In Vitro Microscopical and Microbiological Assessment of the Sealing Ability of Calcium Silicate-Based Root Canal Sealers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tooth Specimens
2.2. Randomization, Experimental Groups, and Root Canal Filling
| Experimental Group | Name and Manufacturer | Composition | Class |
|---|---|---|---|
| AH | AH Plus® (Dentsply DeTrey GmbH, Konstanz, Germany) | Epoxide paste: Diepoxide, Calcium tungstate, Zirconium oxide, Aerosil, Pigment Amine paste: 1-adamantane amine, N,N′-dibenzyl-5-oxa-nonandiamine-1,9, TCD-Diamine, Calcium tungstate, Zirconium oxide, Aerosil, Silicone oil (manufacturer data sheet) | Epoxy-amin resin sealer (2-component material) |
| PR | ProRoot® MTA (Dentsply DeTrey GmbH, Konstanz, Germany) | Bismuth oxide, tricalcium silicate, dicalcium silicate, calcium aluminate, calcium sulfate dyhydrated, trace elements (Fe, Ni, Cu, Sr) [26] | Calcium silicate-based sealer (2-component material) |
| MC | Medcem MTA (Medcem GmbH, Weinfelden, Switzerland) | Portland cement: tricalcium aluminate and silicate, dicalcium silicate, tetracalcium aluminoferrite, calcium oxide + zirconium oxide (radio opacifier) (manufacturer data sheet) | Calcium silicate-based sealer (2-component material) |
| TF | Total Fill® BC SealerTM/BC-coated gutta-percha (American Dental Systems, Vaterstetten, Germany) | Zirconium oxide, dicalcium and tricalcium silicates, calcium phosphate, calcium hydroxide, filler, thickening agents [17,27] | Bioceramic calcium silicate- based sealer (1-component material) |
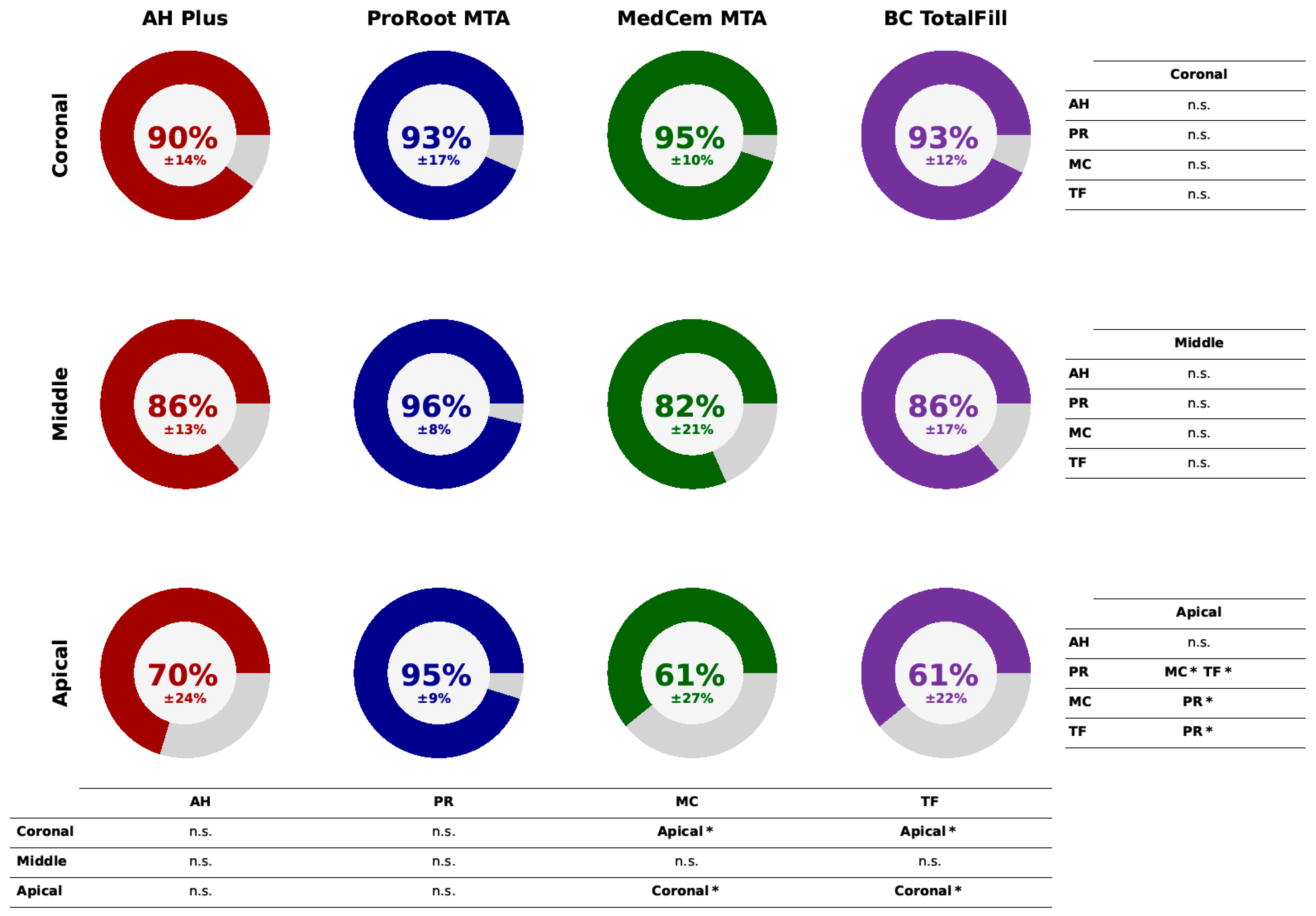
2.3. Solubility Measurements
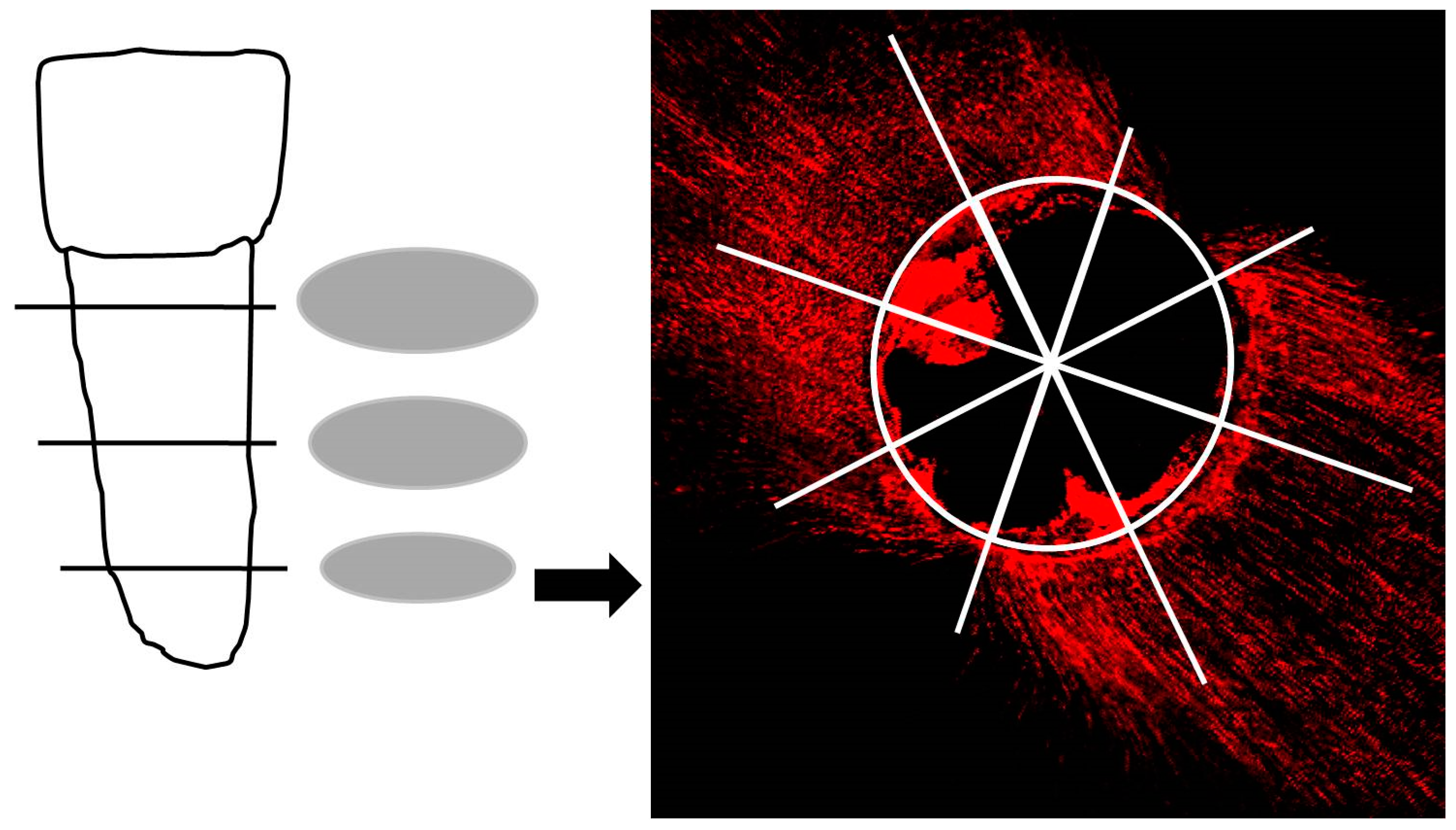
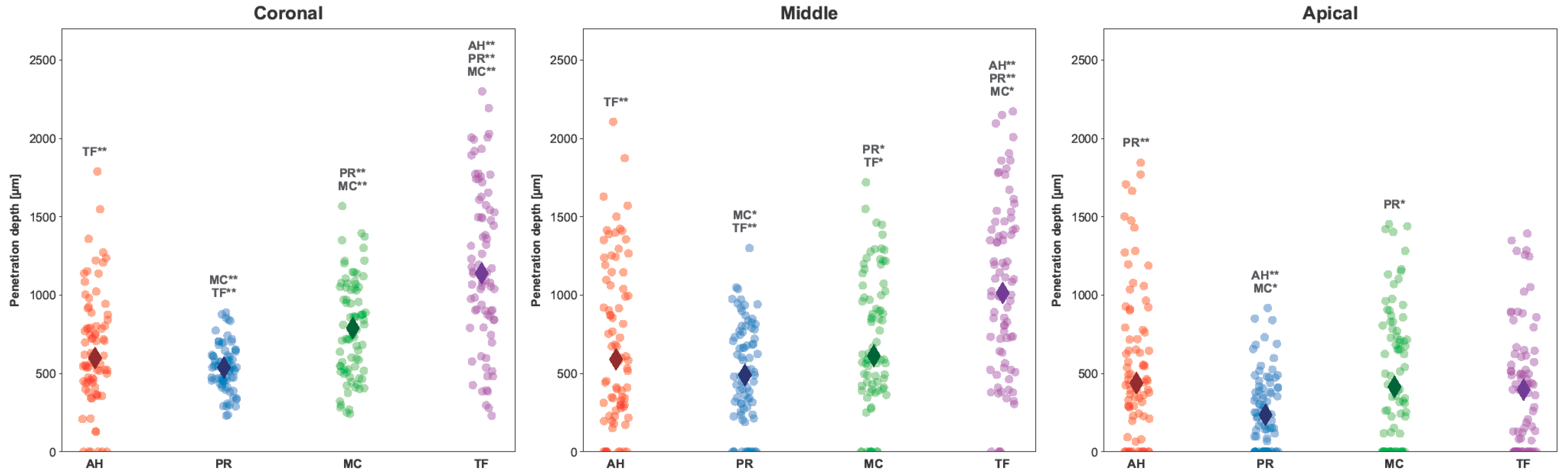
2.4. Fluorescence Microscopic Analysis
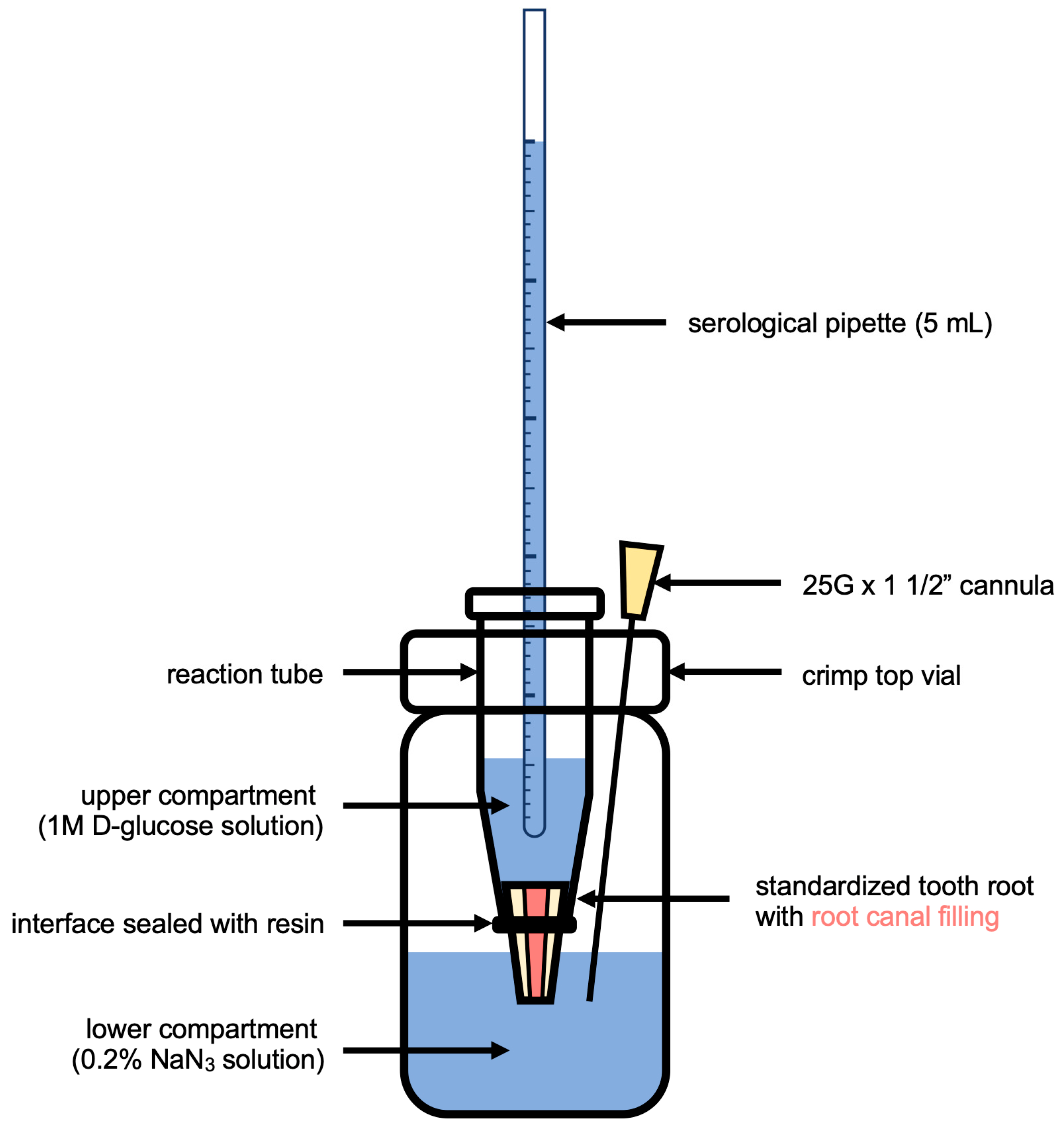
