Surface-Mediated Modulation of Different Biological Responses on Anatase-Coated Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Surface Preparation
2.2. Physico-Chemical Characterization
2.2.1. Wettability Analysis
2.2.2. Surface Roughness
2.2.3. Characterization of Surface Structure
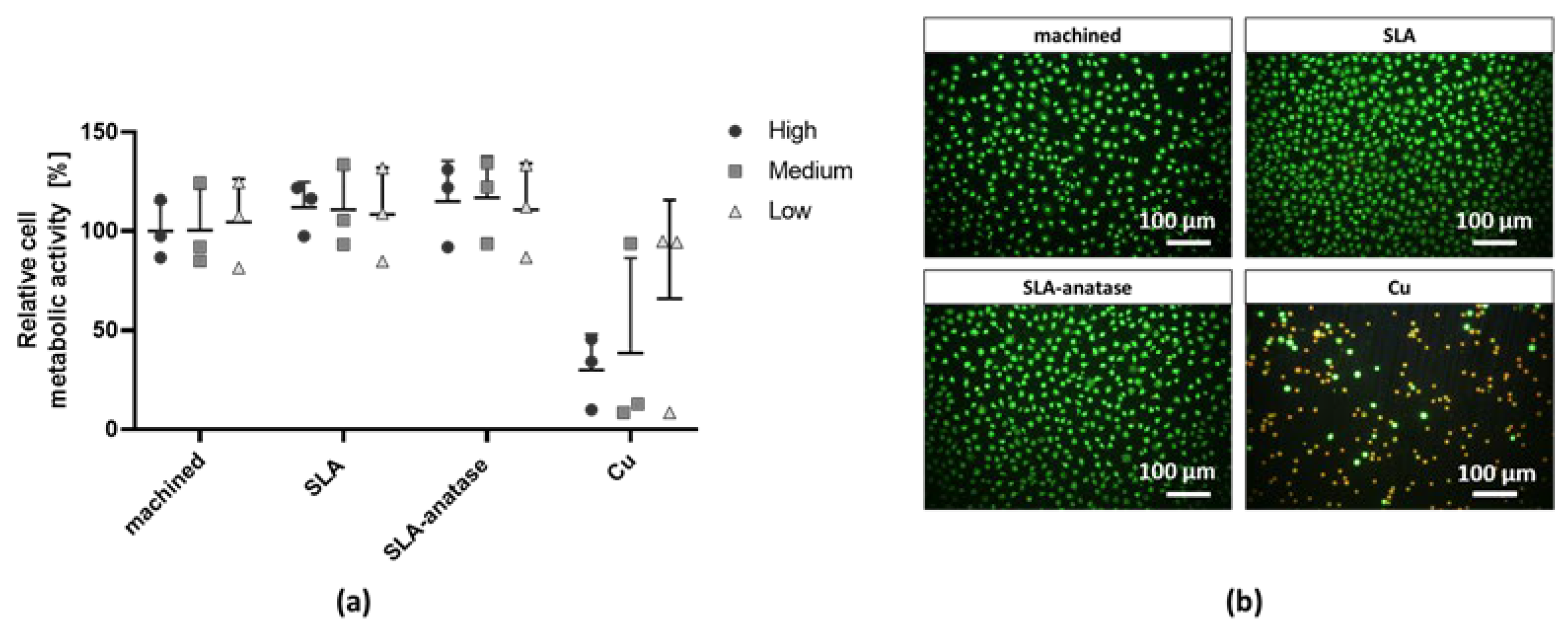
2.3. Cytotoxicity Testing with L929 Fibroblasts
2.4. Osteoblast Responses to Sample Surfaces
2.5. Hemocompatibility Testing
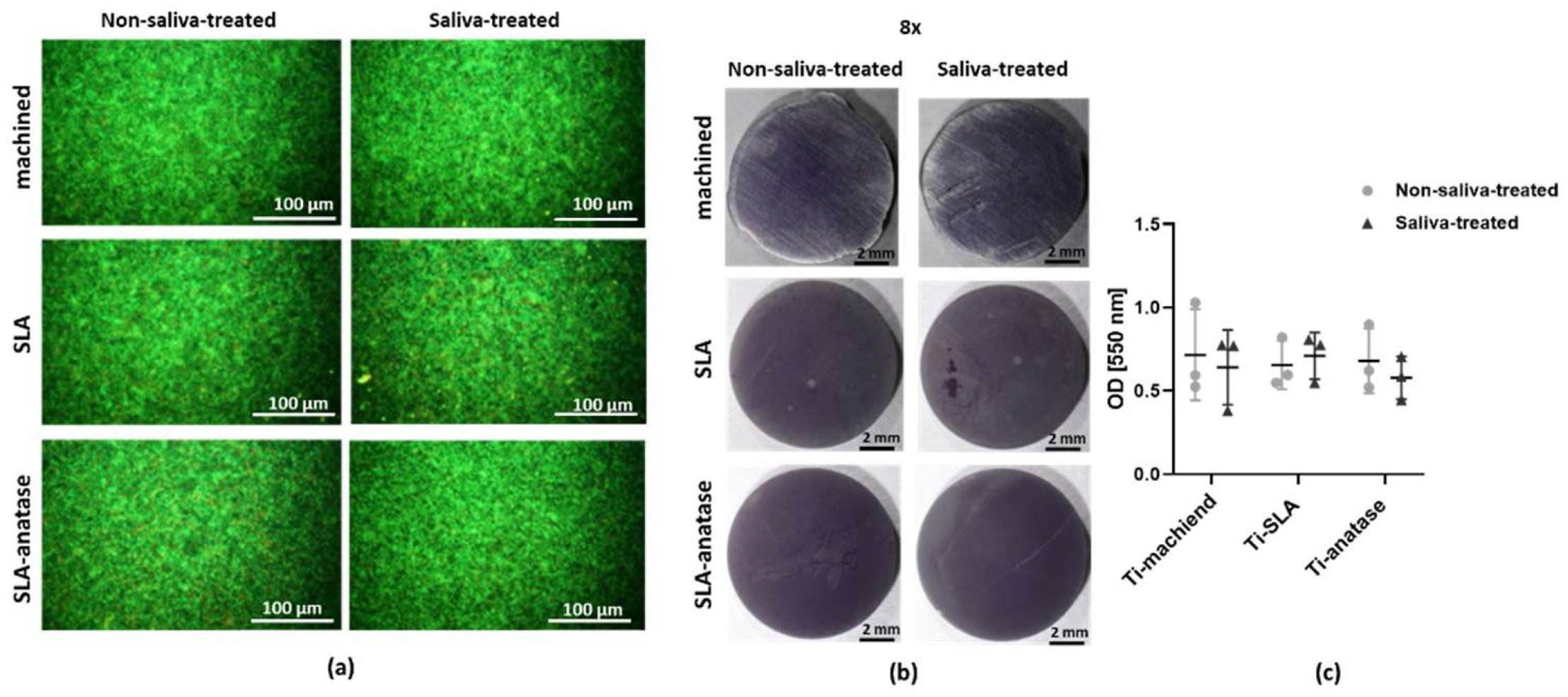
2.6. Bacterial Interactions
2.7. Statistical Analysis
3. Results
3.1. Surface Generation and Characterization
3.1.1. Surface Wettability
3.1.2. Morphology and Topography Analysis
3.1.3. Surface Roughness
3.2. Cytotoxicity Testing with L929 Fibroblasts
3.3. Osteoblast Responses to Sample Surfaces
3.4. Hemocompatibility Testing
3.5. Bacterial Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roberto, L.L.; Crespo, T.S.; Monteiro-Junior, R.S.; Martins, A.M.; De Paula, A.M.; Ferreira, E.F.; Haikal, D.S. Sociodemographic determinants of edentulism in the elderly population: A systematic review and meta-analysis. Gerodontology 2019, 36, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.-S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.-I.; Breine, U.; Adell, R.; Hansson, B.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses: I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50. [Google Scholar] [CrossRef] [PubMed]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Hong, D.G.K.; Oh, J.-h. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin. Implant. Dent. Relat. Res. 2018, 20, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhou, N.; Liu, H.; Huang, H.; Yang, G.; Chen, L.; Ding, M.; Mou, Y. Satisfaction analysis of patients with single implant treatments based on a questionnaire survey. Patient Prefer. Adherence 2019, 13, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, X.; Liu, B. A systematic review and meta-analysis on influencing factors of failure of oral implant restoration treatment. Ann. Palliat. Med. 2021, 10, 12664–12677. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Krieg, P.; Rupp, F.; Kimmerle-Müller, E.; Spintzyk, S.; Richter, M.; Richter, G.; Killinger, A.; Geis-Gerstorfer, J.; Scheideler, L. Osteoblast Response to Different UVA-Activated Anatase Implant Coatings. Adv. Mater. Interfaces 2019, 6, 1801720. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental implant complications. In Seminars in Ultrasound, CT and MRI; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Silva, R.C.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.; Santos, L.R.; Vasconcelos, N.F.; Machado, G. Titanium dental implants: An overview of applied nanobiotechnology to improve biocompatibility and prevent infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Barthes, J.; Ciftci, S.; Ponzio, F.; Knopf-Marques, H.; Pelyhe, L.; Gudima, A.; Kientzl, I.; Bognár, E.; Weszl, M.; Kzhyshkowska, J. The potential impact of surface crystalline states of titanium for biomedical applications. Crit. Rev. Biotechnol. 2018, 38, 423–437. [Google Scholar] [CrossRef]
- He, L.; Dai, D.; Xie, L.; Chen, Y.; Zhang, C. Biological effects, applications and strategies of nanomodification of dental metal surfaces. Mater. Des. 2021, 207, 109890. [Google Scholar] [CrossRef]
- Lupi, S.M.; Albini, B.; Rodriguez y Baena, A.; Lanfrè, G.; Galinetto, P. Anatase forming treatment without surface morphological alteration of dental implant. Materials 2020, 13, 5280. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Lindahl, C.; Lausmaa, J.; Engqvist, H. Biomimetic hydroxyapatite deposition on titanium oxide surfaces for biomedical application. Adv. Biomim. 2011, 20, 429–452. [Google Scholar]
- Ovenstone, J.; Yanagisawa, K. Effect of hydrothermal treatment of amorphous titania on the phase change from anatase to rutile during calcination. Chem. Mater. 1999, 11, 2770–2774. [Google Scholar] [CrossRef]
- Wang, Q.M.; Zhang, T.F.; Kwon, S.H.; Kim, K.H. Fabrication of TiO2 films on glass substrates by a pulsed dc reactive magnetron sputtering. Appl. Mech. Mater. 2011, 71, 5050–5053. [Google Scholar] [CrossRef]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement and market trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Rupp, F.; Haupt, M.; Klostermann, H.; Kim, H.-S.; Eichler, M.; Peetsch, A.; Scheideler, L.; Doering, C.; Oehr, C.; Wendel, H. Multifunctional nature of UV-irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010, 6, 4566–4577. [Google Scholar] [CrossRef]
