Calcium Silicate Promoting the Upcycling Potential of Polysulfone Medical Waste in Load-Bearing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Components
2.2. Preparation of Composites
2.3. Phase Composition and Surface Morphology
2.4. Measurement of Mechanical Properties
2.5. Cytotoxicity
2.6. Bacteria Response
2.7. Statistical Analysis
3. Results
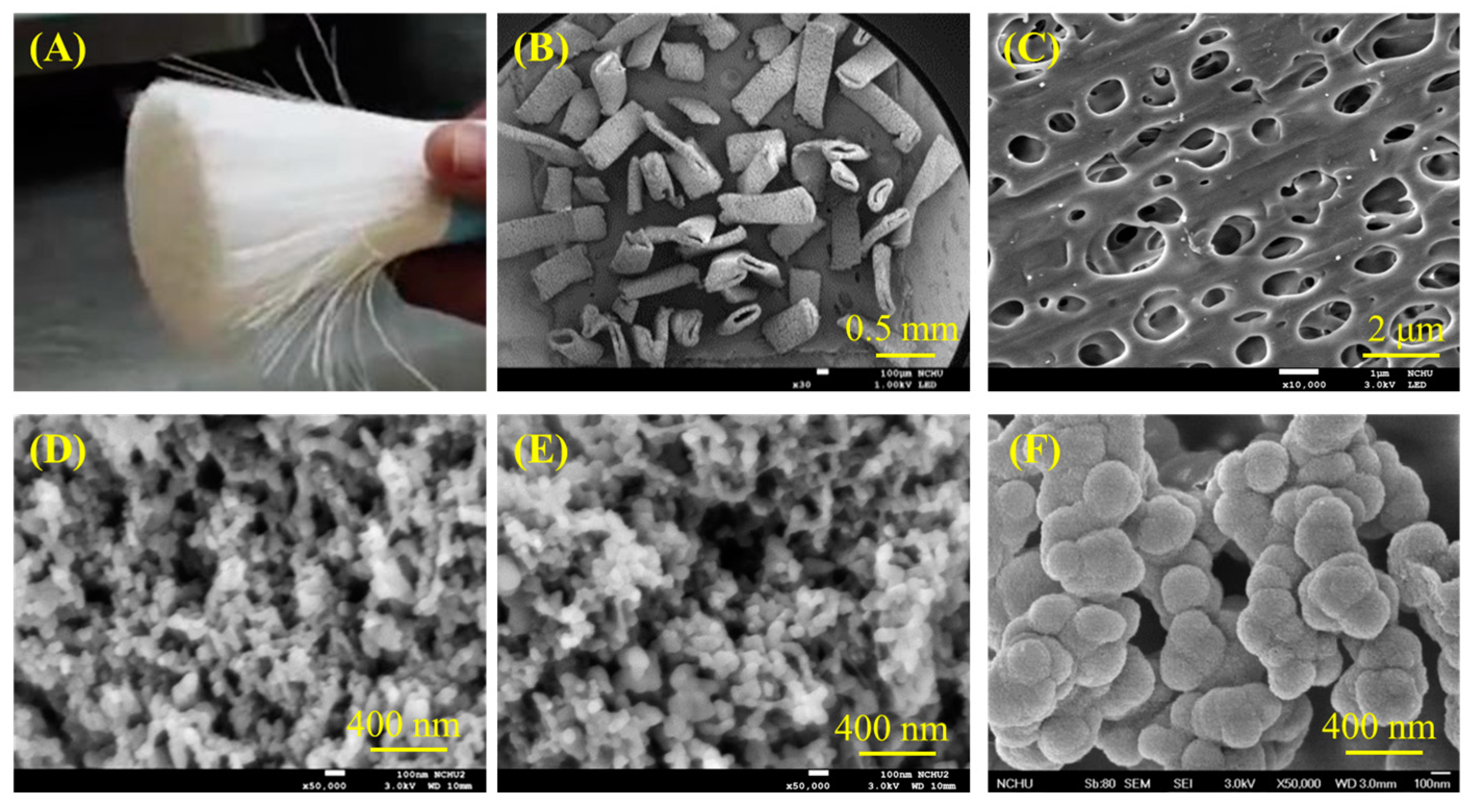
