The Use of Functional Biomaterials in Aesthetic and Functional Restoration in Orbital Surgery
Abstract
:1. Introduction
2. Use of Functional Biomaterials in the Repair of Orbital Floor Injuries
2.1. Overview of Orbital Floor Injuries and Reconstruction
2.2. Indications for Treatment
2.3. Ideal Properties of Biomaterials for Orbital Floor Repair
- Biocompatibility and safety: The material should be non-allergenic and non-carcinogenic. It should mimic the physical properties of the tissue it is replacing.
- Long-term acceptance: The material should be permanently accepted by the body.
- Chemical stability: It should be chemically inert, and capable of being sterilized without deteriorating its chemical properties. The choice of biomaterial should consider its inherent antibacterial properties.
- Manipulability and stability: The material should be easily manipulated during surgical procedures and retain its form post-implantation.
- Fixation capability: The material should allow for secure fixation to the host bone using screws, wire, suture, or adhesive.
- Non-potentiating: It should not encourage microbial growth or the resorption of the underlying bone. It should also not distort adjacent structures.
- Radio-opacity: For clear post-surgical evaluation, the material should be radiopaque.
- Cost-effectiveness: Particularly for alloplastic materials, the cost should be reasonable.
- Porosity: This characteristic is vital for promoting tissue ingrowth and vascularization. Higher porosity enhances cell infiltration, nutrient exchange, and integration with surrounding tissues.
- Mechanical strength and elasticity: These properties should match those of native tissue to prevent implant failure or tissue damage.
- Appropriate biodegradability: Biodegradable materials should enable gradual replacement by native tissues at a rate that aligns with tissue healing and remodeling processes.
- Optimal surface roughness: The surface roughness of certain materials, such as titanium, has the potential to enhance bone-to-material contact, resulting in accelerated osteointegration and increased adhesion strength. However, it is crucial to strike a balance, as heightened surface roughness also poses a risk of bacterial adhesion to the materials.
- Hydrophilicity: Hydrophilic biomaterials offer advantages such as enhanced water retention, promoting cell adhesion and tissue integration, but careful consideration is necessary to avoid excessive hydrophilicity, which could increase susceptibility to biofilm formation.
2.4. Current State: Current Gold Standard, Alternative Options, and Types of Biomaterials in Orbital Floor Repair
2.4.1. Autogenous Bone
2.4.2. Autogenous Cartilage
2.4.3. Allogenic Materials
2.4.4. Alloplastic Materials
2.4.5. Metals
2.4.6. Hydroxyapatite (HA)
2.4.7. Non-Absorbable Polymers
Silicone
Polytetrafluoroethylene (PTFE)
Porous Polyethylene (PE)
Polyethylene/Titanium Composite
Polyethylene Reinforced with Hydroxyapatite (HAPEX™)
Smooth Nylon Foil
2.4.8. Absorbable Polymers
Polycaprolactone
Polylactic Acid-Based Materials
Polyglycolic Acid-Based Biomaterials
Polydiaxanone (PDO)
Polyglactin 910/PDO Copolymers
| Material Type | Key Features | Advantages | Challenges | References |
|---|---|---|---|---|
| Autologous Materials | ||||
| Autologous bone | -Sourced from mandibular coronoid process, anterior maxillary wall, mandibular symphysis, rib, scapula, cranium, and iliac crest | -Intrinsic strength -Flexibility Radio-pacity -Biocompatibility -Tissue tolerance after implantation -Low immune reactivity | -Complex shaping -Donor site morbidity -Unpredictable resorption -Limitations on available donor sites | [11,12,13] |
| Autologous cartilage | -Primarily sourced from nasal septum and conchal cartilage for orbital floor repair but also harvested from auricular and rib cartilage | -Easier to harvest and contour compared to bone -Lasting support -Minimal resorption even after several years -Minimal donor site morbidity | -Lacking radio-opacity -Less structural support than bone -May revert to its previous shape | [13,14,15,16,17,18] |
| Allogenic Materials | ||||
| Titanium | -Wide use in orthopedics and craniofacial reconstruction -Cone-beamed CT can aid in precise positioning -Direct metal sintering techniques emerging to overcome challenges related to these materials | -Good biocompatibility -Corrosion resistance -Mechanical properties resembling bone -Suitable for permanent stability in large defects (i.e., orbitozyggomatic or orbitofrontal reconstructions) -Comparable or superior outcome to autogenous materials | -Requires manual adaptions during surgery which can be time-consuming or error-prone -Associated complications include implant rupture, corrosion, screw weakening, and bone resorption -Fibrotic adherence between titanium materials and orbital structures can lead to diplopia and eyelid retraction -Challenges with shaping and bending | [27,28,29,30,31,32,33,34] |
| Hydroxyapatite (HA) | -Calcium phosphate salt analogous to bone material -Widely used in craniofacial reconstruction | -Excellent biocompatability -Limited resorption | -Low tensile strength -Brittleness -Difficulty stabilizing HA implants -Incompatibility with rigid fixation -Associated with post-operative enophthalmos, intraoperative failures and infections | [50,51,53,54] |
| Non-Absorbable Polymers | ||||
| Silicone | -Used in orbital reconstruction for nearly 50 years | -Biologically and chemically inert -Flexible -Easy to handle -Cost-effective material -Positive post-operative outcomes such as reduced infection and need for repeat surgeries | -Risk of implant-related complications such as infraorbital cyst formation, infection, extrusion, and implant displacement | [55,56,57] |
| Polytetraflouroethylene (PTFE) | -Used in orbital reconstruction for smaller defects (<1.5 cm) | -Biologically and chemically inert -Non-antigenic -Sterilizable via autoclaving -Easily moldable -Proven safe and effective for post-traumatic enophthalmos | -Some reports of late complications such as fistula formations -Less evidence on reliability since it is not used as frequently | [59,60,61,62] |