- Rupp, F.; Haupt, M.; Eichler, M.; Doering, C.; Klostermann, H.; Scheideler, L.; Lachmann, S.; Oehr, C.; Wendel, H.; Decker, E. Formation and photocatalytic decomposition of a pellicle on anatase surfaces. J. Dent. Res. 2012, 91, 104–109. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Wu, X.; Alnazzawi, A.; Watson, J.; Watts, D. Surface characteristics and biocompatibility of cranioplasty titanium implants following different surface treatments. Dent. Mater. 2018, 34, 676–683. [Google Scholar] [CrossRef]
- Rodriguez y Baena, R.; Rizzo, S.; Manzo, L.; Lupi, S.M. Nanofeatured titanium surfaces for dental implantology: Biological effects, biocompatibility, and safety. J. Nanomater. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Svetina, M.; Ciacchi, L.C.; Sbaizero, O.; Meriani, S.; De Vita, A. Deposition of calcium ions on rutile (110): A first-principles investigation. Acta Mater. 2001, 49, 2169–2177. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, V.; Pezzetti, F.; Scarano, A.; Piattelli, A.; Massari, L.; Brunelli, G.; Carinci, F. Anatase coating improves implant osseointegration in vivo. J. Craniofacial Surg. 2007, 18, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, A.; Polimeni, A.; Di Iorio, D.; Carinci, F. Bacterial adhesion on commercially pure titanium and anatase-coated titanium healing screws: An in vivo human study. J. Periodontol. 2010, 81, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Dorkhan, M.; Hall, J.; Uvdal, P.; Sandell, A.; Svensäter, G.; Davies, J.R. Crystalline anatase-rich titanium can reduce adherence of oral streptococci. Biofouling 2014, 30, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Bianchi, S.; Botticelli, G.; Rastelli, E.; Tomei, A.; Palmerini, M.; Continenza, M.; Macchiarelli, G. Scanning electron microscopy and microbiological approaches for the evaluation of salivary microorganisms behaviour on anatase titanium surfaces: In vitro study. Morphologie 2018, 102, 1–6. [Google Scholar] [CrossRef]
- Giordano, C.; Saino, E.; Rimondini, L.; Pedeferri, M.P.; Visai, L.; Cigada, A.; Chiesa, R. Electrochemically induced anatase inhibits bacterial colonization on Titanium Grade 2 and Ti6Al4V alloy for dental and orthopedic devices. Colloids Surf. B Biointerfaces 2011, 88, 648–655. [Google Scholar] [CrossRef]