2.5. Glucose Penetration Assay for Leakage Analysis
2.6. Statistical Analysis
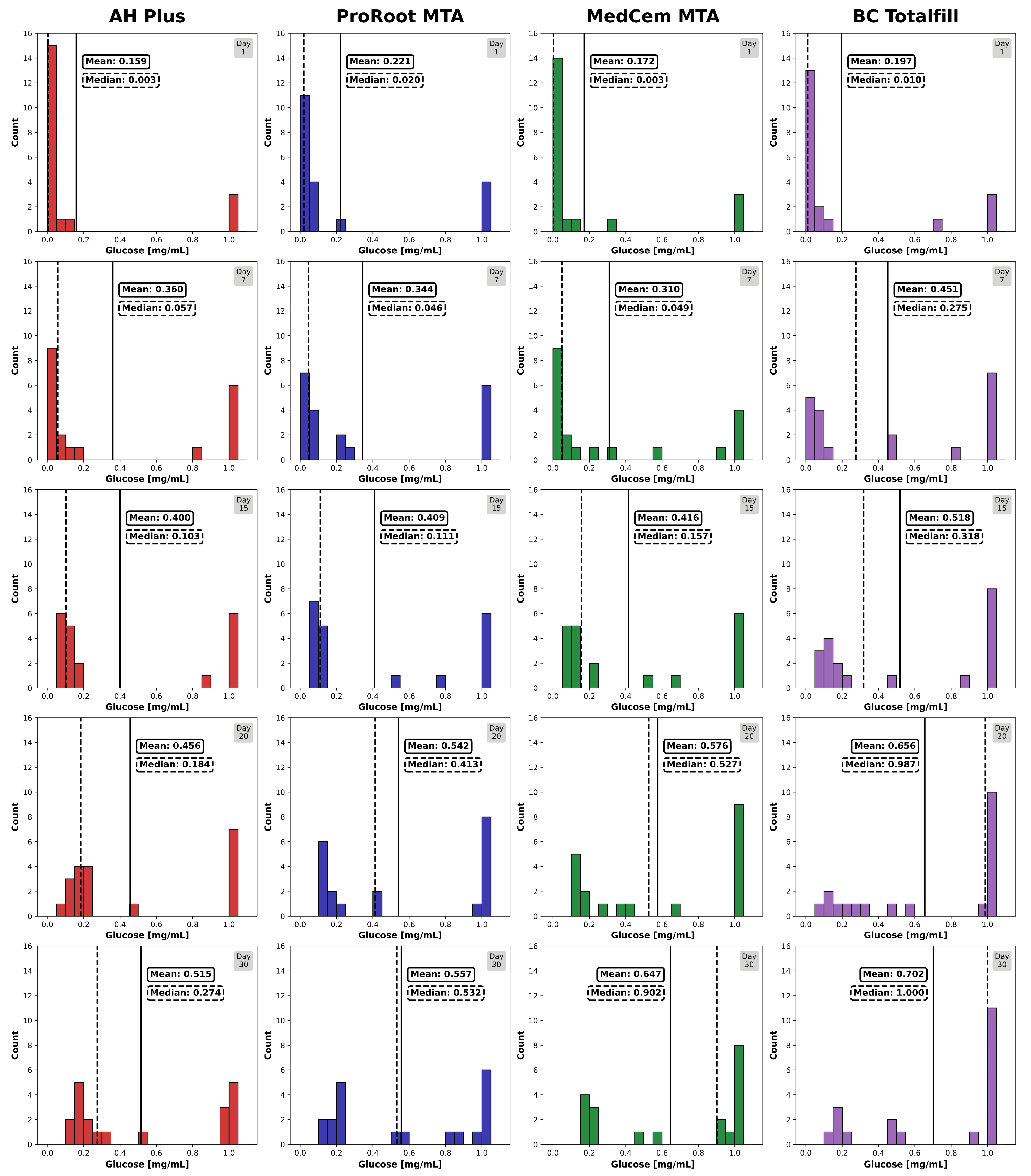
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, P.N.R. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Gulabivala, K.; Ng, Y.L. Factors that affect the outcomes of root canal treatment and retreatment-A reframing of the principles. Int. Endod. J. 2023, 56, 82–115. [Google Scholar] [CrossRef] [PubMed]
- Khayat, A.; Lee, S.J.; Torabinejad, M. Human saliva penetration of coronally unsealed obturated root canals. J. Endod. 1993, 19, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Barborka, B.J.; Woodmansey, K.F.; Glickman, G.N.; Schneiderman, E.; He, J. Long-term Clinical Outcome of Teeth Obturated with Resilon. J. Endod. 2017, 43, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, R.; Moinzadeh, A.T.; Camilleri, L.; Wesselink, P.R.; Tanomaru Filho, M.; Camilleri, J. Porosity and sealing ability of root fillings with gutta-percha and BioRoot RCS or AH Plus sealers. Evaluation by three ex vivo methods. Int. Endod. J. 2016, 49, 774–782. [Google Scholar] [CrossRef]
- Santos, J.N.; Tjäderhane, L.; Ferraz, C.C.; Zaia, A.; Alves, M.; De Goes, M.; Carrilho, M. Long-term sealing ability of resin-based root canal fillings: Sealing ability of endodontic fillings. Int. Endod. J. 2010, 43, 455–460. [Google Scholar] [CrossRef]
- Prestegaard, H.; Portenier, I.; Ørstavik, D.; Kayaoglu, G.; Haapasalo, M.; Endal, U. Antibacterial activity of various root canal sealers and root-end filling materials in dentin blocks infected ex vivo with Enterococcus faecalis. Acta Odontol. Scand. 2014, 72, 970–976. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Diegritz, C.; Hickel, R.; Huth, K.C.; Kollmuss, M. A comprehensive in vitro comparison of the biological and physicochemical properties of bioactive root canal sealers. Clin. Oral Investig. 2022, 26, 6209–6222. [Google Scholar] [CrossRef]
