H.; Hosseinzadeh, S.A.; Molasalehi, S.; Atyabi, S.M.; Norouzian, D. Improved Anti-Biofilm Activity and Long-Lasting Effects of Novel Serratiopeptidase Immobilized on Cellulose Nanofibers. Appl. Microbiol. Biotechnol. 2023, 107, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Mahdiani, H.; Yazdani, F.; Khoramipour, M.; Valizadeh, V.; Bakhshandeh, H.; Dinarvand, R. Preparation and Physicochemical Characterization of Hyaluronic Acid-Lysine Nanogels Containing Serratiopeptidase to Control Biofilm Formation. Sci. Rep. 2024, 14, 6111. [Google Scholar] [CrossRef]
- Filby, B.W.; Weldrick, P.J.; Paunov, V.N. Overcoming Beta-Lactamase-Based Antimicrobial Resistance by Nanocarrier-Loaded Clavulanic Acid and Antibiotic Cotreatments. ACS Appl. Bio Mater. 2022, 5, 3826–3840. [Google Scholar] [CrossRef]
- Papa, R.; Artini, M.; Cellini, A.; Tilotta, M.; Galano, E.; Pucci, P.; Amoresano, A.; Selan, L. A New Anti-Infective Strategy to Reduce the Spreading of Antibiotic Resistance by the Action on Adhesion-Mediated Virulence Factors in Staphylococcus Aureus. Microb. Pathog. 2013, 63, 44–53. [Google Scholar] [CrossRef]
- Mantravadi, H.B. Effectivity of Titanium Oxide Based Nano Particles on E. Coli from Clinical Samples. J. Clin. Diagn. Res. 2017, 11, DC37–DC40. [Google Scholar] [CrossRef]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef]
- González-Restrepo, D.; Zuluaga-Vélez, A.; Orozco, L.M.; Sepúlveda-Arias, J.C. Silk Fibroin-Based Dressings with Antibacterial and Anti-Inflammatory Properties. Eur. J. Pharm. Sci. 2024, 195, 106710. [Google Scholar] [CrossRef] [PubMed]

| Tauc Equation | Units | |
|---|---|---|
| A | Absorbance | a.u |
| α | ||
| c | ||
| a.u | ||
| eV | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchor-Moncada, J.J.; Vasquez-Giraldo, S.; Zuluaga-Vélez, A.; Orozco, L.M.; Veloza, L.A.; Sepúlveda-Arias, J.C. Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties. J. Funct. Biomater. 2024, 15, 300. https://doi.org/10.3390/jfb15100300
Melchor-Moncada JJ, Vasquez-Giraldo S, Zuluaga-Vélez A, Orozco LM, Veloza LA, Sepúlveda-Arias JC. Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties. Journal of Functional Biomaterials. 2024; 15(10):300. https://doi.org/10.3390/jfb15100300
Chicago/Turabian StyleMelchor-Moncada, Jhon Jairo, Santiago Vasquez-Giraldo, Augusto Zuluaga-Vélez, Lina Marcela Orozco, Luz Angela Veloza, and Juan Carlos Sepúlveda-Arias. 2024. "Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties" Journal of Functional Biomaterials 15, no. 10: 300. https://doi.org/10.3390/jfb15100300
APA StyleMelchor-Moncada, J. J., Vasquez-Giraldo, S., Zuluaga-Vélez, A., Orozco, L. M., Veloza, L. A., & Sepúlveda-Arias, J. C. (2024). Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties. Journal of Functional Biomaterials, 15(10), 300. https://doi.org/10.3390/jfb15100300