3.1. Morphology of Raw Materials
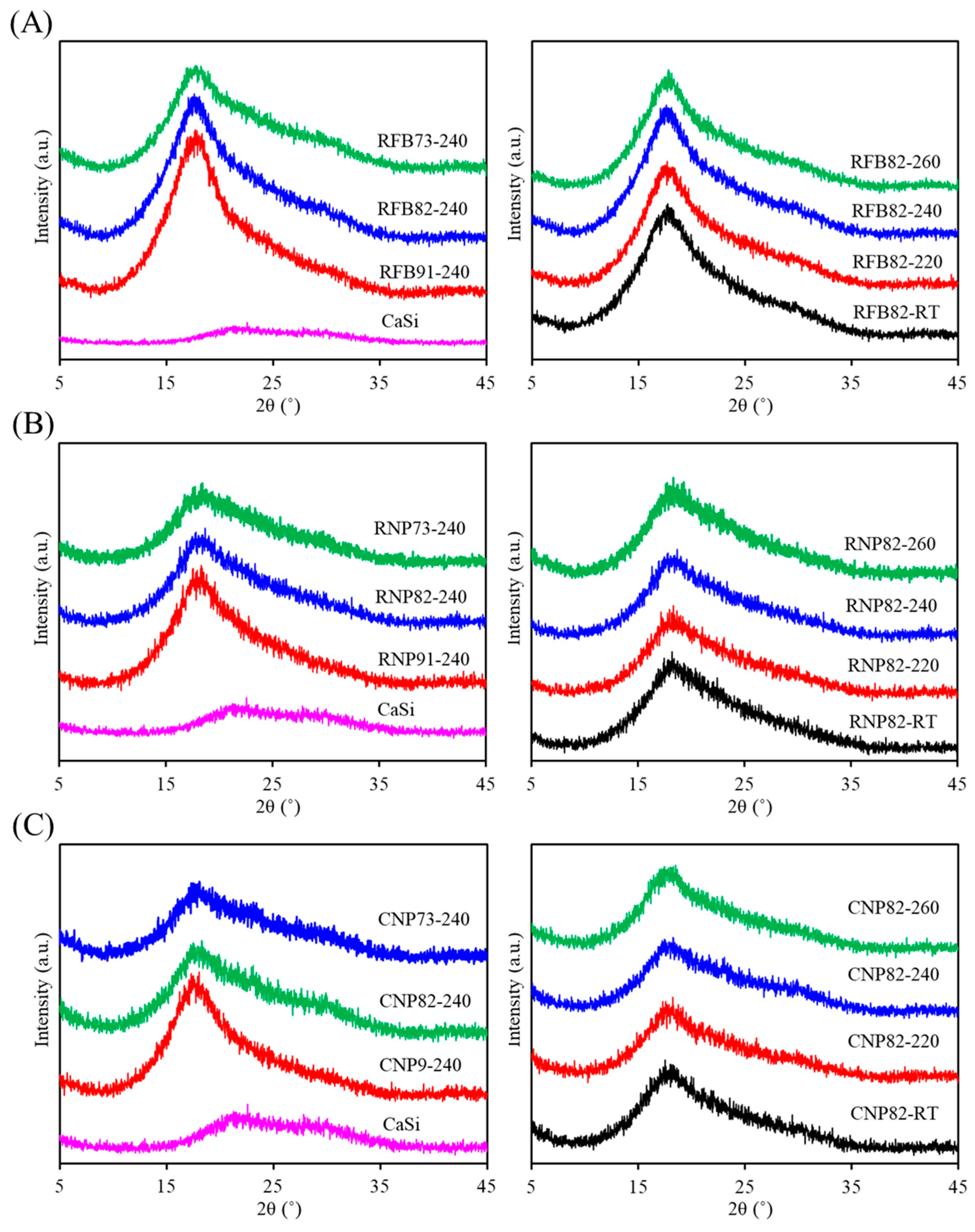
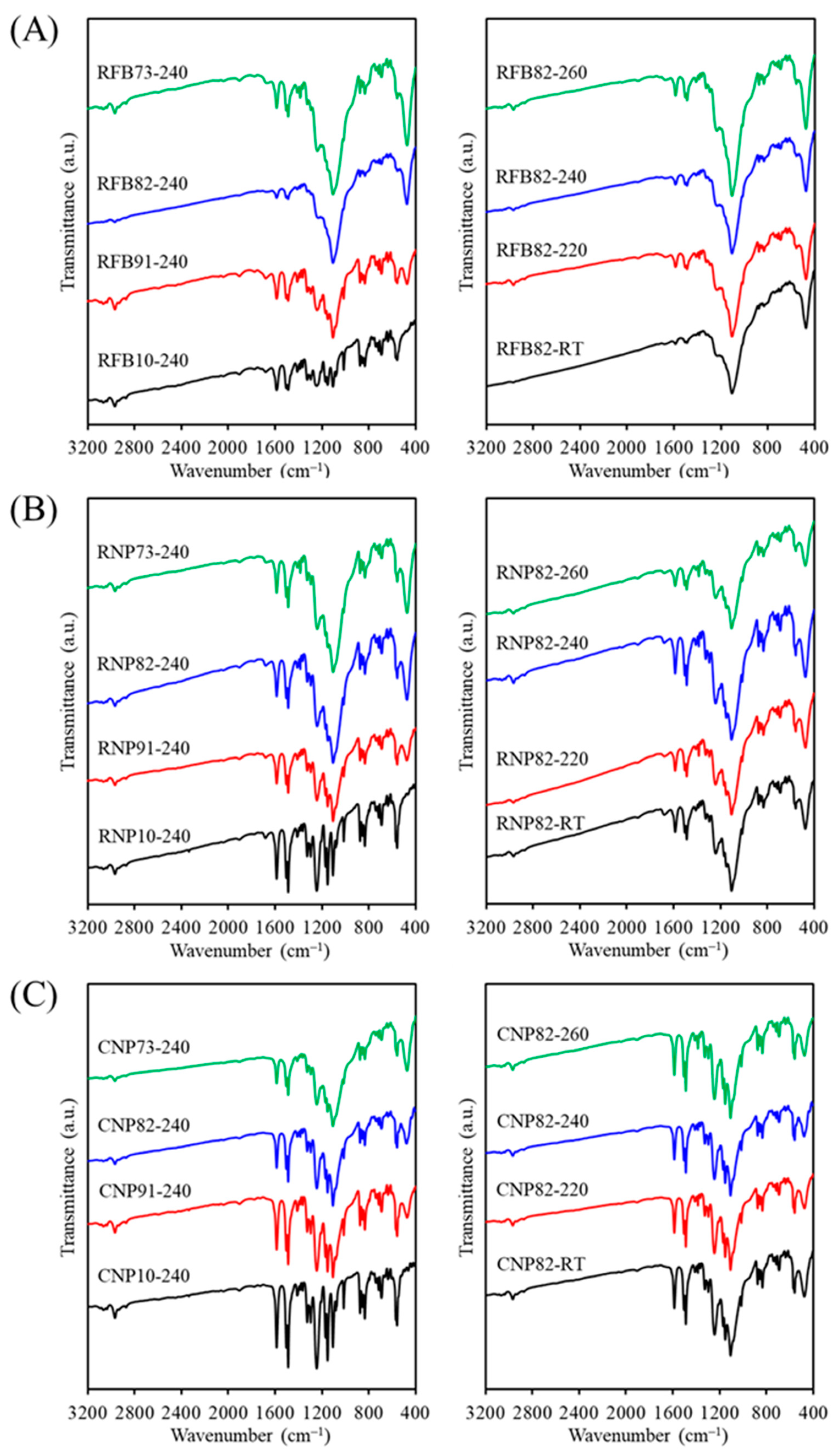
3.2. Phase Composition
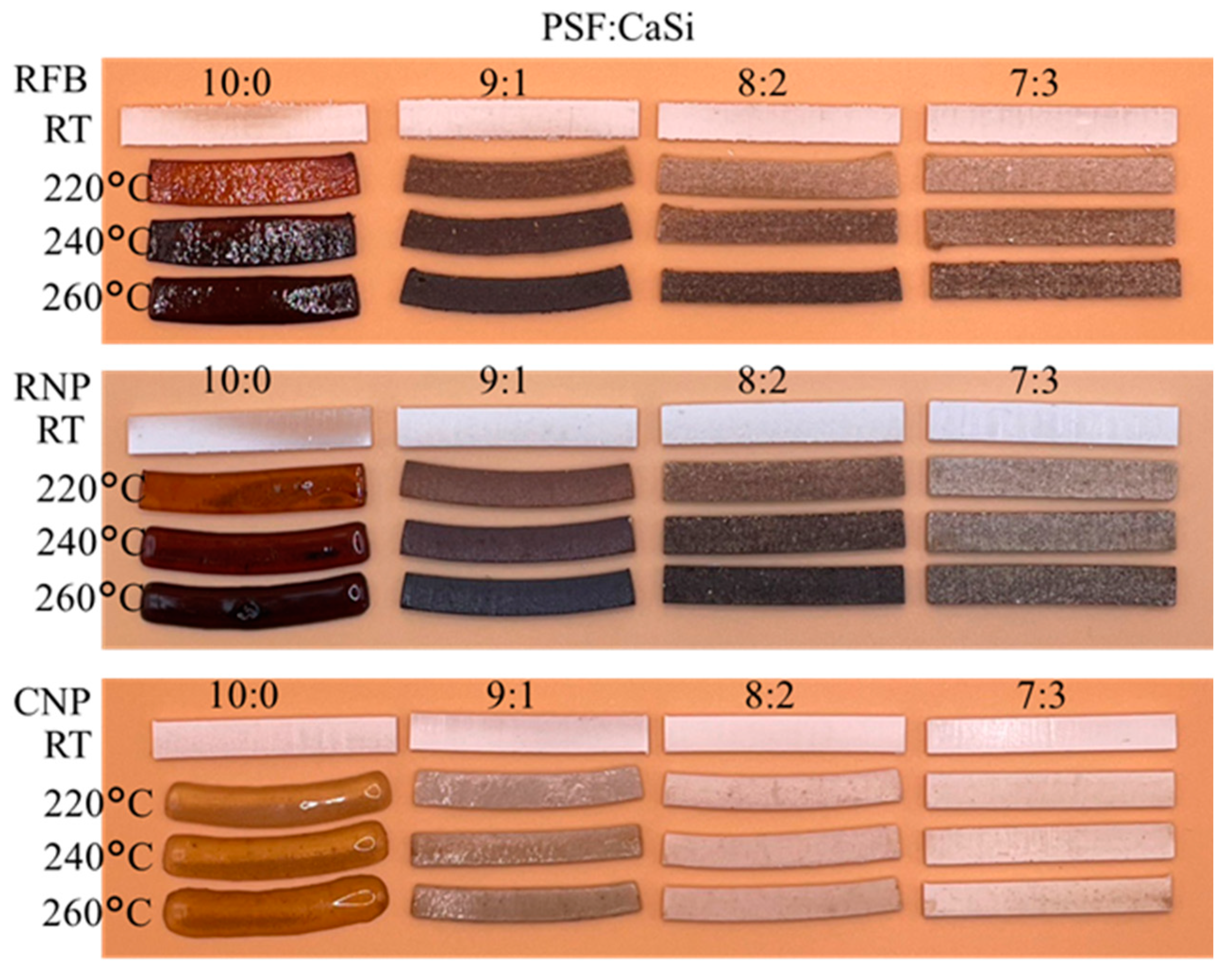
3.3. Formability of Composites
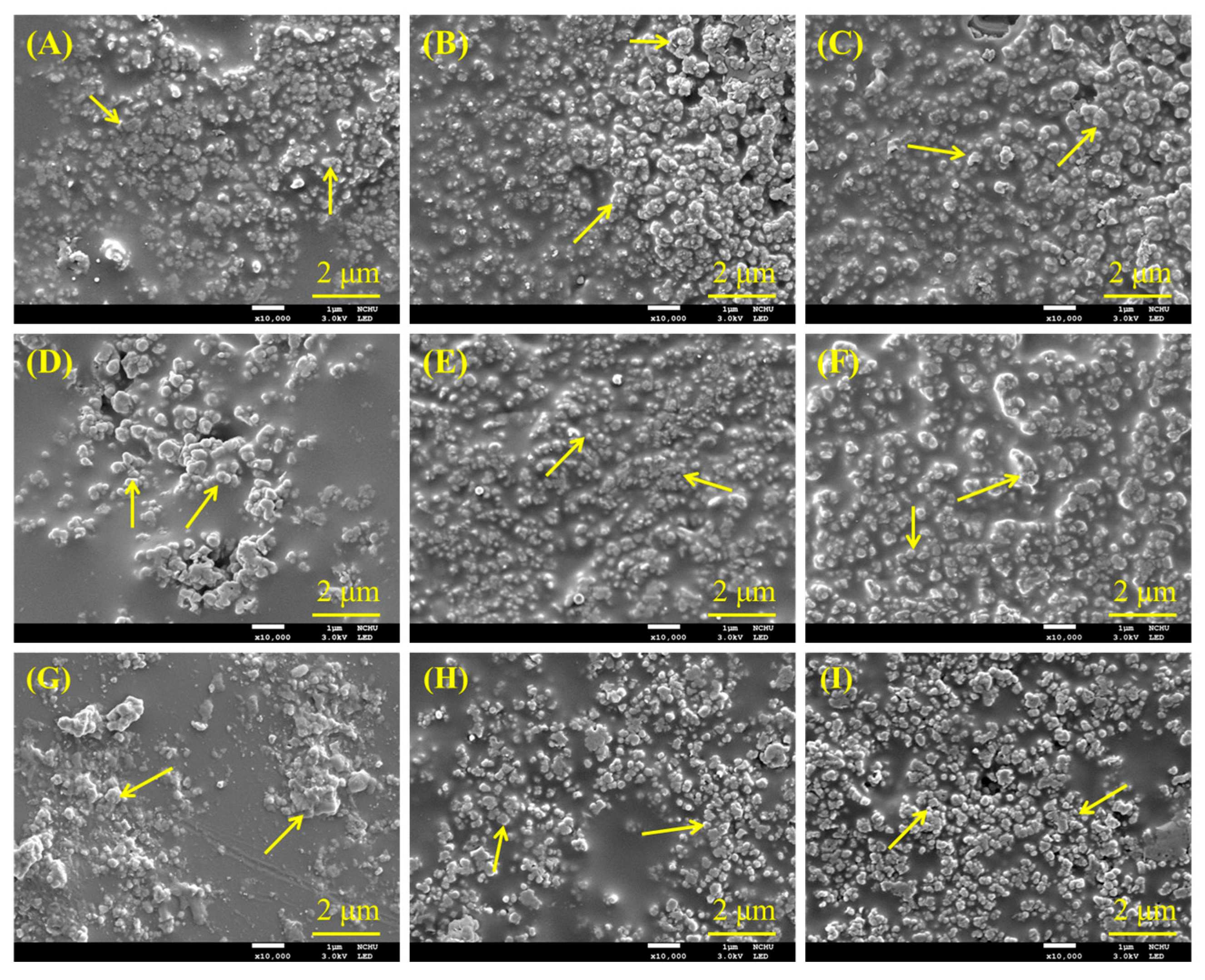
3.4. Morphology of Composites
3.5. Mechanical Properties
3.5.1. Compressive Properties
3.5.2. Tensile Properties
3.5.3. Three-Point Bending Properties
3.6. L929 Cytotoxicity
3.7. Bacterial Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jambeck, J.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Fang, X.; Zhang, Z.; Li, S.; Sun, J. Healable and recyclable polymeric materials with high mechanical robustness. ACS Mater. Lett. 2022, 4, 554–571. [Google Scholar] [CrossRef]
- Available online: https://www.tsn.org.tw/twrds.html?page=&year=2022# (accessed on 1 January 2022).