- Camargo, C.H.R.; Oliveira, T.R.; Silva, G.O.; Rabelo, S.B.; Valera, M.C.; Cavalcanti, B.N. Setting Time Affects In Vitro Biological Properties of Root Canal Sealers. J. Endod. 2014, 40, 530–533. [Google Scholar] [CrossRef]
- Konjhodzic-Prcic, A.; Gorduysus, O.; Kucukkaya, S.; Atila, B.; Muftuoglu, S.; Zeybek, D. In Vitro Comparison of Cytotoxicity of Four Root Canal Sealers on Human Gingival Fibroblasts. Med. Arh. 2015, 69, 24–27. [Google Scholar] [CrossRef]
- Chaudhari, P.S.; Chandak, M.G.; Jaiswal, A.A.; Mankar, N.P.; Paul, P. A Breakthrough in the Era of Calcium Silicate-Based Cements: A Critical Review. Cureus 2022, 14, e28562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial Activity of Endodontic Sealers by Modified Direct Contact Test Against Enterococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material: Cytotoxicity of iRoot SP. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Assmann, E.; Böttcher, D.E.; Hoppe, C.B.; Grecca, F.S.; Kopper, P.M.P. Evaluation of Bone Tissue Response to a Sealer Containing Mineral Trioxide Aggregate. J. Endod. 2015, 41, 62–66. [Google Scholar] [CrossRef] [PubMed]
- McMichael, G.E.; Primus, C.M.; Opperman, L.A. Dentinal Tubule Penetration of Tricalcium Silicate Sealers. J. Endod. 2016, 42, 632–636. [Google Scholar] [CrossRef]
- Mestieri, L.B.; Gomes-Cornélio, A.L.; Rodrigues, E.M.; Salles, L.P.; Bosso-Martelo, R.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Biocompatibility and bioactivity of calcium silicate-based endodontic sealers in human dental pulp cells. J. Appl. Oral Sci. 2015, 23, 467–471. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Schemkämper, P.; Bürklein, S.; Schäfer, E. Short and Long-Term Solubility, Alkalizing Effect, and Thermal Persistence of Premixed Calcium Silicate-Based Sealers: AH Plus Bioceramic Sealer vs. Total Fill BC Sealer. Materials 2022, 15, 7320. [Google Scholar] [CrossRef]