| Polyethylene (PE; Medpor) | -Used in orbital floor repair over the past two decades -Porous structure that vascular components and connective tissue can grow into -Enhanced by patient-specific 3D printed models | -Customizable material -Porous structure enables formation of fibrovascular networks -Reduced infection and implant displacement risks | -Reports of immediate and long-term complications, such as surgical site infection, cyst formation, hematoma, and implant extrusion | [26,28,63,64,65,66,67,68,69,70,72,73] |
| Polyethylene/Titanium (Medpor Titan) | -Combination of titanium mesh and porous PE | -Leverages titanium’s strength, radio-opacity, and memory along with PE’s ability to enable fibrovascular ingrowth -Smooth surface reducing abrasions -Enhanced stability | -Reports of late-onset infection | [63,67,72,73,74] |
| Polyethylene with hydroxyapatite (HAPEX) | -High-density porous PE reinforced by HA | -Biologically inert -Can stimulate bone integration -Good stability -Good integration between implant and supporting bone | -Brittleness -Inadequate strength, modulus, and toughness to substitute load-bearing bone | [75] |
| Nylon foil (SupraFOIL) | -Non-absorbable clear sheeting derived from nylon suture biomaterial | -Found to be safe and effective -No findings of post-operative enophthalmos in small and medium-sized fractures -May prevent orbital fixation syndrome | -Some cases of intracapsular hemorrhage, orbital hematoma, and orbital inflammation reported | [76,77,78,79] |
| Absorbable Polymers | ||||
| Polycaprolactone (PCL) | -Semi-rigid mesh structure | -Structural stability -Highly malleable enabling precise anatomical adaptation -Hydrolyzes into metabolites with mild acidity | -Some complications observed | [36,103,104] |
| Polylactic acid (PLA) based materials | -High molecular weight -Bioresorbable osteosynthetic material | -Ease of contouring -Mechanical integrity -Avoidance of donor-site morbidity -Stable shelf life of healed bone or soft tissue after complete resorption -Resistant to hydrolysis -Comparable outcomes to autologous bone | -Limitations in use for medium or larger-sized defects | [57,82,83,84,85,86,87,89] |
| Poly glycolic acid (PGA) biomaterials | -Biodegradable polymer -Rapid degradation within 2 months and >90% resorption within 9 months making it less suitable for standalone use -Often used in combination with PLA | -Suitable for orbital floor repair of smaller defects and where faster degradation (within 6 months) is needed -Low risk of delayed infection or migration | -Not suitable for large defects -Less suitable for severe orbital trauma cases due to lack of stability | [89,90,91,92,93] |
| Polydiaxanone (PDO) | -Semicrystalline polymer -Available in plate, foil, and sheet forms | -Positive outcomes for smaller defects | -Evidence of post-operative complications such as hematoma, diplopia, extrusion, and enophthalmos -May be suboptimal for larger defects | [94,95,96,97,98,99] |
| Polyglactin 910/PDO (Ethisorb) | -Flexible membranes that offer strength and long-term resorption | -Positive results for small to moderate-sized orbital floor defects | -May be unsuitable for larger-sized defects | [100,101,102,103] |
2.5. What’s New? Emerging Biomaterials and Their New Applications in Orbital Floor Repair
2.5.1. Additives and Coatings
2.5.2. Nanoparticles
2.5.3. Tissue Engineering
2.5.4. Patient-Specific Implants (PSI) and 3D Printing
| Material Type | Key Features | Advantages | Challenges | References |
|---|---|---|---|---|
| Additives and coatings | -Surface modifications of metals, ceramics and polymers -Include surface-treated titanium and porous coatings | -Some coatings offer antibacterial properties and promote osteointegration | -Potential cytotoxicity -Challenges with stability and long-term performance | [105] |
| Nanoparticles | -Incorporation of polyurethane (PU), HA, and antimicrobial metal ions into materials | -HA nanoparticles into cyclic acetal hydrogels have shown positive in vivo bone growth -HA synthesized with ionic substitutions can enhance bioactivity and cell viability -Antimicrobial ions enhance antimicrobial properties | -Potential cytotoxicity -Unclear stability -Short-half life | [105,106,107,109,110] |
| Tissue engineering | -Include the application of recombinant bone morphogenetic proteins (BMPs) and bone marrow-derived mesenchymal stem cells | -Shown to promote tissue regeneration and accelerated healing | -Limited research in human trials | [81,110,111,112,113,114,115,116,117,118] |
| Patient-Specific Implants (PSI) and 3D Printing | -Crafted from biomaterials such as poly(trimethylene carbonate) and titanium mesh | -Offer customizability and surgical precision -Shown to foster rapid neovascularization and bone growth | -Further research required to confirm long-term efficacy | [46,119,120,121] |
2.6. Challenges and Barriers
2.7. Gaps in Knowledge and Future Directions
3. Role of Functional Biomaterials in Orbital Implants and Prothesis
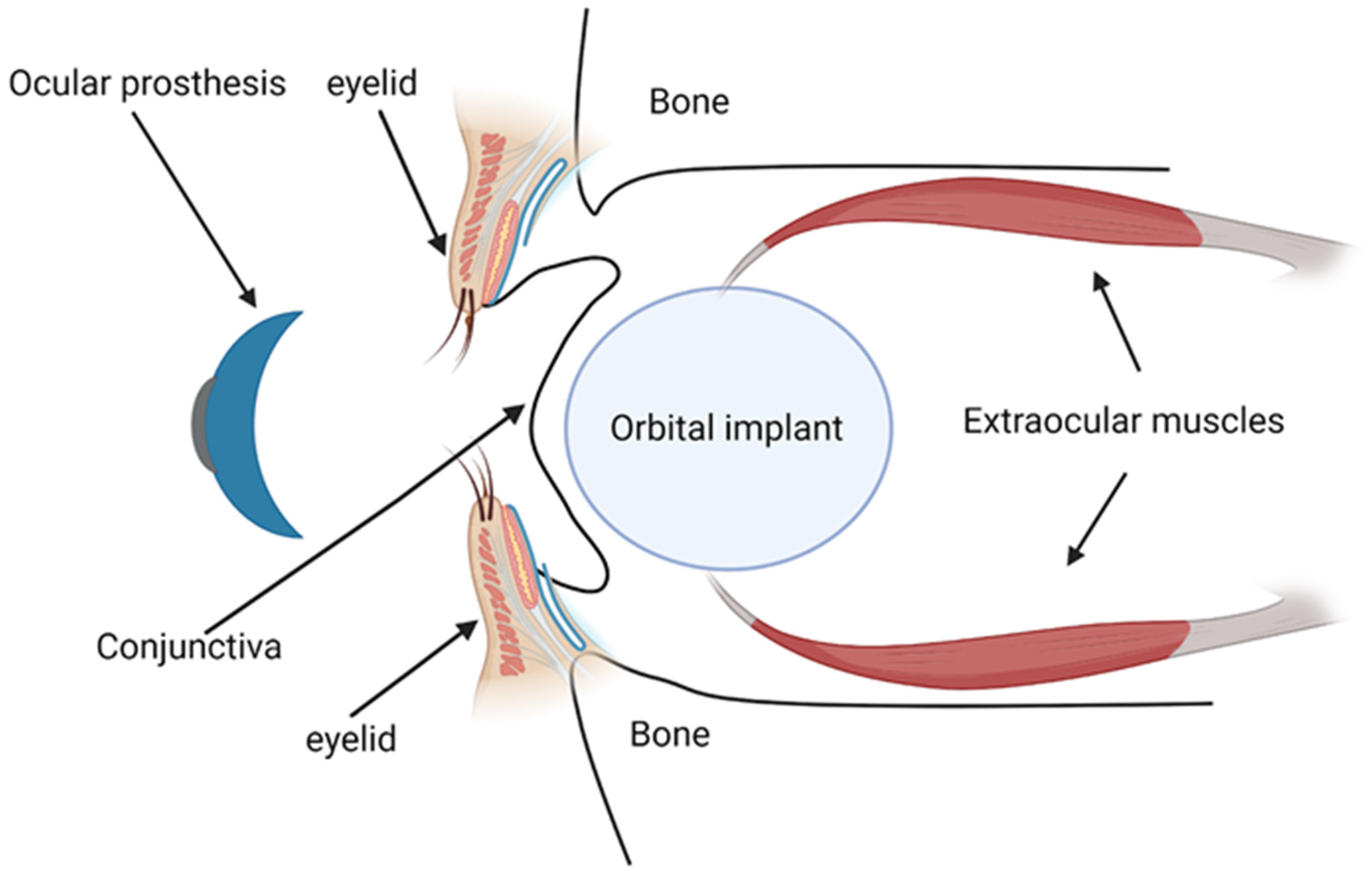
3.1. Overview of Orbital Implants and Prosthesis
3.2. Indications for the Use of Orbital Implants and Prosthesis
3.3. Ideal Properties of Biomaterials for Orbital Implants and Prosthesis
- Biocompatibility: The material should be non-allergenic, non-toxic, and not incite an adverse immune response from the host tissue.
- Long-term acceptance: The material should either be permanently accepted by the body.
- Manipulability and stability: The material should be easily manipulated during surgical procedures and retain its form post-implantation. It should maintain sufficient volume to maintain the natural structure of the orbit.
- Mechanical stability and motility: Implants should allow natural movement of the prosthesis for optimal aesthetic outcomes.
- Proper support for ocular prosthesis: Implants should hold and support the prosthesis appropriately.
- Cost-effectiveness: Implants should be economically accessible for a wide range of patients.
- An optimal ocular prosthesis should possess the following ideal attributes:
- Lightweight: To promote comfort, the prosthesis should not be heavy.
- Color match: The prosthesis should match the color of the contralateral eye for a natural appearance.
- Hygiene: The design should facilitate easy and effective cleaning.
- Texture: The prosthesis should mimic the natural eye to provide a realistic look and feel.
- Availability: The prosthesis should be easily accessible for replacements or adjustments as needed.