- Nakashima, A.; Ogata, S.; Doi, S.; Yamahira, M.; Naraki, S.; Takasugi, N.; Ohmoto, T.; Ito, T.; Masaki, T.; Yorioka, N. Performance of polysulfone membrane dialyzers and dialysate flow pattern. Clin. Exp. Nephrol. 2006, 10, 210–215. [Google Scholar] [CrossRef]
- Azizah, D.A.; Kusworo, T.D.; Kumoro, A.C. Developing UV-light driven photocatalytic PSf/Ni-doped ZnO/PDA membrane with superior antifouling, self-cleaning, and self-protecting performances for handmade batik wastewater treatment. Mater. Today Sustain. 2024, 26, 100721. [Google Scholar] [CrossRef]
- Filimon, A.; Albu, R.M.; Stoica, I.; Avram, E. Blends based on ionic polysulfones with improved conformational and microstructural characteristics: Perspectives for biomedical applications. Compos. B 2016, 93, 1–11. [Google Scholar] [CrossRef]
- Stannat, S.; Bahlmann, J.; Kiessling, D.; Koch, K.; Deicher, H.; Peter, H.H. Complement activation during hemodialysis. Comparison of polysulfone and cuprophan membranes. Contrib. Nephrol. 1985, 46, 102–108. [Google Scholar]
- Schaefer, R.M.; Heidland, A.; Hörl, W.H. Release of leukocyte elestase during hemodialysis. Effect of different dialysis membranes. Contrib. Nephrol. 1985, 46, 109–117. [Google Scholar]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Isildak, I.; Oprea, O.; Cristache, C.M. Non-resorbable nanocomposite membranes for guided bone regeneration based on polysulfone-quartz fiber grafted with nano-TiO2. Nanomaterials 2019, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of metal orthopaedic implants. J. Bone Jt. Surg. Am. 1998, 80, 268–282. [Google Scholar] [CrossRef]
- Kettunen, J.; Makela, E.A.; Miettinen, H.; Nevalainen, T.; Heikkilä, M.; Pohjonen, T.; Törmälä, P.; Rokkanen, P. Mechanical properties and strength retention of carbon fibre-reinforced liquid crystalline polymer (LCP/CF) composite: An experimental study on rabbits. Biomaterials 1998, 19, 1219–1228. [Google Scholar] [CrossRef]
- Wei, C.K.; Ding, S.J. Acid-resistant calcium silicate-based composite implants with high-strength as load-bearing bone graft substitutes and fracture fixation devices. J. Mech. Behav. Biomed. Mater. 2016, 62, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Chou, Y.-S.; Lee, T.-M.; Fu, Y.-C.; Ou, S.-F.; Chen, S.-H.; Lee, T.-C.; Wang, Y.-H. The uniform distribution of hydroxyapatite in a polyurethane foam-based scaffold (PU/HAp) to enhance bone repair in a calvarial defect model. Int. J. Mol. Sci. 2024, 25, 6440. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.M.; De Bruijn, W.C. Foreign body reactions to resorbable poly(l-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jones, I.A.; Parsons, A.J.; Bernard, J.; Farmer, J.; Scotchford, C.A.; Walker, G.S.; Rudd, C.D. Composites for bone repair: Phosphate glass fibre reinforced PLA with varying fibre architecture. J. Mater. Sci. Mater. Med. 2011, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Comp. Sci. Tech. 2004, 64, 789–817. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Wang, M.; Bonfield, W. Chemically coupled hydroxyapatite–polyethylene composites: Structure and properties. Biomaterials 2001, 22, 1311–1320. [Google Scholar] [CrossRef]
- Dalby, M.J.; Kayser, M.V.; Bonfield, W.; Di Silvio, L. Initial attachment of osteoblasts to an optimised HAPEX topography. Biomaterials 2002, 23, 681–690. [Google Scholar] [CrossRef]
- Huang, H.; Liu, X.; Wang, J.; Suo, M.; Zhang, J.; Sun, T.; Wang, H.; Liu, C.; Li, Z. Strategies to improve the performance of polyetheretherketone (PEEK) as orthopedic implants: From surface modification to addition of bioactive materials. J. Mater. Chem. B 2024, 12, 4533–4552. [Google Scholar] [CrossRef]