- Urban, K.; Neuhaus, J.; Donnermeyer, D.; Schäfer, E.; Dammaschke, T. Solubility and pH Value of 3 Different Root Canal Sealers: A Long-term Investigation. J. Endod. 2018, 44, 1736–1740. [Google Scholar] [CrossRef]
- Bose, R.; Ioannidis, K.; Foschi, F.; Bakhsh, A.; Kelly, R.D.; Deb, S.; Mannocci, F.; Niazi, S.A. Antimicrobial Effectiveness of Calcium Silicate Sealers against a Nutrient-Stressed Multispecies Biofilm. J. Clin. Med. 2020, 249, 2722. [Google Scholar] [CrossRef]
- De-Deus, G.; Santos, G.O.; Monteiro, I.Z.; Cavalcante, D.M.; Simões-Carvalho, M.; Belladonna, F.G.; Silva, E.J.N.L.; Souza, E.M.; Licha, R.; Zogheib, C.; et al. Micro-CT assessment of gap-containing areas along the gutta- percha-sealer interface in oval-shaped canals. Int. Endod. J. 2022, 55, 795–807. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Souza, E.M.; Silva, E.J.N.L.; Belladonna, F.G.; Simões-Carvalho, M.; Cavalcante, D.M.; Versiani, M.A. A critical analysis of research methods and experimental models to study root canal fillings. Int. Endod. J. 2022, 55 (Suppl. S2), 384–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, M.W.; Fan, B.; Cheung, G.S.; Hu, H.L. A new quantitative method using glucose for analysis of endodontic leakage. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kollmuss, M.; Preis, C.E.; Kist, S.; Hickel, R.; Huth, K.C. Differences in physical characteristics and sealing ability of three tricalcium silicate-based cements used as root-end-filling materials. Am. J. Dent. 2017, 30, 185–189. [Google Scholar] [PubMed]