3.4. Current Gold Standard, Alternative Options, and Types of Biomaterials in Orbital Implants and Prosthesis
3.4.1. Ceramics
3.4.2. Autologous Materials
3.4.3. Polymers
Silicone
Poly(methylmethacrylate) (PMMA)
Poly-HEMA (2-Hydroxyethyl Methacralate) Implant (Alphasphere)
Polytetrafluoroethylene (ePTFE or Gore-Tex) Based Materials
| Material Type | Key Features | Advantages | Challenges | References |
|---|---|---|---|---|
| Ceramics | -Initially derived from glass -HA and alumina more commonly applied | -Porous designs permit fibrovascular growth that enhances implant longevity -Good outcomes when combined with autologous tissues | -Porous ceramic structure complicates suturing -Rough surfaces can impact biocompatibility -Limited application in children due to growth-related concerns | [132,133,134,135,136,137,138,139,140,141,142,143,144,145] |
| Autologous materials | -Include fat, bone, skin, cartilage and muscle grafts | -More affordable than synthetic implants -Useful for patients who cannot tolerate synthetic materials or children whose anatomical structures evolve over time -Used to wrap or salvage exposed implants | -Unpredictable success rate -Potential post-operative complications at tissue extraction sites | [146,147,148,149,150,151,152,153,154,155] |
| Silicone | -Various designs including solid, grooved strips and sponge-based implants -Porous and non-porous forms | -Biologically inert, reducing risk of adverse reactions -Flexibility and ease of handling | -Limited prosthetic movement compared to other implant types -Risk of migration without proper wrapping or connections | [156,157,158] |
| Poly(methylmethacrylate) (PMMA) | -Non-porous and hallow variations | -Highly biocompatible and transparent to visible light -Provide good volume correction -Offer stability and customizability -Positive aesthetic results | -Hydrophobicity can increase deposits of tear proteins and other debris near eyelids -Risk of poor wettability leading to dryness, lacrimal drainage blockage, meibomian gland dysfunction, excessive mucoid discharge, and lagophthalmos | [133,159,160,161,162] |
| Poly-HEMA (2-hydroxyethyl methacralate) implant (Alphasphere) | -Two-phase structure with a porous sponge at the front and non-porous gel at the back | -Does not require tissue wrapping -Facilitates direct muscle suturing -Enhanced mobility | -Reports of host tissue reactions and implant failure | [163,164,173] |
| Polytetrafluoroethylene (ePTFE or Gore-Tex) based materials | -Porous quasi-integrated enucleation implant -Siliconized non-porous posterior surface -Composites of Teflon/alumina and Teflon/carbon fibres | -Composite materials have shown reduced complication risks -Enhanced motility | -Inflammatory complications limit application of pure Teflon implants -Risk of infection | [165,166,167,168,169,170,171,172] |
| Porous PE (Medpor) | -Made from ultra-high molecular weight polyetheylene | -Minimal inflammation and fibrosis -Cost-effective -Smoother surface -Affordable | -Slower vascularization than other implant materials -Poor tissue in-growth can limit antibiotic penetration, necessitating implant removal in cases of infection -Advanced designs with added functionalities are more costly | [174,175] |
Polyethylene (PE)
3.5. What’s New? Emerging Biomaterials and Their New Applications in Orbital Implants and Prosthesis
3.5.1. Surface Coatings
Coatings to Improve Vascularization
Coatings to Improve Antimicrobial Activity
3.5.2. Bioactive Glasses (BGs)
3.5.3. Biosilicate-Derived Implants
| Material Type | Key Features | Advantages | Challenges | References |
|---|---|---|---|---|
| Coatings to improve vascularization | -Include synthetic HA, fibroblast growth factor (bFGF), and VEGF functionalized collagen | -bFGF coated with antibiotic drops has demonstrated improved healing rates and increased fibrovascular growth -Porous HA scaffolds coated with VEGF functionalized collagen may have enhanced mechanical strength, increased cell proliferation, and pronounced angiogenesis compared to uncoated HA scaffolds | -Lack of benefit of HA-coated implants -Current research only involves animal and in vitro studies | [176,177,178,179,180,181,182,183,184,185] |
| Coatings to improve antimicrobial activity | -Include silver, silver/silica, and copper-oxide nanoparticles | -Antimicrobial benefits | -Risk of toxicity | [186,187,188] |
| Bioactive glasses | -Porous and non-porous forms that can release therapeutic ions | -Biocompatible -Incorporation of ions enables antibacterial and anti-inflammatory effects -Induce angiogenesis and fibrovascularization | -Lack of long-term clinical evidence | [189,190,192,193,194,195] |
| Biosilicate-derived implants | -Enhanced glass composite with Na-Ca silicate | -Highly biocompatible -Reduced inflammatory response | -Novel application in ocular prostheses with limited evidence to date | [197,199] |
3.6. Challenges, Barriers, Gaps in Knowledge and Future Directions
3.7. Gaps in Knowledge and Future Directions
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstine, M.A. Clinical Recommendations for Repair of Isolated Orbital Floor Fractures: An Evidence-Based Analysis1 1The Author Has No Financial Interest in Any of the Materials Discussed. Ophthalmology 2002, 109, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Regan, W.F. Blow-Out Fracture of the Orbit*: Mechanism and Correction of Internal Orbital Fracture. Am. J. Ophthalmol. 1957, 44, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Kulwin, D.R.; Leadbetter, M.G. Orbital Rim Trauma Causing a Blowout Fracture. Plast. Reconstr. Surg. 1984, 73, 969. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, N.; Lyne, J.; Urdang, M.; Garey, L. An Investigation into the Mechanism of Orbital Blowout Fractures. Br. J. Plast. Surg. 1999, 52, 607–612. [Google Scholar] [CrossRef]
- Warwar, R.E.; Bullock, J.D.; Ballal, D.R.; Ballal, R.D. Mechanisms of Orbital Floor Fractures: A Clinical, Experimental, and Theoretical Study. Ophthal. Plast. Reconstr. Surg. 2000, 16, 188. [Google Scholar] [CrossRef]
- Kersten, R.C.; Vagefi, M.R.; Bartley, G.B. Orbital “Blowout” Fractures: Time for a New Paradigm. Ophthalmology 2018, 125, 796–798. [Google Scholar] [CrossRef]
- 2020–2021 BCSC Basic and Clinical Science CourseTM. Available online: https://www.aao.org/education/bcscsnippetdetail.aspx?id=b879ee48-8b97-4985-a5b9-af8f9125d2c6 (accessed on 4 August 2023).