- ISO 10993-12; Standardization, I. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- Straub, A.; Wendel, H.P.; Dietz, K.; Schiebold, D.; Peter, K.; Schoenwaelder, S.M.; Ziemer, G. Selective inhibition of the platelet phosphoinositide 3-kinase p110β as promising new strategy for platelet protection during extracorporeal circulation. Thromb. Haemost. 2008, 99, 609–615. [Google Scholar]
- Zhang, B.; Li, B.; Gao, S.; Li, Y.; Cao, R.; Cheng, J.; Li, R.; Wang, E.; Guo, Y.; Zhang, K. Y-doped TiO2 coating with superior bioactivity and antibacterial property prepared via plasma electrolytic oxidation. Mater. Des. 2020, 192, 108758. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Z.; Yao, X.; Zhou, Y.; Xiong, Y.; Li, Y.; Xu, J.; Dargusch, M.S.; Yan, M. From bio-inertness to osseointegration and antibacterial activity: A one-step micro-arc oxidation approach for multifunctional Ti implants fabricated by additive manufacturing. Mater. Des. 2022, 221, 110962. [Google Scholar] [CrossRef]
- Vrakatseli, V.; Farsari, E.; Mataras, D. Wetting properties of transparent anatase/rutile mixed phase glancing angle magnetron sputtered nano-TiO2 films. Micromachines 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Dastan, D.; Liu, L.; Wu, L.; Shi, Z.; Chu, Q.-Q.; Altaf, F.; Mohammed, M.K. Surface wettability of various phases of titania thin films: Atomic-scale simulation studies. J. Mol. Graph. Model. 2023, 118, 108335. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, Y.D.; Zheng, Y. In vitro biocompatibility study of nano TiO2 materials. Adv. Mater. Res. 2008, 47, 1438–1441. [Google Scholar] [CrossRef]
- Sangeetha, S.; Kathyayini, S.R.; Raj, P.D.; Dhivya, P.; Sridharan, M. Biocompatibility studies on TiO2 coated Ti surface. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; IEEE: Piscataway Township, NJ, USA, 2013. [Google Scholar]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, B.; López-Huerta, F.; Vega, R.; Hernández-Torres, J.; García-González, L.; Salceda, E.; Herrera-May, A.L.; Soto, E. Cytotoxicity evaluation of anatase and rutile TiO2 thin films on CHO-K1 cells in vitro. Materials 2016, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, Y. Osteoblast-like Cell Proliferation, ALP Activity and Photocatalytic Activity on Sintered Anatase and Rutile Titanium Dioxide. Materials 2021, 14, 4414. [Google Scholar] [CrossRef] [PubMed]