- Unal, G.C.; Kececi, A.D.; Kaya, B.U.; Tac, A.G. Quality of root canal fillings performed by undergraduate dental students. Eur. J. Dent. 2011, 5, 324–330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belío-Reyes, I.A.; Bucio, L.; Cruz-Chavez, E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder diffraction. J. Endod. 2009, 35, 875–878. [Google Scholar] [CrossRef]
- Yap, W.Y.; Che Ab Aziz, Z.A.; Azami, N.H.; Al-Haddad, A.Y.; Khan, A.A. An in vitro Comparison of Bond Strength of Different Sealers/Obturation Systems to Root Dentin Using the Push-Out Test at 2 Weeks and 3 Months after Obturation. Med. Princ. Pract. 2017, 26, 464–469. [Google Scholar] [CrossRef]
- Van Rossum, G.; Drake, F.L. PYTHON 3 Reference Manual; CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2009. [Google Scholar]
- Mjör, I.A.; Smith, M.R.; Ferrari, M.; Mannocci, F. The structure of dentine in the apical region of human teeth. Int. Endod. J. 2001, 34, 346–353. [Google Scholar] [CrossRef]
- Tuncel, B.; Nagas, E.; Cehreli, Z.; Uyanik, O.; Vallittu, P.; Lassila, L. Effect of endodontic chelating solutions on the bond strength of endodontic sealers. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Verma, A.; Arora, A.; Taneja, S. Comparative evaluation of dentinal tubule penetration and push-out bond strength of new injectable hydraulic calcium disilicate based root canal sealer: A single blinded in vitro study. J. Oral Biol. Craniofacial Res. 2024, 14, 143–151. [Google Scholar] [CrossRef]
- Akcay, M.; Arslan, H.; Durmus, N.; Mese, M.; Capar, I.D. Dentinal tubule penetration of AH Plus, iRoot SP, MTA fillapex, and guttaflow bioseal root canal sealers after different final irrigation procedures: A confocal microscopic study. Laser Surg. Med. 2016, 48, 70–76. [Google Scholar] [CrossRef] [PubMed]