- Poeschl, P.W.; Baumann, A.; Dorner, G.; Russmueller, G.; Seemann, R.; Fabian, F.; Ewers, R. Functional Outcome after Surgical Treatment of Orbital Floor Fractures. Clin. Oral Investig. 2012, 16, 1297–1303. [Google Scholar] [CrossRef]
- Waite, P.D.; Carr, D.D. The Transconjunctival Approach for Treating Orbital Trauma. J. Oral Maxillofac. Surg. 1991, 49, 499–503. [Google Scholar] [CrossRef]
- Baumann, A.; Ewers, R. Use of the Preseptal Transconjunctival Approach in Orbit Reconstruction Surgery. J. Oral Maxillofac. Surg. 2001, 59, 287–291. [Google Scholar] [CrossRef]
- Saha, A.K.; Samaddar, S.; Kumar, A.; Chakraborty, A.; Deb, B. A Comparative Study of Orbital Blow out Fracture Repair, Using Autogenous Bone Graft and Alloplastic Materials. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 542–549. [Google Scholar] [CrossRef]
- Moubarak, M.A.; Saied, S.; Shoeib, M.A.; Hassanyn, M.A. Reconstruction of Orbital Blow-Out Fracture by Titanium Mesh Versus Autogenous Bone. Egypt. J. Plast. Reconstr. Surg. 2023, 47, 123–132. [Google Scholar] [CrossRef]
- Saluja, H.; Sachdeva, S.; Shah, S.; Dadhich, A.; Tandon, P.; More, V. Autogenous Grafts for Orbital Floor Reconstruction: A Review. Int. J. Oral Craniofac. Sci. 2017, 3, 046–052. [Google Scholar]
- Mangan, M.S.; Goker, A.E.; Yurttaser Ocak, S.; Uyar, Y. Comparison of Nasoseptal Cartilage Graft versus Titanium Mesh in Reconstruction of Pure Orbital Blowout Fractures. J. Craniofac. Surg. 2021, 32, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Deep, M.; Baroudi, I.; Assad, M. Assessment of the Efficacy of Auricular Conchal Cartilage Graft in Repairing Orbital FLoor Fractures and Its Effect on Diplopia: A Nonrandomized Clinical Trial. Ann. Med. Surg. 2023, 85, 3538–3544. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, G.-M. Repairing Orbital Floor Fracture with Autologous Nasal Septal Cartilage by Using Endoscope through Transoral Maxillary Sinus. J. Coll. Physicians Surg. Pak. JCPSP 2022, 32, 1372–1373. [Google Scholar]
- Kim, T.W.; Kim, M.J. Application of One Piece Autologous Rib Cartilage Graft in Hollow Space of Complete Naso-Ethmoid Orbital Fracture. J. Craniofac. Surg. 2022, 33, 1028–1031. [Google Scholar] [CrossRef]
- Alasady, M.S.; Kanj, A. Evaluation the Outcomes of Using Iliac Bone Graft for Reconstruction of Traumatic Orbital Floor Fractures. J. Popul. Ther. Clin. Pharmacol. 2022, 29, e71–e78. [Google Scholar] [CrossRef]
- Waite, P.D.; Clanton, J.T. Orbital Floor Reconstruction with Lyophilized Dura. J. Oral Maxillofac. Surg. 1988, 46, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Webster, K. Orbital Floor Repair with Lyophilized Porcine Dermis. Oral Surg. Oral Med. Oral Pathol. 1988, 65, 161–164. [Google Scholar] [CrossRef]
- Sallam, M.M.; Hashem, H.A.; Shokier, H.M.R. Use of Demineralized Bone Sheets in Reconstruction of Orbital Floor Trap Door Fracture. J. Appl. Sci. Res. 2010, 6, 653–658. [Google Scholar]
- Chen, J.M.; Zingg, M.; Laedrach, K.; Raveh, J. Early Surgical Intervention for Orbital Floor Fractures: A Clinical Evaluation of Lyophilized Dura and Cartilage Reconstruction. J. Oral Maxillofac. Surg. 1992, 50, 935–941. [Google Scholar] [CrossRef]
- Guerra, M.F.M.; Pérez, J.S.; Rodriguez-Campo, F.J.; Gías, L.N. Reconstruction of Orbital Fractures with Dehydrated Human Dura Mater. J. Oral Maxillofac. Surg. 2000, 58, 1361–1366. [Google Scholar] [CrossRef]
- Sakr, H.; Shaaban, A.; Aly, T.; Elshiekh, S. Clinical Evaluation of Using Cortical Lamina Xenograft in Reconstruction of Orbital Floor. Alex. Dent. J. 2021, 46, 8–12. [Google Scholar] [CrossRef]
- Tak, K.S.; Jung, M.S.; Lee, B.H.; Kim, J.H.; Ahn, D.K.; Jeong, H.S.; Park, Y.K.; Suh, I.S. Combination of Absorbable Mesh and Demineralized Bone Matrix in Orbital Wall Fracture for Preventing Herniation of Orbit. J. Craniofac. Surg. 2014, 25, e352–e356. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Saba, E.S.; Gupta, N.; Hendricks, T.M.; Singh, D.J. Alloplastic Reconstruction of Orbital Floor Fractures: A Systematic Review and Pooled Outcomes Analysis. Eur. J. Plast. Surg. 2020, 43, 109–116. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Dev, V.; Pilania, D.; Harsh, A. Reconstruction of Orbital Floor Fractures with Titanium Micromesh: Our Experience. J. Maxillofac. Oral Surg. 2022, 21, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Marella, V.; Rohit; Khetrapal, P.; Gangasani, A.; Bhanot, R.; Uppal, A. Titanium Mesh versus Medpor Implant in Orbital Floor Reconstructions: A Comparative Study. J. Pharm. Bioallied Sci. 2021, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Munoli, A.; Bhanushali, J.; Jagannathan, M. Outcome of Precontoured Titanium Mesh in the Reconstruction of Orbital Blowout Fractures. Indian J. Plast. Surg. 2023, 56, 062–067. [Google Scholar] [CrossRef] [PubMed]
- Seen, S.; Young, S.; Lang, S.S.; Lim, T.-C.; Amrith, S.; Sundar, G. Orbital Implants in Orbital Fracture Reconstruction: A Ten-Year Series. Craniomaxillofacial Trauma Reconstr. 2021, 14, 56–63. [Google Scholar] [CrossRef]
- Ye, L.-X.; Sun, X.-M.; Zhang, Y.-G.; Zhang, Y. Materials to Facilitate Orbital Reconstruction and Soft Tissue Filling in Posttraumatic Orbital Deformaties. Plast. Aesthetic Res. 2016, 3, 86. [Google Scholar] [CrossRef]
- Essig, H.; Dressel, L.; Rana, M.; Rana, M.; Kokemueller, H.; Ruecker, M.; Gellrich, N.-C. Precision of Posttraumatic Primary Orbital Reconstruction Using Individually Bent Titanium Mesh with and without Navigation: A Retrospective Study. Head Face Med. 2013, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Su, Y.-X.; Yan, Y.; Choi, W.S.; Yang, W.-F.; Zhang, C.; Chen, X.; Curtin, J.P.; Ouyang, J.; Zhang, B. A Systematic Approach for Making 3D-Printed Patient-Specific Implants for Craniomaxillofacial Reconstruction. Engineering 2020, 6, 1291–1301. [Google Scholar] [CrossRef]
- Purnell, C.A.; Vaca, E.E.; Ellis, M.F. Orbital Fracture Reconstruction Using Prebent, Anatomic Titanium Plates: Technical Tips to Avoid Complications. J. Craniofac. Surg. 2018, 29, e515–e517. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, I.; Lee, J.; Oh, D.Y.; Jun, Y.J.; Rhie, J.W.; Shim, J.; Moon, S. Restoration of the Inferomedial Orbital Strut Using a Standardized Three-dimensional Printing Implant. J. Anat. 2020, 236, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Application of the Three-Dimensionally Printed Biodegradable Polycaprolactone (PCL) Mesh in Repair of Orbital Wall Fractures. J. Cranio-Maxillofac. Surg. 2019, 47, 1065–1071. [Google Scholar] [CrossRef]
- Choi, J.H.; Baek, W. Late Reconstruction of Post-Traumatic Enophthalmos and Hypoglobus Using Three-Dimensional Implants: A Case Series. Arch. Craniofacial Surg. 2022, 23, 232–236. [Google Scholar] [CrossRef]
- Mommaerts, M.Y.; Büttner, M.; Vercruysse, H.; Wauters, L.; Beerens, M. Orbital Wall Reconstruction with Two-Piece Puzzle 3D Printed Implants: Technical Note. Craniomaxillofacial Trauma Reconstr. 2016, 9, 055–061. [Google Scholar] [CrossRef]