- Oréfice, R.; Clark, A.; West, J.; Brennan, A.; Hench, L. Processing, properties, and in vitro bioactivity of polysulfone-bioactive glass composites. J. Biomed. Mater. Res. A 2007, 80, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yue, C.Y.; Chua, B. Production and evaluation of hydroxyapatite reinforced polysulfone for tissue replacement. J. Mate. Sci. Mater. Med. 2001, 12, 821–826. [Google Scholar] [CrossRef]
- Ding, S.J. Biodegradation behavior of chitosan/calcium phosphate composites. J. Non-Crystal. Solids 2007, 353, 2367–2373. [Google Scholar] [CrossRef]
- Bhat, K.A.; Prakash, P.L.; Manoharan, N.; Lakshmibai, A.; Sangeetha, D. Fabrication of polymethyl methacrylate/polysulfone/nanoceramic composites for orthopedic applications. J. Appl. Polym. Sci. 2013, 127, 2764–2775. [Google Scholar] [CrossRef]
- Babar Munir, H.M.; Yasin, S.; Iqbal, T.; Qamar, S.; Ahmad, A.; Mahmood, H.; Moniruzzaman, M. Thermomechanical evaluation of zinc oxide/hydroxyapatite/high-density polyethylene hybrid composites. J. Appl. Polym. Sci. 2024, 141, e55683. [Google Scholar] [CrossRef]
- Cao, J.; Yang, S.; Liao, Y.; Wang, Y.; He, J.; Xiong, C.; Shi, K.; Hu, X. Evaluation of polyetheretherketone composites modified by calcium silicate and carbon nanotubes for bone regeneration: Mechanical properties, biomineralization and induction of osteoblasts. Front. Bioeng. Biotechnol. 2023, 11, 1271140. [Google Scholar] [CrossRef]
- Robinson, P., II; Wilson, C., II; Mecholsky, J., Jr. Processing and mechanical properties of hydroxyapatite–polysulfone laminated composites. J. Eur. Ceram. Soc. 2014, 34, 1387–1396. [Google Scholar] [CrossRef]
- Marcolongo, M.; Ducheyne, P.; Garino, J.; Schepers, E. Bioactive glass fiber/polymeric composites bond to bone tissue. J. Biomed. Mater. Res. 1998, 39, 161–170. [Google Scholar] [CrossRef]
- Huang, S.C.; Wu, B.C.; Ding, S.J. Stem cell differentiation-induced calcium silicate cement with bacteriostatic activity. J. Mater. Chem. B 2015, 3, 570–580. [Google Scholar] [CrossRef]
- Wu, I.T.; Chu, Y.H.; Huang, Y.R.; Chen, C.C.; Ding, S.J. Antibacterial ability and osteogenic activity of polyphenols-tailored calcium silicate bone cement. J. Mater. Chem. B 2022, 10, 4640–4649. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Xia, L.; Zhao, C.; Chen, L.; Yi, D.; Chang, J.; Huang, L.; Zheng, X.; Zhu, H.; et al. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surf. B 2015, 126, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.T.; Kao, P.F.; Huang, Y.R.; Ding, S.J. In vitro and in vivo osteogenesis of gelatin-modified calcium silicate cement with washout resistance. Mater. Sci. Eng. C 2020, 117, 111297. [Google Scholar] [CrossRef] [PubMed]
- Edanami, N.; Takenaka, S.; Ibn Belal, R.S.; Yoshiba, K.; Takahara, S.; Yoshiba, N.; Ohkura, N.; Noiri, Y. In vivo assessment of the apatite-forming ability of new-generation hydraulic calcium silicate cements using a rat subcutaneous implantation model. J. Funct. Biomater. 2023, 14, 213. [Google Scholar] [CrossRef]
- Huang, Y.R.; Wu, I.T.; Chen, C.C.; Ding, S.J. In vitro comparisons of microscale and nanoscale calcium silicate particles. J. Mater. Chem. B 2020, 8, 6034–6047. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wu, I.T.; Chen, C.C.; Ding, S.J. Synergistic effect of polyethylene glycol and lactic acid on handling properties and antibacterial efficacy of premixed calcium silicate cement. J. Funct. Biomater. 2024, 15, 187. [Google Scholar] [CrossRef]