- Uboldi, C.; Urbán, P.; Gilliland, D.; Bajak, E.; Valsami-Jones, E.; Ponti, J.; Rossi, F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol. Vitr. 2016, 31, 137–145. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Carballo-Vila, M.; Moreno-Burriel, B.; Chinarro, E.; Jurado, J.R.; Casañ-Pastor, N.; Collazos-Castro, J.E. Titanium oxide as substrate for neural cell growth. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 94–105. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, K.H.; Choy, K.C.; Oh, K.T.; Kim, K.N. Photocatalytic antibacterial effect of TiO2 film formed on Ti and TiAg exposed to Lactobacillus acidophilus. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007, 80, 353–359. [Google Scholar] [CrossRef]
 : machined,
: machined,  : SLA,
: SLA,  : SLA-anatase). (a) Quantitative results of static contact angle measurements of different surfaces. The grid line represents the borderline of 90 ° between hydrophilicity and hydrophobicity, *** p < 0.001; (n = 5). (b) Surface roughness Sa of sample discs (n = 5; **** p < 0.0001). (c) Representative SEM images of the test surfaces at two different magnifications (5000× and 10,000×). SEM images demonstrate typical surface microstructures: machined, SLA and SLA-anatase surfaces. ns: not significant.
: SLA-anatase). (a) Quantitative results of static contact angle measurements of different surfaces. The grid line represents the borderline of 90 ° between hydrophilicity and hydrophobicity, *** p < 0.001; (n = 5). (b) Surface roughness Sa of sample discs (n = 5; **** p < 0.0001). (c) Representative SEM images of the test surfaces at two different magnifications (5000× and 10,000×). SEM images demonstrate typical surface microstructures: machined, SLA and SLA-anatase surfaces. ns: not significant.
 : machined,
: machined,  : SLA,