- Gonzalez Alvarez, A.; Ananth, S.; Dovgalski, L.; Evans, P.L. Custom Three-Dimensional Printed Orbital Plate Composed of Two Joined Parts with Variable Thickness for a Large Orbital Floor Reconstruction after Post-Traumatic Zygomatic Fixation. Br. J. Oral Maxillofac. Surg. 2020, 58, e341–e342. [Google Scholar] [CrossRef]
- Day, K.M.; Phillips, P.M.; Sargent, L.A. Correction of a Posttraumatic Orbital Deformity Using Three-Dimensional Modeling, Virtual Surgical Planning with Computer-Assisted Design, and Three-Dimensional Printing of Custom Implants. Craniomaxillofacial Trauma Reconstr. 2018, 11, 078–082. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Zhang, D.; Hua, W.; Yin, J.; Jin, Y.; Chen, M. Theoretical Model of Pediatric Orbital Trapdoor Fractures and Provisional Personalized 3D Printing-Assisted Surgical Solution. Bioact. Mater. 2021, 6, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Tel, A.; Sembronio, S.; Costa, F.; Stenico, A.S.; Bagatto, D.; D’Agostini, S.; Robiony, M. Endoscopically Assisted Computer-Guided Repair of Internal Orbital Floor Fractures: An Updated Protocol for Minimally Invasive Management. J. Cranio-Maxillofac. Surg. 2019, 47, 1943–1951. [Google Scholar] [CrossRef]
- Vehmeijer, M.; Van Eijnatten, M.; Liberton, N.; Wolff, J. A Novel Method of Orbital Floor Reconstruction Using Virtual Planning, 3-Dimensional Printing, and Autologous Bone. J. Oral Maxillofac. Surg. 2016, 74, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Longeac, M.; Depeyre, A.; Pereira, B.; Barthelemy, I.; Pham Dang, N. Virtual Surgical Planning and Three-Dimensional Printing for the Treatment of Comminuted Zygomaticomaxillary Complex Fracture. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Luo, T.-Y.; Ma, J.; Dong, Z.; Cao, G.; Xu, J.; Liu, B.-Y.; Zhang, Q.-R.; Zhang, S.-L. Application of Three-Dimensional Printing Technology in the Orbital Blowout Fracture Reconstruction. J. Craniofac. Surg. 2019, 30, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Jeong, W.S.; Park, T.; Choi, J.W.; Koh, K.S.; Oh, T.S. The Accuracy of Patient Specific Implant Prebented with 3D-Printed Rapid Prototype Model for Orbital Wall Reconstruction. J. Cranio-Maxillofac. Surg. 2017, 45, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.S.; Jeong, W.S.; Chang, T.J.; Koh, K.S.; Choi, J.-W. Customized Orbital Wall Reconstruction Using Three-Dimensionally Printed Rapid Prototype Model in Patients With Orbital Wall Fracture. J. Craniofac. Surg. 2016, 27, 2020–2024. [Google Scholar] [CrossRef]
- Nekooei, S.; Sardabi, M.; Razavi, M.; Nekooei, A.; Kiarudi, M. Implantation of Customized, Preshaped Implant for Orbital Fractures with the Aid of Three-Dimensional Printing. Middle East Afr. J. Ophthalmol. 2018, 25, 56. [Google Scholar] [CrossRef]
- Mathur, K.K.; Tatum, S.A.; Kellman, R.M. Carbonated Apatite and Hydroxyapatite in Craniofacial Reconstruction. Arch. Facial Plast. Surg. 2003, 5, 379–383. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Lemke, B.N.; Kikkawa, D.O. Repair of Orbital Floor Fractures with Hydroxyapatite Block Scaffolding; LWW: Philadelphia, PA, USA, 1999; Volume 15, pp. 161–165. ISBN 0740-9303. [Google Scholar]
- Reyes, J.P.; Celorico, J.R.; Dela Cuesta, L.C.; Filo, J.M.; Daan, L.G.; Bernardo, S.T.; Abano, J. Bioceramic Orbital Plate Implant. Philipp. J. Sci. 2000, 129, 93–99. [Google Scholar]
- Nam, S.-B.; Bae, Y.-C.; Moon, J.-S.; Kang, Y.-S. Analysis of the Postoperative Outcome in 405 Cases of Orbital Fracture Using 2 Synthetic Orbital Implants. Ann. Plast. Surg. 2006, 56, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Prowse, S.J.B.; Hold, P.M.; Gilmour, R.F.; Pratap, U.; Mah, E.; Kimble, F.W. Orbital Floor Reconstruction: A Case for Silicone. A 12 Year Experience. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Klisovic, D.D.; Katz, S.E.; Lubow, M. The Wayward Implant: Orbital Silicone Plate Extrusion Associated with Squamous Epithelial Downgrowth and Infection. Orbit 2002, 21, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Arisawa, K.; Arisawa, Y.; Morishima, Y. Symptomatic Cystic Lesions as Late Post-Operative Complications of Silicone Implantation for Orbital Wall Fracture Reconstruction: A Long-Term Follow-up Study. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Vichitvejpaisal, P.; Dalvin, L.A.; Lally, S.E.; Shields, C.L. Delayed Implant Infection with Cutibacterium Acnes (Propionibacterium Acnes) 30 Years after Silicone Sheet Orbital Floor Implant. Orbit 2020, 39, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.J.; Donovan, M.G.; Hellstein, J.W.; Dickerson, N.C. Experimental Evaluation of Expanded Polytetrafluoroethylene for Reconstruction of Orbital Floor Defects. J. Oral Maxillofac. Surg. 1994, 52, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Elmazar, H.; Jackson, I.T.; Degner, D.; Miyawaki, T.; Barakat, K.; Andrus, L.; Bradford, M. The Efficacy of Gore-Tex vs. Hydroxyapatite and Bone Graft in Reconstruction of Orbital Floor Defects. Eur. J. Plast. Surg. 2003, 25, 362–368. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, T.; Zhong, Y.; Yu, Z.; Duan, H.; Li, D.; Xu, L.; Yuan, J.; Wei, M. Efficacy and Safety of Expanded Polytetrafluoroethylene Implantation in the Correction of Long-Term Posttraumatic Enophthalmos. Plast. Reconstr. Surg. 2023, 152, 1313–1318. [Google Scholar] [CrossRef]
- Goslin, K.N.; Miller, N.J.; Nguyen, E.V.; Hassan, A.S. Complex Fistula Formations After Orbital Fracture Repair With Teflon: A Review of 3 Case Reports. Ann. Plast. Surg. 2021, 86, 421–423. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.-I.; Kwon, Y.H.; Min, S.; Shin, H.W. Coating Medpor® Implant with Tissue-Engineered Elastic Cartilage. J. Funct. Biomater. 2020, 11, 34. [Google Scholar] [CrossRef]
- Demir, C.İ.; Yaşar, E.K.; Arıcı, Z.; Alagöz, M.Ş. Porous Polyethylene Implants in Orbital Floor Reconstruction: Outcome and Complications. Kocaeli Med. J. 2022, 11, 114–121. [Google Scholar] [CrossRef]
- Parikh, A.; Pfeiffer, M.; Yim, C.; Burnstine, M. Implants and Spacers for Paralytic Ectropion: Literature Review and Assessment of a Thin-Profile Porous Polyethylene Implant. Indian J. Ophthalmol. 2023, 71, 444. [Google Scholar] [CrossRef] [PubMed]
- Ram, H.; Singh, R.K.; Mohammad, S.; Gupta, A.K. Efficacy of Iliac Crest vs. Medpor in Orbital Floor Reconstruction. J. Maxillofac. Oral Surg. 2010, 9, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, B.; Beaulieu, R. Late-Onset Orbital Infection Following Blowout Fracture Repair with a Medpor Titan Implant: A Case Report. Investig. Ophthalmol. Vis. Sci. 2022, 63, 602-A0303. [Google Scholar]
- Suller, A.L.; Parikh, R.N.; Zhao, J.; Mahoney, N.R.; Campbell, A.A.; Siadati, S.; Eberhart, C.G.; Fu, R. Chocolate Cysts Associated With Porous Polyethylene Orbital Implants. Ophthal. Plast. Reconstr. Surg. 2021, 37, e75–e80. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, N.K.; ERÇÖÇEN, A. Reconstruction of Orbital Floor Fractures Using a Porous Polyethylene Implant: Outcomes in the Early, Intermediate and Late Postoperative Periods. ENT Updat. 2020, 10, 321–325. [Google Scholar]