- El Hachem, R.; Khalil, I.; Le Brun, G.; Pellen, F.; Le Jeune, B.; Daou, M.; El Osta, N.; Naaman, A.; Abboud, M. Dentinal tubule penetration of AH Plus, BC Sealer and a novel tricalcium silicate sealer: A confocal laser scanning microscopy study. Clin. Oral Investig. 2019, 23, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Caceres, C.; Larrain, M.R.; Monsalve, M.; Pena Bengoa, F. Dentinal tubule penetration and adaptation of bio-C sealer and AH-plus: A Comparative SEM evaluation. Eur. Endod. J. 2021, 6, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Arikatla, S.K.; Chalasani, U.; Mandava, J.; Yelisela, R.K. Interfacial adaptation and penetration depth of bioceramic endodontic sealers. J. Conserv. Dent. 2018, 21, 373–377. [Google Scholar] [CrossRef]
- Kim, H.; Kim, E.; Lee, S.J.; Shin, S.J. Comparisons of the retreatment efficacy of calcium silicate and epoxy resin-based sealers and residual sealer in dentinal tubules. J. Endod. 2015, 41, 2025–2030. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Qiu, Y.; Xu, D.; Cui, L.; Wu, B. The tubular penetration depth and adaption of four sealers: A scanning electron microscopic study. BioMed Res. Int. 2017, 2017, 2946524. [Google Scholar] [CrossRef]
- Zhou, H.M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef]
- Awati, A.S.; Dhaded, N.S.; Mokal, S.; Doddwad, P.K. Analysis of the depth of penetration of an epoxy resin-based sealer following a final rinse of irrigants and use of activation systems: An in vitro study. J. Conserv. Dent. Endod. 2024, 27, 87–94. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Schmidt, S.; Rohrbach, A.; Berlandi, J.; Bürklein, S.; Schäfer, E. Debunking the Concept of Dentinal Tubule Penetration of Endodontic Sealers: Sealer Staining with Rhodamine B Fluorescent Dye Is an Inadequate Method. Materials 2021, 14, 3211. [Google Scholar] [CrossRef]