: SLA,  : SLA-anatase). (a) Quantitative results of static contact angle measurements of different surfaces. The grid line represents the borderline of 90 ° between hydrophilicity and hydrophobicity, *** p < 0.001; (n = 5). (b) Surface roughness Sa of sample discs (n = 5; **** p < 0.0001). (c) Representative SEM images of the test surfaces at two different magnifications (5000× and 10,000×). SEM images demonstrate typical surface microstructures: machined, SLA and SLA-anatase surfaces. ns: not significant.
: SLA-anatase). (a) Quantitative results of static contact angle measurements of different surfaces. The grid line represents the borderline of 90 ° between hydrophilicity and hydrophobicity, *** p < 0.001; (n = 5). (b) Surface roughness Sa of sample discs (n = 5; **** p < 0.0001). (c) Representative SEM images of the test surfaces at two different magnifications (5000× and 10,000×). SEM images demonstrate typical surface microstructures: machined, SLA and SLA-anatase surfaces. ns: not significant.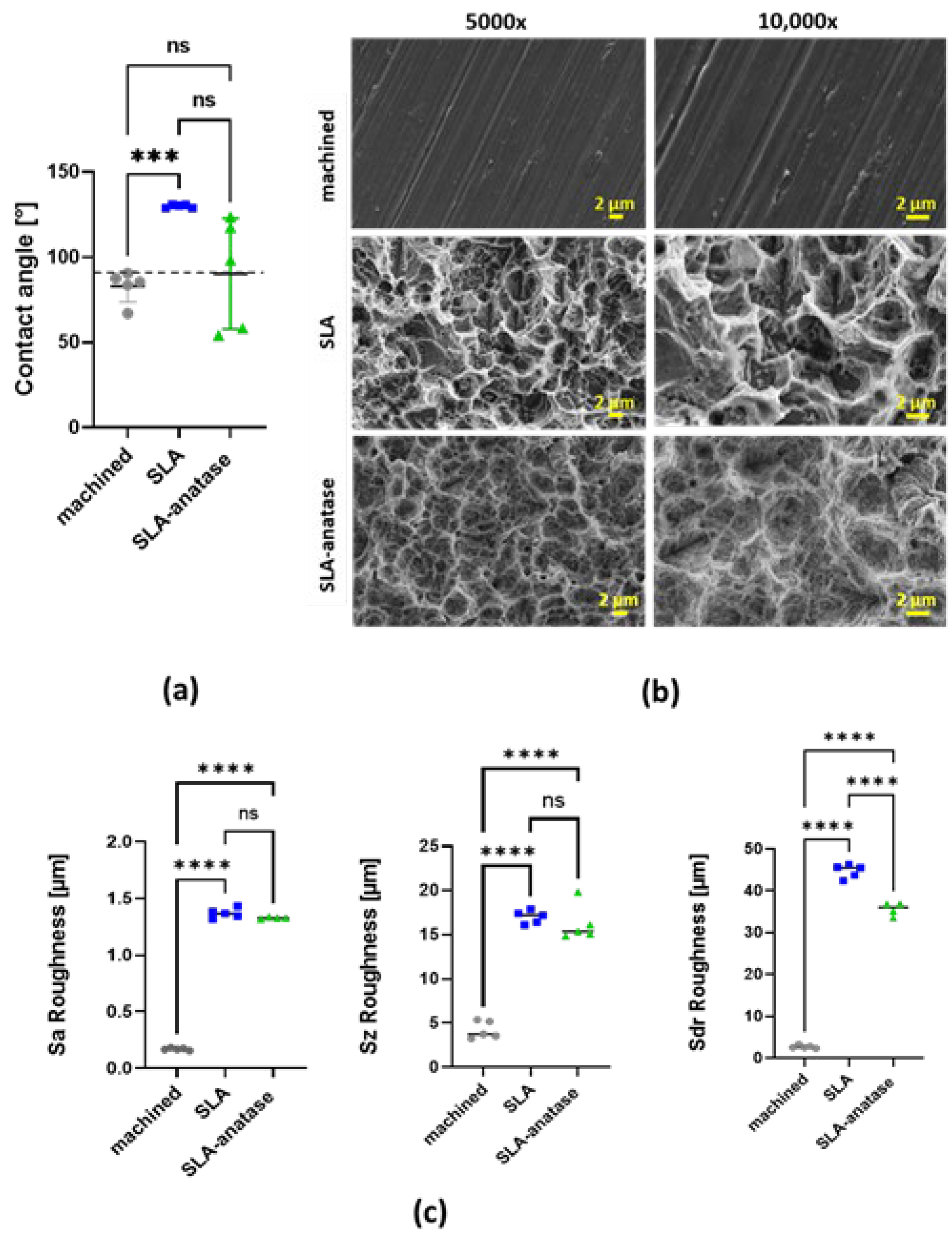

 : machined,
: machined,  : SLA,
: SLA,  : SLA-anatase). (a) Representative images of sample surface coverage by alizarin red stained calcium phosphate deposits after 14 days in culture under 8× and 32× magnification. (b) Following photodocumentation, the stain was dissolved from the cells and quantified with a spectrophotometer. The machined group was used as a control and set to 100%. The bar graph shows the mean ± SD (n = 3; * p < 0.05). ns: not significant.
: SLA-anatase). (a) Representative images of sample surface coverage by alizarin red stained calcium phosphate deposits after 14 days in culture under 8× and 32× magnification. (b) Following photodocumentation, the stain was dissolved from the cells and quantified with a spectrophotometer. The machined group was used as a control and set to 100%. The bar graph shows the mean ± SD (n = 3; * p < 0.05). ns: not significant.
 : machined,
: machined,  : SLA,
: SLA,  : SLA-anatase). (a) Representative images of sample surface coverage by alizarin red stained calcium phosphate deposits after 14 days in culture under 8× and 32× magnification. (b) Following photodocumentation, the stain was dissolved from the cells and quantified with a spectrophotometer. The machined group was used as a control and set to 100%. The bar graph shows the mean ± SD (n = 3; * p < 0.05). ns: not significant.