- Slentz, D.H.; Rajjoub, L.; Domanski, M. Atraumatic Delayed Orbital Hematoma Sixteen Years after Orbital Floor Fracture Repair with Porous Polyethylene Implant. J. Craniofac. Surg. 2019, 30, 539–540. [Google Scholar] [CrossRef]
- Pang, S.S.Y.; Fang, C.; Chan, J.Y.W. Application of Three-Dimensional Printing Technology in Orbital Floor Fracture Reconstruction. Trauma Case Rep. 2018, 17, 23–28. [Google Scholar] [CrossRef]
- Peng, M.Y.; Merbs, S.L.; Grant, M.P.; Mahoney, N.R. Orbital Fracture Repair Outcomes with Preformed Titanium Mesh Implants and Comparison to Porous Polyethylene Coated Titanium Sheets. J. Cranio-Maxillofac. Surg. 2017, 45, 271–274. [Google Scholar] [CrossRef]
- Tabrizi, R.; Ozkan, T.B.; Mohammadinejad, C.; Minaee, N. Orbital Floor Reconstruction. J. Craniofac. Surg. 2010, 21, 1142–1146. [Google Scholar] [CrossRef]
- Blessing, N.W.; Rong, A.J.; Tse, B.C.; Erickson, B.P.; Lee, B.W.; Johnson, T.E. Orbital Bony Reconstruction With Presized and Precontoured Porous Polyethylene–Titanium Implants. Ophthal. Plast. Reconstr. Surg. 2021, 37, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Downes, R.N.; Vardy, S.; Tanner, K.E.; Bonfield, W. Hydroxyapatite-Polyethylene Composite in Orbital Surgery. In Bioceramics; Elsevier: Amsterdam, The Netherlands, 1991; pp. 239–246. [Google Scholar]
- Campbell, B.C.; Shipchandler, T.Z.; Ting, J.Y.; Vernon, D.; Torabi, R.S.; Miller, M.; Lee, H.H. Wraparound Nylon Foil Implant for Isolated Orbital Floor Fractures. Am. J. Otolaryngol. 2022, 43, 103229. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.J.; Garibaldi, D.C.; Iliff, N.T.; Grant, M.P.; Merbs, S.L. Smooth Nylon Foil (SupraFOIL) Orbital Implants in Orbital Fractures: A Case Series of 181 Patients. Ophthal. Plast. Reconstr. Surg. 2008, 24, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Jaru-Ampornpan, P.; Joseph, S.S.; Grisolia, A.B.D.; Briceno, C.A. Warfarin-Associated Delayed Orbital Hemorrhage after Orbital Fracture Repair with Smooth Nylon Foil Implant. Orbit 2019, 38, 519–523. [Google Scholar] [CrossRef]
- Jensen, A.D.; Hodgson, N.M.; Parikh, R.; Eberhart, C.G.; Henderson, A.D.; Fu, R. Orbital Inflammation in the Setting of a Nylon Foil Implant. Orbit 2022, 41, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.U.; Kim, S.Y. Biodegradable Implants for Orbital Wall Fracture Reconstruction. Arch. Craniofacial Surg. 2020, 21, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Asamura, S.; Ikada, Y.; Matsunaga, K.; Wada, M.; Isogai, N. Treatment of Orbital Floor Fracture Using a Periosteum–Polymer Complex. J. Cranio-Maxillofac. Surg. 2010, 38, 197–203. [Google Scholar] [CrossRef]
- Young, S.M.; Sundar, G.; Lim, T.-C.; Lang, S.S.; Thomas, G.; Amrith, S. Use of Bioresorbable Implants for Orbital Fracture Reconstruction. Br. J. Ophthalmol. 2017, 101, 1080–1085. [Google Scholar] [CrossRef]
- Al-Sukhun, J.; Lindqvist, C. A Comparative Study of 2 Implants Used to Repair Inferior Orbital Wall Bony Defects: Autogenous Bone Graft Versus Bioresorbable Poly-L/DL-Lactide [P(L/DL)LA 70/30] Plate. J. Oral Maxillofac. Surg. 2006, 64, 1038–1048. [Google Scholar] [CrossRef]
- Lieger, O.; Schaller, B.; Zix, J.; Kellner, F.; Iizuka, T. Repair of Orbital Floor Fractures Using Bioresorbable Poly-L/DL-Lactide Plates. Arch. Facial Plast. Surg. 2010, 12, 399–404. [Google Scholar] [CrossRef]
- Rozema, F.R.; Bos, R.R.; Pennings, A.J.; Jansen, H.W. Poly (L-Lactide) Implants in Repair of Defects of the Orbital Floor: An Animal Study. J. Oral Maxillofac. Surg. 1990, 48, 1305–1309. [Google Scholar] [CrossRef]
- Cordewener, F.W.; Bos, R.R.M.; Rozema, F.R.; Houtman, W.A. Poly(l-Lactide) Implants for Repair of Human Orbital Floor Defects: Clinical and Magnetic Resonance Imaging Evaluation of Long-Term Results. J. Oral Maxillofac. Surg. 1996, 54, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Esmail, M.E.K.; Ibrahiem, M.F.K.; Abdallah, R.M.A.; Elshafei, A.M.K.; Gawdat, T.I. Resorbable Polylactic Acid Polymer Plates in Repair of Blow-out Orbital Floor Fractures. Eur. J. Ophthalmol. 2021, 31, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Ishihara, T.; Tsuboi, Y.; Morishima, Y. Intermediate Outcomes of Orbital Wall Reconstruction Using Different Alloplastic Materials: Which Is Ideal? Plast. Reconstr. Surg. 2022, 150, 865–875. [Google Scholar] [CrossRef]
- Enislidis, G.; Pichorner, S.; Kainberger, F.; Ewers, R. Lactosorb Panel and Screws for Repair of Large Orbital Floor Defects. J. Cranio-Maxillofac. Surg. 1997, 25, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.V.; Walsh, J.; Brook, I.M. The Response of Cultured Bone Cells to Resorbable Polyglycolic Acid and Silicone Membranes for Use in Orbital Floor Fracture Repair. Clin. Mater. 1994, 17, 71–80. [Google Scholar] [CrossRef]
- Lin, J.; German, M.; Wong, B. Use of Copolymer Polylactic and Polyglycolic Acid Resorbable Plates in Repair of Orbital Floor Fractures. Facial Plast. Surg. 2014, 30, 581–586. [Google Scholar] [CrossRef]
- Eppley, B.L.; Sadove, M.A.; Havlik, R.J. Resorbable Plate Fixation in Pediatric Craniofacial Surgery. Plast. Reconstr. Surg. 1997, 100, 1–7. [Google Scholar] [CrossRef]
- Mauriello, J.A., Jr.; Wasserman, B.; Kraut, R. Use of Vicryl (Polyglactin-910) Mesh Implant for Repair of Orbital Floor Fracture Causing Diplopia: A Study of 28 Patients over 5 Years. Ophthal. Plast. Reconstr. Surg. 1993, 9, 191–195. [Google Scholar] [CrossRef]
- Beck-Broichsitter, B.E.; Acar, C.; Kandzia, C.; Jochens, A.; Wiltfang, J.; Becker, S.T. Reconstruction of the Orbital Floor with Polydioxanone: A Long-Term Clinical Survey of up to 12 Years. Br. J. Oral Maxillofac. Surg. 2015, 53, 736–740. [Google Scholar] [CrossRef]
- Becker, S.T.; Terheyden, H.; Fabel, M.; Kandzia, C.; Möller, B.; Wiltfang, J. Comparison of Collagen Membranes and Polydioxanone for Reconstruction of the Orbital Floor after Fractures. J. Craniofac. Surg. 2010, 21, 1066–1068. [Google Scholar] [CrossRef]
- Gierloff, M.; Seeck, N.G.K.; Springer, I.; Becker, S.; Kandzia, C.; Wiltfang, J. Orbital Floor Reconstruction with Resorbable Polydioxanone Implants. J. Craniofac. Surg. 2012, 23, 161–164. [Google Scholar] [CrossRef]
- Mikkonen, P.; Paukku, P.; Lindqvist, C. Reconstruction of Orbital Floor with Polydioxanone Plate. Int. J. Oral Maxillofac. Surg. 1991, 20, 83–87. [Google Scholar]