- Li, B.; Bian, X.; Hu, W.; Wang, X.; Li, Q.; Wang, F.; Sun, M.; Ma, K.; Zhang, C.; Chang, J.; et al. Regenerative and protective effects of calcium silicate on senescent fibroblasts induced by high glucose. Wound Rep. Reg. 2020, 28, 315–325. [Google Scholar] [CrossRef]
- Cheng, W.; Chang, J. Fabrication and characterization of polysulfone–dicalcium silicate composite films. J. Biomater. Appl. 2006, 20, 361–376. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Progress in dental materials: Application of natural ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Chen, B.; Berretta, S.; Evans, K.; Smith, K.; Ghita, O. A primary study into graphene/polyether ether ketone (PEEK) nanocomposite for laser sintering. Appl. Surf. Sci. 2018, 428, 1018–1028. [Google Scholar] [CrossRef]
- Bouchareb, S.; Doufnoune, R.; Riahi, F.; Cherif-Silini, H.; Belbahri, L. High performance of polysulfone/graphene oxide-silver nanocomposites with excellent antibacterial capability for medical applications. Mater. Today Commun. 2021, 27, 102297. [Google Scholar] [CrossRef]
- Fiaschini, N.; Giuliani, C.; Vitali, R.; Tammaro, L.; Valerini, D.; Rinaldi, A. Design and manufacturing of antibacterial electrospun polysulfone membranes functionalized by Ag nanocoating via magnetron sputtering. Nanomaterials 2022, 12, 3962. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. B 2014, 59, 133–139. [Google Scholar] [CrossRef]
- Dong, X.; Jeong, T.J.; Kline, E.; Banks, L.; Grulke, E.; Harris, T.; Escobar, I.C. Eco-friendly solvents and their mixture for the fabrication of polysulfone ultrafiltration membranes: An investigation of doctor blade and slot die casting methods. J. Membr. Sci. 2020, 614, 118510. [Google Scholar] [CrossRef]
- Mehta, R.; Brahmbhatt, H.; Mukherjee, M.; Bhattacharya, A. Tuning separation behavior of tailor-made thin film poly(piperazine-amide) composite membranes for pesticides and salts from water. Desalination 2017, 404, 280–290. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, D.; Wang, W. Mechanical properties and long-term durability of recycled polysulfone plastic. Waste Manag. 2019, 84, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Zambianchi, M.; Aluigi, A.; Capobianco, M.L.; Corticelli, F.; Elmi, I.; Zampolli, S.; Stante, F.; Bocchi, L.; Belosi, F.; Navacchia, M.L.; et al. Polysulfone hollow porous granules prepared from wastesof ultrafiltration membranes as sustainable adsorbent for water and air remediation. Adv. Sustain. Syst. 2017, 1, 1700019. [Google Scholar] [CrossRef]
- Khaliha, S.; Tunioli, F.; Foti, L.; Bianchi, A.; Kovtun, A.; Marforio, T.D.; Zambianchi, M.; Bettini, C.; Briñas, E.; Vázquez, E.; et al. Upcycling of plastic membrane industrial scraps and reuse as sorbent for emerging contaminants in water. Environ. Sci. Water Res. Technol. 2024, 10, 1097–1107. [Google Scholar] [CrossRef]
- Yaszem, M.J.; Paynet, R.G.; Hayes, W.C.; Lange, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef]
- Peroglio, M.; Gremillard, L.; Gauthier, C.; Chazeau, L.; Verrier, S.; Alini, M.; Chevalier, J. Mechanical properties and cytocompatibility of poly(ε-caprolactone)-infiltrated biphasic calcium phosphate scaffolds with bimodal pore distribution. Acta Biomater. 2010, 6, 4369–4379. [Google Scholar] [CrossRef]
- Ding, S.J.; Chu, Y.H.; Chen, P.T. Mechanical biocompatibility, osteogenic activity and antibacterial efficacy of calcium silicate-zirconia biocomposites. ACS Omega 2021, 6, 7106–7118. [Google Scholar] [CrossRef]