: SLA-anatase). (a) Representative images of sample surface coverage by alizarin red stained calcium phosphate deposits after 14 days in culture under 8× and 32× magnification. (b) Following photodocumentation, the stain was dissolved from the cells and quantified with a spectrophotometer. The machined group was used as a control and set to 100%. The bar graph shows the mean ± SD (n = 3; * p < 0.05). ns: not significant.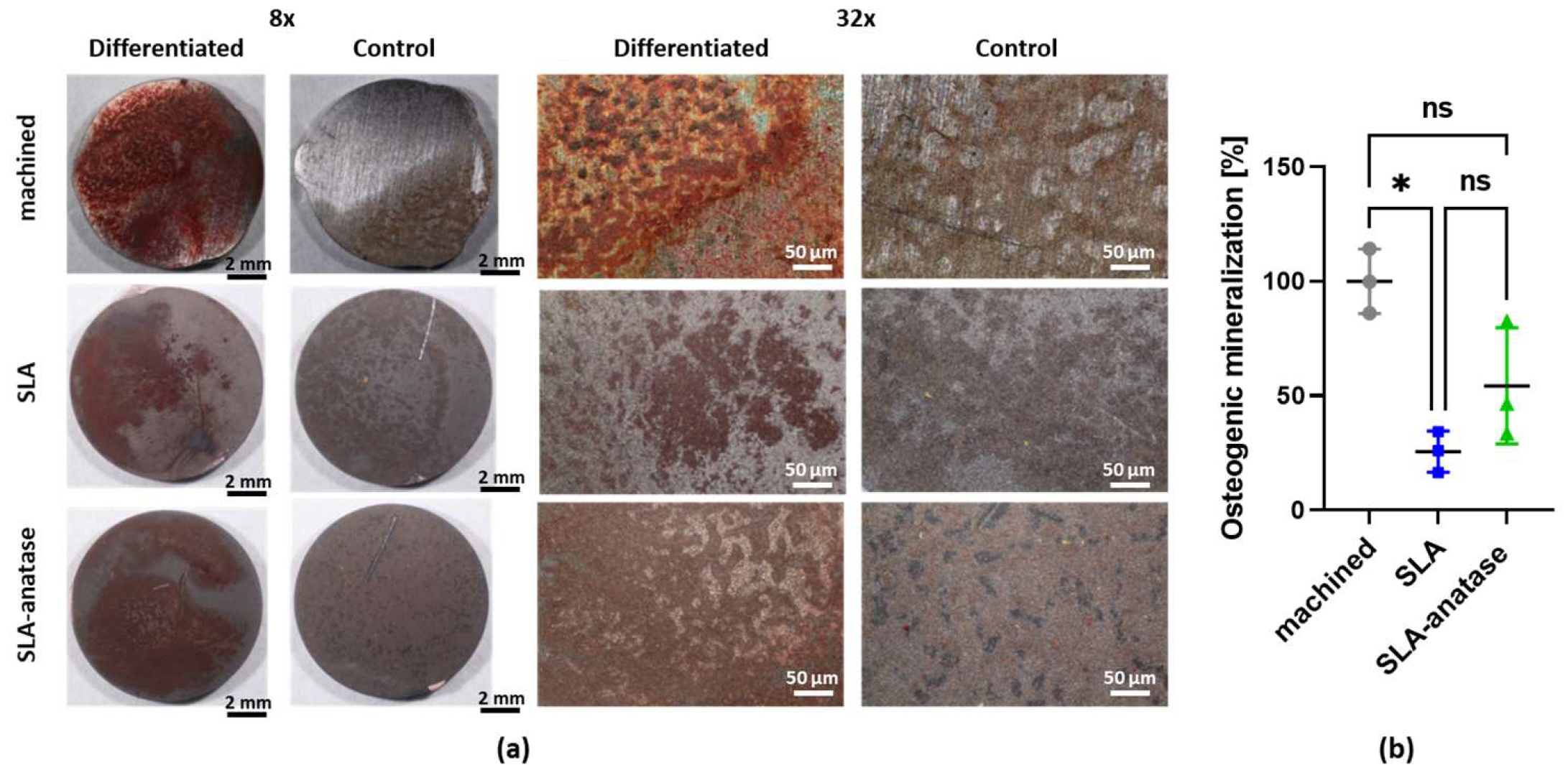
 : baseline, ■: control,
: baseline, ■: control,  : machined,
: machined,  : SLA,
: SLA,  : SLA-anatase). Before and after the incubation of the specimen with heparinized human whole blood for 60 min under agitation (35 rpm), white blood cells (a), red blood cells (b), platelets (c) and beta-thromboglobulin (d) were evaluated (n = 5, * p > 0.05). Baseline: directly after venipuncture. Control: tube only. (e) Representative SEM images of the three surfaces after incubation with human whole blood are depicted (magnification 1000× and 2500×). ns: not significant.