- Baumann, A.; Burggasser, G.; Gauss, N.; Ewers, R. Orbital Floor Reconstruction with an Alloplastic Resorbable Polydioxanone Sheet. Int. J. Oral Maxillofac. Surg. 2002, 31, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Kontio, R.; Suuronen, R.; Salonen, O.; Paukku, P.; Konttinen, Y.T.; Lindqvist, C. Effectiveness of Operative Treatment of Internal Orbital Wall Fracture with Polydioxanone Implant. Int. J. Oral Maxillofac. Surg. 2001, 30, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Büchel, P.; Rahal, A.; Seto, I.; Iizuka, T. Reconstruction of Orbital Floor Fracture with Polyglactin 910/Polydioxanon Patch (Ethisorb): A Retrospective Study. J. Oral Maxillofac. Surg. 2005, 63, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Steinmassl, O.; Laimer, J.; Offermanns, V.; Wildauer, M.; Steinmassl, P.-A.; Grams, A.E.; Kofler, F.; Rasse, M.; Bruckmoser, E. Clinical Outcome Following Surgical Repair of Small versus Large Orbital Floor Fractures Using Polyglactin 910/Polydioxanone (Ethisorb®). Materials 2020, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Blake, F.; Blessmann, M.; Smeets, R.; Friedrich, R.; Schmelzle, R.; Heiland, M.; Eichhorn, W. Long-Term Follow-up of Blowout Fractures of the Orbital Floor Reconstructed with a Polyglactin 910/PDS Implant. Eur. J. Trauma Emerg. Surg. 2011, 37, 609–613. [Google Scholar] [CrossRef]
- Tabrizi, R.; Langner, N.J.; Pouzesh, A.; Arabion, H. Evaluation of the Biodegradable Plates (PG910/PDO) for Reconstruction of Various Sizes of Orbital Floor Defects in the Blow-Out Fractures. Craniomaxillofacial Trauma Reconstr. 2013, 6, 187–190. [Google Scholar] [CrossRef]
- Gerressen, M.; Gilleßen, S.; Riediger, D.; Hölzle, F.; Modabber, A.; Ghassemi, A. Radiologic and Facial Morphologic Long-Term Results in Treatment of Orbital Floor Fracture With Flexible Absorbable Alloplastic Material. J. Oral Maxillofac. Surg. 2012, 70, 2375–2385. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- AL-Hamoudi, F.; Rehman, H.U.; Almoshawah, Y.A.; Talari, A.C.S.; Chaudhry, A.A.; Reilly, G.C.; Rehman, I.U. Bioactive Composite for Orbital Floor Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 10333. [Google Scholar] [CrossRef]
- Patel, M.; Betz, M.W.; Geibel, E.; Patel, K.J.; Caccamese, J.F.; Coletti, D.P.; Sauk, J.J.; Fisher, J.P. Cyclic Acetal Hydroxyapatite Nanocomposites for Orbital Bone Regeneration. Tissue Eng. Part A 2010, 16, 55–65. [Google Scholar] [CrossRef]
- Alhamoudi, F.; Akhtar, H.; Almoshawah, Y.; Talari, A.; Chaudhry, A.; Reilly, G.; Rehman, I.U. Bioactive Nano Hydroxyapatites for Orbital Floor Repair and Regeneration. J. Nanomed. 2021, 4, 1038. [Google Scholar]
- Sarfaraz, S.; Khan, A.; Hameed, F.; Arshad, A.; Mutahir, Z.; Zeeshan, R.; Ijaz, K.; Chaudhry, A.A.; Khalid, H.; Rehman, I.; et al. Osteogenic and Antibacterial Scaffolds of Silk Fibroin/Ce-Doped ZnO for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1205–1216. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Facts, Challenges, and Future Perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- Ramly, E.P.; Alfonso, A.R.; Kantar, R.S.; Wang, M.M.; Siso, J.R.D.; Ibrahim, A.; Coelho, P.G.; Flores, R.L. Safety and Efficacy of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Craniofacial Surgery. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2347. [Google Scholar] [CrossRef]
- Ristiniemi, J.; Flinkkilä, T.; Hyvönen, P.; Lakovaara, M.; Pakarinen, H.; Jalovaara, P. RhBMP-7 Accelerates the Healing in Distal Tibial Fractures Treated by External Fixation. J. Bone Joint Surg. Br. 2007, 89, 265–272. [Google Scholar] [CrossRef]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical Applications of BMP-7/OP-1 in Fractures, Nonunions and Spinal Fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef]
- Wen, Y.-D.; Jiang, W.-M.; Yang, H.-L.; Shi, J.-H. Exploratory Meta-Analysis on Dose-Related Efficacy and Complications of rhBMP-2 in Anterior Cervical Discectomy and Fusion: 1,539,021 Cases from 2003 to 2017 Studies. J. Orthop. Transl. 2020, 24, 166–174. [Google Scholar] [CrossRef]
- Chen, T.-M.; Tzeng, Y.-S.; Tsai, J.-C.; Burnouf, T. Single-Donor Allogeneic Platelet Fibrin Glue and Osteoconductive Scaffold in Orbital Floor Fracture Reconstruction. Ann. Plast. Surg. 2013, 70, 370–374. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, X.; Zhou, H.; Deng, Y.; Sun, J.; Xiao, C.; Gu, P.; Fan, X. Repair of Orbital Bone Defects in Canines Using Grafts of Enriched Autologous Bone Marrow Stromal Cells. J. Transl. Med. 2014, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, H.; Gu, P.; Fan, X. Repair of Canine Medial Orbital Bone Defects With miR-31–Modified Bone Marrow Mesenchymal Stem Cells. Investig. Opthalmology Vis. Sci. 2014, 55, 6016. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, O.; Geven, M.A.; Varjas, V.; Varga, P.; Gehweiler, D.; Stadelmann, V.A.; Smidt, T.; Zeiter, S.; Sprecher, C.; Bos, R.R.M.; et al. Orbital Floor Repair Using Patient Specific Osteoinductive Implant Made by Stereolithography. Biomaterials 2020, 233, 119721. [Google Scholar] [CrossRef] [PubMed]
- Blumer, M.; Pejicic, R.; Gander, T.; Johner, J.P.; Held, U.; Wagner, M.E. Customized Titanium Reconstruction of Orbital Fractures Using a Mirroring Technique for Virtual Reconstruction and 3D Model Printing. J. Oral Maxillofac. Surg. 2021, 79, 200.e1–200.e9. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-S.; Shin, H.J. Comparison of Orbital Reconstructive Effect between Customized Orbital Implants Using Three-Dimensional Printed Templates and Conventional Manual-Bending Implants in Blowout Fracture Surgery. Appl. Sci. 2023, 13, 9012. [Google Scholar] [CrossRef]
- Chowdhury, K.; Krause, G.E. Selection of Materials for Orbital Floor Reconstruction. Arch. Otolaryngol. Neck Surg. 1998, 124, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.; Enninghorst, N. The Dilemma of Reconstructive Material Choice for Orbital Floor Fracture: A Narrative Review. Medicines 2022, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghi, D.M.; Moshfeghi, A.A.; Finger, P.T. Enucleation. Surv. Ophthalmol. 2000, 44, 277–301. [Google Scholar] [CrossRef]
- Dortzbach, R.K.; Woog, J.J. Choice of Procedure: Enucleation, Evisceration, or Prosthetic Fitting Over Globes. Ophthalmology 1985, 92, 1249–1255. [Google Scholar] [CrossRef]
- Kostick, D.A.; Linberg, J.V. Evisceration with Hydroxyapatite Implant: Surgical Technique and Review of 31 Case Reports. Ophthalmology 1995, 102, 1542–1549. [Google Scholar] [CrossRef]
- Walter, W.L. Update on Enucleation and Evisceration Surgery*. Ophthal. Plast. Reconstr. Surg. 1985, 1, 243. [Google Scholar] [CrossRef]
- Sokoya, M.; Cohn, J.E.; Kohlert, S.; Lee, T.; Kadakia, S.; Ducic, Y. Considerations in Orbital Exenteration. Semin. Plast. Surg. 2019, 33, 103–105. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Yang, X.; Fan, X.-L. The Evolution of Orbital Implants and Current Breakthroughs in Material Design, Selection, Characterization, and Clinical Use. Front. Bioeng. Biotechnol. 2022, 9, 800998. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, H.A.; Sabt, B.I.; Al-Mujaini, A.S. Orbital Implant Exposure Following Enucleation or Evisceration. Oman J. Ophthalmol. 2017, 10, 87–90. [Google Scholar] [CrossRef]
- Quaranta-Leoni, F.M.; Fiorino, M.G.; Quaranta-Leoni, F.; Di Marino, M. Anophthalmic Socket Syndrome: Prevalence, Impact and Management Strategies. Clin. Ophthalmol. Auckl. NZ 2021, 15, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Mules, P. Evisceration of the Giobe with Artificial Vitreous. Ophthalmol. Soc. UK 1885, 5, 200–208. [Google Scholar]