- Weller, R.N.; Tay, K.C.; Garrett, L.V. Microscopic appearance and apical seal of root canals filled with gutta-percha and ProRoot Endo Sealer after immersion in a phosphate-containing fluid. Int. Endod. J. 2008, 41, 977–986. [Google Scholar] [CrossRef]
- Zamparini, F.; Siboni, F.; Prati, C.; Taddei, P.; Gandolfi, M.G. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin. Oral Investig. 2019, 23, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Deniz Sungur, D.; Purali, N.; Cosgun, E.; Calt, S. Push-out bond strength and dentinal tubule penetration of different root canal sealers used with coated core materials. Restor. Dent. Endod. 2016, 41, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yanpiset, K.; Banomyong, D.; Chotvorrarak, K.; Srisatjaluk, R.L. Bacterial leakage and micro-computed tomography evalua- tion in round-shaped canals obturated with bioceramic cone and sealer using matched single cone technique. Restor. Dent. Endod. 2018, 43, e30. [Google Scholar] [CrossRef] [PubMed]
- Keleş, A.; Keskin, C. Presence of voids after warm vertical compaction and single-cone obturation in band-shaped isthmuses using micro-computed tomography: A phantom study. Microsc. Res. Tech. 2020, 83, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Gibson, R.; Butler, J.; Pacheco, R.; Askar, M.; Paurazas, S. Volumetric Evaluation of 5 Root Canal Obturation Methods in TrueTooth 3-dimensional–Printed Tooth Replicas Using Nano–computed Tomography. J. Endod. 2021, 47, 485–491.e4. [Google Scholar] [CrossRef] [PubMed]
- Moinzadeh, A.T.; Farack, L.; Wilde, F.; Shemesh, H.; Zaslansky, P. Synchrotron-based Phase Contrast-enhanced Micro–Computed Tomography Reveals Delaminations and Material Tearing in Water-expandable Root Fillings Ex Vivo. J. Endod. 2016, 42, 776–781. [Google Scholar] [CrossRef]