- Jeyachandran, P.; Bontha, S.; Bodhak, S.; Balla, V.K.; Kundu, B.; Doddamani, M. Mechanical behaviour of additively manufactured bioactive glass/high density polyethylene composites. J. Mech. Behav. Biomed. Mater. 2020, 108, 103830. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, P.; Shu, Z.; Chen, A.; Su, J.; Wu, H.; Chen, Z.; Yang, L.; Yan, C.; Shi, Y. Laser powder bed fusion of poly-ether-ether-ketone/bioactive glass composites: Processability, mechanical properties, and bioactivity. Compos. Sci. Technol. 2023, 231, 109805. [Google Scholar] [CrossRef]
- Hu, Q.; Li, B.; Wang, M.; Shen, J. Preparation and characterization of biodegradable chitosan/hydroxyapatite nanocomposite rods via in situ hybridization: A potential material as internal fixation of bone fracture. Biomaterials 2004, 25, 779–785. [Google Scholar] [CrossRef]
- Costa, M.R.; Filho, J.A.C.; Luna, C.B.B.; Dantas, G.M.P.; Costa, A.C.F.d.M.; Oliveira, N.M.d.S. Toward the Production of Hydroxyapatite/Poly(Ether-Ether-Ketone) (PEEK) Biocomposites: Exploring the Physicochemical, Mechanical, Cytotoxic and Antimicrobial Properties. Polymers 2024, 16, 2520. [Google Scholar] [CrossRef]
- Danilova, S.N.; Yarusova, S.B.; Kulchin, Y.N.; Zhevtun, I.G.; Buravlev, I.Y.; Okhlopkova, A.A.; Gordienko, P.S.; Subbotin, E.P. UHMWPE/CaSiO3 Nanocomposite: Mechanical and Tribological Properties. Polymers 2021, 13, 570. [Google Scholar] [CrossRef]
- Senra, M.R.; Vieira Marques, M.F.; Saboya Souza, D.H. Ultra-high molecular weight polyethylene bioactive composites with carbonated hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2020, 110, 103938. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Peitl, O.; Oréfice, R.L.; Hench, L.L.; Brennan, A.B. Effect of the crystallization of bioactive glass reinforcing agents on the mechanical properties of polymer composites. Mater. Sci. Eng. A 2004, 372, 245–251. [Google Scholar] [CrossRef]
- Oréfice, R.; West, J.; LaTorre, G.; Hench, L.; Brennan, A. Effect of long-term in vitro testing on the properties of bioactive glass-polysulfone composites. Biomacromolecules 2010, 11, 657–665. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Goodship, A.E.; Kenwright, J. The influence of induced micromovement upon the healing of experimental tibiai fractures. J. Bone Jt. Surg. 1985, 678, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Molster, A.; Grjerdet, N.R.; Raugstad, T.S.; Hvidsten, K.; Alho, A.; Bang, G. Effect of instability on experimental fracture healing. Acta Orthop. Scand. 1982, 53, 521–526. [Google Scholar] [CrossRef]
- Mailhot, J.M.; Sharawy, M.M.; Galal, M.; Oldham, A.M.; Russell, C.M. Porous polysulfone coated with platelet-derived growth factor-BB stimulates proliferation of human periodontal ligament fibroblasts. J. Periodontol. 1996, 67, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, A.; Karlik, W.; Wiechetek, M.; Werynski, A. Attachment and metabolic activity of hepatocytes cultivated on selected polymeric membranes. Int. J. Artif. Organs 1998, 21, 460–466. [Google Scholar] [CrossRef]
- Fonsato, V.; Herrera, M.B.; Buttiglieri, S.; Gatti, S.; Camussi, G.; Tetta, C. Use of a rotary bioartificial liver in the differentiation of human liver stem cells. Tissue Eng. C Methods 2010, 16, 123–132. [Google Scholar] [CrossRef]