: SLA-anatase). Before and after the incubation of the specimen with heparinized human whole blood for 60 min under agitation (35 rpm), white blood cells (a), red blood cells (b), platelets (c) and beta-thromboglobulin (d) were evaluated (n = 5, * p > 0.05). Baseline: directly after venipuncture. Control: tube only. (e) Representative SEM images of the three surfaces after incubation with human whole blood are depicted (magnification 1000× and 2500×). ns: not significant.
 : baseline, ■: control,
: baseline, ■: control,  : machined,
: machined,  : SLA,
: SLA,  : SLA-anatase). Before and after the incubation of the specimen with heparinized human whole blood for 60 min under agitation (35 rpm), white blood cells (a), red blood cells (b), platelets (c) and beta-thromboglobulin (d) were evaluated (n = 5, * p > 0.05). Baseline: directly after venipuncture. Control: tube only. (e) Representative SEM images of the three surfaces after incubation with human whole blood are depicted (magnification 1000× and 2500×). ns: not significant.
: SLA-anatase). Before and after the incubation of the specimen with heparinized human whole blood for 60 min under agitation (35 rpm), white blood cells (a), red blood cells (b), platelets (c) and beta-thromboglobulin (d) were evaluated (n = 5, * p > 0.05). Baseline: directly after venipuncture. Control: tube only. (e) Representative SEM images of the three surfaces after incubation with human whole blood are depicted (magnification 1000× and 2500×). ns: not significant.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadnejad, L.; Theurer, A.; Alber, J.; Illing, B.; Kimmerle-Mueller, E.; Schultheiss, J.; Krajewski, S.; Rupp, F. Surface-Mediated Modulation of Different Biological Responses on Anatase-Coated Titanium. J. Funct. Biomater. 2024, 15, 29. https://doi.org/10.3390/jfb15020029
Mohammadnejad L, Theurer A, Alber J, Illing B, Kimmerle-Mueller E, Schultheiss J, Krajewski S, Rupp F. Surface-Mediated Modulation of Different Biological Responses on Anatase-Coated Titanium. Journal of Functional Biomaterials. 2024; 15(2):29. https://doi.org/10.3390/jfb15020029
Chicago/Turabian StyleMohammadnejad, Leila, Antonia Theurer, Julia Alber, Barbara Illing, Evi Kimmerle-Mueller, Jacob Schultheiss, Stefanie Krajewski, and Frank Rupp. 2024. "Surface-Mediated Modulation of Different Biological Responses on Anatase-Coated Titanium" Journal of Functional Biomaterials 15, no. 2: 29. https://doi.org/10.3390/jfb15020029
APA StyleMohammadnejad, L., Theurer, A., Alber, J., Illing, B., Kimmerle-Mueller, E., Schultheiss, J., Krajewski, S., & Rupp, F. (2024). Surface-Mediated Modulation of Different Biological Responses on Anatase-Coated Titanium. Journal of Functional Biomaterials, 15(2), 29. https://doi.org/10.3390/jfb15020029