- Moinzadeh, A.T.; Zerbst, W.; Boutsioukis, C.; Shemesh, H.; Zaslansky, P. Porosity distribution in root canals filled with gutta percha and calcium silicate cement. Dent. Mater. 2015, 31, 1100–1108. [Google Scholar] [CrossRef]
- Soares, A.P.; Bitter, K.; Lagrange, A.; Rack, A.; Shemesh, H.; Zaslansky, P. Gaps at the interface between dentine and self-adhesive resin cement in post-endodontic restorations quantified in 3D by phase contrast-enhanced micro-CT. Int. Endod. J. 2020, 53, 392–402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huth, K.C.; Wuersching, S.N.; Benz, L.; Kist, S.; Kollmuss, M. In Vitro Microscopical and Microbiological Assessment of the Sealing Ability of Calcium Silicate-Based Root Canal Sealers. J. Funct. Biomater. 2024, 15, 341. https://doi.org/10.3390/jfb15110341
Huth KC, Wuersching SN, Benz L, Kist S, Kollmuss M. In Vitro Microscopical and Microbiological Assessment of the Sealing Ability of Calcium Silicate-Based Root Canal Sealers. Journal of Functional Biomaterials. 2024; 15(11):341. https://doi.org/10.3390/jfb15110341
Chicago/Turabian StyleHuth, Karin Christine, Sabina Noreen Wuersching, Leander Benz, Stefan Kist, and Maximilian Kollmuss. 2024. "In Vitro Microscopical and Microbiological Assessment of the Sealing Ability of Calcium Silicate-Based Root Canal Sealers" Journal of Functional Biomaterials 15, no. 11: 341. https://doi.org/10.3390/jfb15110341
APA StyleHuth, K. C., Wuersching, S. N., Benz, L., Kist, S., & Kollmuss, M. (2024). In Vitro Microscopical and Microbiological Assessment of the Sealing Ability of Calcium Silicate-Based Root Canal Sealers. Journal of Functional Biomaterials, 15(11), 341. https://doi.org/10.3390/jfb15110341