- Stankova, L.; Fraczek-Szczypta, A.; Blazewicz, M.; Filova, E.; Blazewicz, S.; Lisa, V.; Bacakova, L. Human osteoblast-like MG 63 cells on polysulfone modified with carbon nanotubes or carbon nanohorns. Carbon 2014, 37, 578–591. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Kitridis, D.; Savvidis, P.; Cheva, A.; Papalois, A.; Givissis, P.; Chalidis, B. Are absorbable plates more resistant to infection than titanium implants? an experimental pre-clinical trial in rabbits. J. Funct. Biomater. 2023, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Xu, J.J.; Qiu, Y.R. A novel kind of polysulfone material with excellent biocompatibility modified by the sulfonated hydroxypropyl chitosan. Mater. Sci. Eng. C 2017, 79, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Harun, Z.; Yusof, K.N.; Yunos, M.Z.; Shohur, M.F.; Jamalludin, M.R. Antibacterial polysulfone membranes: The effect of eugenol and zinc oxide as additives. Mater. Sci. Forum 2016, 867, 132–138. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, M.; Xu, Z.; Jiang, C.; Shen, C.; Meng, Q. Guanidyl-functionalized graphene/polysulfone mixed matrix ultrafiltration membrane with superior permselective, antifouling and antibacterial properties for water treatment. J. Colloid Interface Sci. 2019, 540, 235–305. [Google Scholar] [CrossRef]


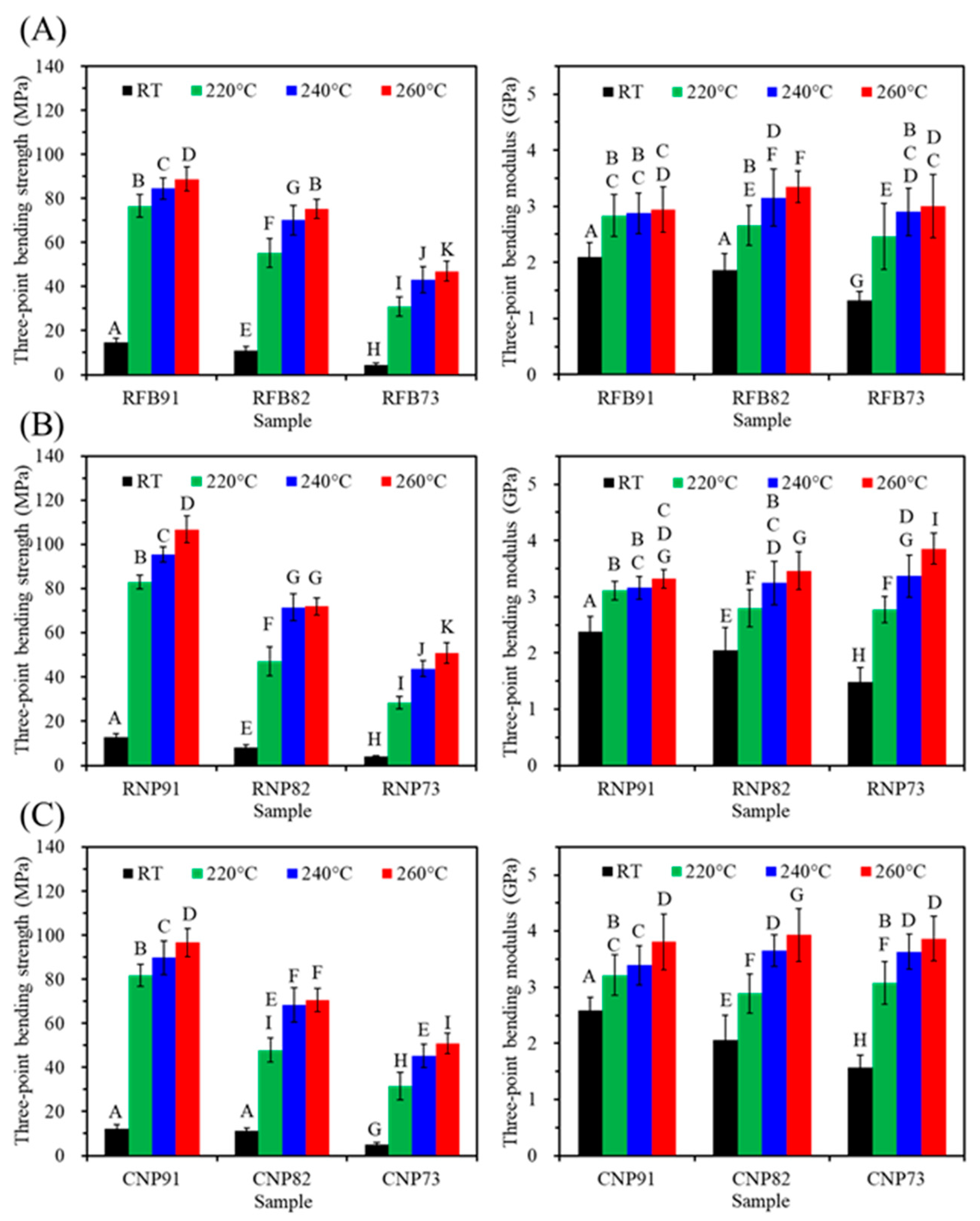
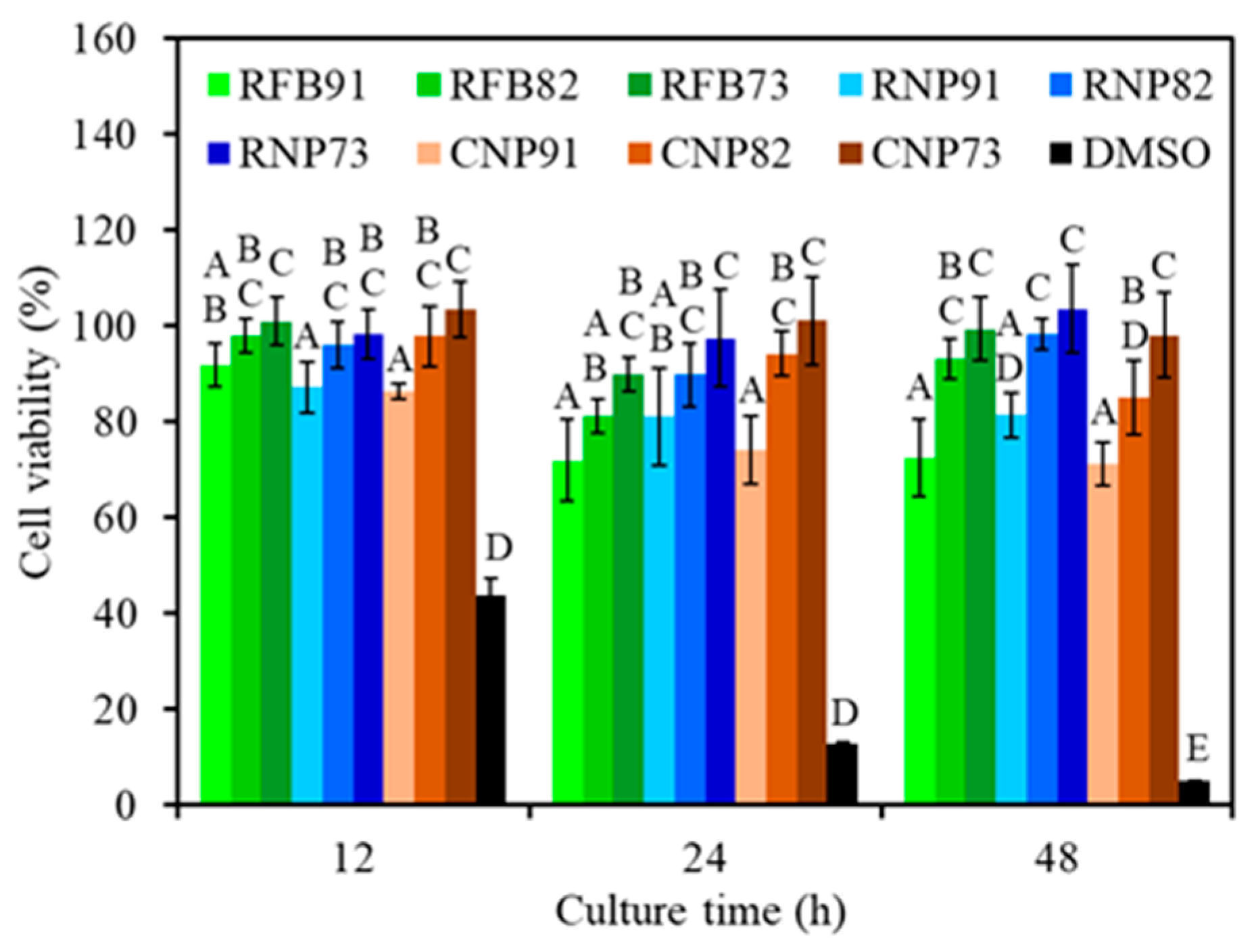

| Sample Code | Compressive | Tensile | Bending | Cell Viability (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Strength (MPa) | Modulus (GPa) | Strength (MPa) | Modulus (GPa) | Strength (MPa) | Modulus (GPa) | 12 h | 24 h | 48 h | |
| RFB | |||||||||
| RFB91 | 71 ± 8 | 2.5 ± 0.3 | 57 ± 5 | 2.8 ± 0.3 | 84 ± 5 | 2.9 ± 0.4 | 92 ± 5 | 72 ± 8 | 73 ± 8 |
| RFB82 | 90 ± 6 | 2.6 ± 0.3 | 42 ± 4 | 2.8 ± 0.4 | 70 ± 7 | 3.2 ± 0.5 | 98 ± 4 | 81 ± 4 | 93 ± 4 |
| RFB73 | 64 ± 6 | 2.3 ± 0.3 | 25 ± 3 | 2.4 ± 0.4 | 43 ± 6 | 2.9 ± 0.4 | 101 ± 5 | 90 ± 4 | 99 ± 7 |
| RNP | |||||||||
| RNP91 | 89 ± 7 | 2.5 ± 0.3 | 70 ± 4 | 2.6 ± 0.5 | 96 ± 4 | 3.2 ± 0.2 | 87 ± 5 | 81 ± 10 | 81 ± 4 |
| RNP82 | 82 ± 6 | 2.6 ± 0.3 | 36 ± 4 | 2.4 ± 0.4 | 72 ± 6 | 3.3 ± 0.4 | 96 ± 5 | 90 ± 7 | 98 ± 3 |
| RNP73 | 62 ± 6 | 2.4 ± 0.3 | 22 ± 4 | 2.7 ± 0.3 | 44 ± 4 | 3.4 ± 0.4 | 98 ± 5 | 98 ± 10 | 104 ± 9 |
| CNP | |||||||||
| CNP91 | 93 ± 5 | 2.5 ± 0.3 | 65 ± 4 | 2.5 ± 0.5 | 90 ± 8 | 3.4 ± 0.4 | 86 ± 2 | 74 ± 7 | 71 ± 5 |
| CNP82 | 82 ± 8 | 2.6 ± 0.3 | 33 ± 4 | 2.5 ± 0.6 | 68 ± 8 | 3.7 ± 0.3 | 98 ± 6 | 94 ± 5 | 85 ± 8 |
| CNP73 | 69 ± 4 | 2.5 ± 0.3 | 21 ± 3 | 2.7 ± 0.4 | 45 ± 5 | 3.6 ± 0.3 | 103 ± 6 | 101 ± 9 | 98 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-N.; Chung, J.-J.; Jiang, H.-Y.; Ding, S.-J. Calcium Silicate Promoting the Upcycling Potential of Polysulfone Medical Waste in Load-Bearing Applications. J. Funct. Biomater. 2024, 15, 323. https://doi.org/10.3390/jfb15110323
Chang C-N, Chung J-J, Jiang H-Y, Ding S-J. Calcium Silicate Promoting the Upcycling Potential of Polysulfone Medical Waste in Load-Bearing Applications. Journal of Functional Biomaterials. 2024; 15(11):323. https://doi.org/10.3390/jfb15110323
Chicago/Turabian StyleChang, Chi-Nan, Jia-Jia Chung, Huei-Yu Jiang, and Shinn-Jyh Ding. 2024. "Calcium Silicate Promoting the Upcycling Potential of Polysulfone Medical Waste in Load-Bearing Applications" Journal of Functional Biomaterials 15, no. 11: 323. https://doi.org/10.3390/jfb15110323
APA StyleChang, C.-N., Chung, J.-J., Jiang, H.-Y., & Ding, S.-J. (2024). Calcium Silicate Promoting the Upcycling Potential of Polysulfone Medical Waste in Load-Bearing Applications. Journal of Functional Biomaterials, 15(11), 323. https://doi.org/10.3390/jfb15110323