- Artioli Schellini, S.; Barbarini Ferraz, L.C.; Rahdar, A.; Baino, F. Applications of Bioceramics in the Management of Orbital Floor Factures and Anophthalmic Cavity: A Review. Iran. J. Mater. Sci. Eng. 2022, 19, 2. [Google Scholar] [CrossRef]
- Dutton, J.J. Coralline Hydroxyapatite as an Ocular Implant. Ophthalmology 1991, 98, 370–377. [Google Scholar] [CrossRef]
- Kundu, B.; Sinha, M.; Mitra, S.; Basu, D. Synthetic Hydroxyapatite-Based Integrated Orbital Implants: A Human Pilot Trial. Indian J. Ophthalmol. 2005, 53, 235. [Google Scholar] [CrossRef] [PubMed]
- Babar, T.F.; Hussain, M.; Zaman, M. Clinico-Pathologic Study of 70 Enucleations. JPMA J. Pak. Med. Assoc. 2009, 59, 612–614. [Google Scholar]
- Owji, N.; Mosallaei, M.; Taylor, J. The Use of Mersilene Mesh for Wrapping of Hydroxyapatite Orbital Implants: Mid-Term Result. Orbit 2012, 31, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Morel, X.; Rias, A.; Briat, B.; El Aouni, A.; D’Hermies, F.; Adenis, J.P.; Legeais, J.M.; Renard, G. Biocompatibility of a Porous Alumina Orbital Implant. Preliminary Results of an Animal Experiment. J. Fr. Ophtalmol. 1998, 21, 163–169. [Google Scholar]
- Noiri, A.; Hoshi, F.; Murakami, H.; Sato, K.; Kawai, S.-I.; Kawai, K. Biocompatibility of a Mobile Alumina-Ceramic Orbital Implant. Folia Ophthalmol. Jpn. 2002, 53, 476–480. [Google Scholar]
- Jordan, D.R.; Gilberg, S.; Mawn, L.A. The Bioceramic Orbital Implant: Experience With 107 Implants. Ophthal. Plast. Reconstr. Surg. 2003, 19, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.A.; Tong, J.; Juniat, V.; Patel, S.; Dhatrak, D.; Selva, D. Extensive Orbital Inflammation in an Anophthalmic Socket: Is the Bioceramic Implant a Bystander or a Participant? Am. J. Ophthalmol. Case Rep. 2022, 28, 101721. [Google Scholar] [CrossRef]
- Ramey, N.; Gupta, D.; Price, K.; Husain, A.; Richard, M.; Woodward, J. Comparison of Complication Rates of Porous Anophthalmic Orbital Implants. Ophthalmic Surg. Lasers Imaging Retina 2011, 42, 434–440. [Google Scholar] [CrossRef]
- Jordan, D.R.; Klapper, S.R.; Gilberg, S.M.; Dutton, J.J.; Wong, A.; Mawn, L. The Bioceramic Implant: Evaluation of Implant Exposures in 419 Implants. Ophthal. Plast. Reconstr. Surg. 2010, 26, 80–82. [Google Scholar] [CrossRef]
- Zigiotti, G.L.; Cavarretta, S.; Morara, M.; Nam, S.M.; Ranno, S.; Pichi, F.; Lembo, A.; Lupo, S.; Nucci, P.; Meduri, A. Standard Enucleation with Aluminium Oxide Implant (Bioceramic) Covered with Patient’s Sclera. Sci. World J. 2012, 2012, 481584. [Google Scholar] [CrossRef]
- Wang, J.-K.; Lai, P.-C. Bioceramic Orbital Implant Exposure Repaired by a Retroauricular Myoperiosteal Graft. Ophthalmic Surg. Lasers Imaging Retina 2008, 39, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Hauck, M.J.; Steele, E.A. Dermis Fat Graft Implantation after Unilateral Enucleation for Retinoblastoma in Pediatric Patients. Ophthal. Plast. Reconstr. Surg. 2015, 31, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Quaranta-Leoni, F.M.; Sposato, S.; Raglione, P.; Mastromarino, A. Dermis-Fat Graft in Children as Primary and Secondary Orbital Implant. Ophthal. Plast. Reconstr. Surg. 2016, 32, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Çoban Karataş, M.; Altan Yaycıoğlu, R.; Canan, H. Orbital Dermis Fat Graft Transplantation: Results in Primary and Secondary Implantation. 2015. Available online: https://jag.journalagent.com/z4/vi.asp?pdir=tjo&plng=eng&un=TJO-55823&look4= (accessed on 16 December 2023).
- Nentwich, M.M.; Schebitz-Walter, K.; Hirneiss, C.; Hintschich, C. Dermis Fat Grafts as Primary and Secondary Orbital Implants. Orbit 2014, 33, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Habal, M.B. Aesthetic Considerations in the Reconstruction of the Anophthalmic Orbit. Aesthetic Plast. Surg. 1987, 11, 229–239. [Google Scholar] [CrossRef]
- Hynes, S.L.; Forrest, C.R.; Borschel, G.H. Use of the Anterolateral Thigh Flap for Reconstruction of the Pediatric Anophthalmic Orbit. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 84–90. [Google Scholar] [CrossRef]
- Wei, Y.H.; Liao, S.L. The Reconstruction of a Contracted Eye Socket Using a Post-Auricular Full-Thickness Skin Graft. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 821–827. [Google Scholar] [CrossRef]
- Shams, P.N.; Bohman, E.; Baker, M.S.; Maltry, A.C.; Kopp, E.D.; Allen, R.C. Chronic Anophthalmic Socket Pain Treated by Implant Removal and Dermis Fat Graft. Br. J. Ophthalmol. 2015, 99, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Winocour, S.; Saksena, A.; Oh, C.; Wu, P.S.; Laungani, A.; Baltzer, H.; Saint-Cyr, M. A Systematic Review of Comparison of Autologous, Allogeneic, and Synthetic Augmentation Grafts in Nipple Reconstruction. Plast. Reconstr. Surg. 2016, 137, 14e–23e. [Google Scholar] [CrossRef]
- Medel, R.; Alonso, T.; Pelaez, F.; Vasquez, L. Periumbilical Fat Auto-Graft Associated to a Porous Orbital Implant for Socket Reconstruction after Enucleation. Orbit 2016, 35, 181–186. [Google Scholar] [CrossRef]
- Baino, F. Scleral Buckling Biomaterials and Implants for Retinal Detachment Surgery. Med. Eng. Phys. 2010, 32, 945–956. [Google Scholar] [CrossRef]
- GIRARD, L.J.; McPHERSON, A.R. Scleral Buckling: Full Thickness and Circumferential, Using Silicon Rubber Rodding and Photocoagulation. Arch. Ophthalmol. 1962, 67, 409–420. [Google Scholar] [CrossRef]
- Schepens, C.L.; Okamura, I.D.; Brockhurst, R.J.; Regan, C.D.J. Scleral Buckling Procedures: V. Synthetic Sutures and Silicone Implants. Arch. Ophthalmol. 1960, 64, 868–881. [Google Scholar] [CrossRef]
- Agahan, A.L.; Tan, A.D. Use of Hollow Polymethylmethacrylate as an Orbital Implant. Philipp. J. Ophthalmol. 2004, 29, 21–25. [Google Scholar]
- Groth, M.J.; Bhatnagar, A.; Clearihue, W.J.; Goldberg, R.A.; Douglas, R.S. Long-Term Efficacy of Biomodeled Polymethyl Methacrylate Implants for Orbitofacial Defects. Arch. Facial Plast. Surg. 2006, 8, 381–389. [Google Scholar] [CrossRef]
- Taneja, S.; Aldoais, T.; Kaliki, S. Primary Orbital Polymethylmethacrylate Implant Following Primary Enucleation for Retinoblastoma: A Study of 321 Cases. Orbit 2021, 40, 127–132. [Google Scholar] [CrossRef]
- Pine, K.R.; De Silva, K.; Zhang, F.; Yeoman, J.; Jacobs, R. Towards Improving the Biocompatibility of Prosthetic Eyes. Heliyon 2021, 7, e06234. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, L.; Boss, J.; Shah, C.T.; Droste, P.J.; Hassan, A.S. Alphasphere as a Successful Ocular Implant in Primary Enucleation and Secondary Orbital Implant Exchange. Orbit 2013, 32, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Jakobiec, F.A.; De Castro, D.K.; Mendoza, P.R.; Fay, A. Extruded, Partially Disintegrated, Poly-HEMA Orbital Implant (AlphaSphere). Ophthal. Plast. Reconstr. Surg. 2014, 30, e86–e91. [Google Scholar] [CrossRef] [PubMed]
- Cas, R.D.; Maus, M.; Bilyk, J.; Chang, W.; Eagle, R.C., Jr.; Rubin, P. Gore-Tex as an Orbital Implant Material. Ophthal. Plast. Reconstr. Surg. 1998, 14, 425–431. [Google Scholar] [CrossRef]
- Mortemousque, B.; Leger, F.; Velou, S.; Graffan, R.; Colin, J.; Korobelnik, J.F. S/e-PTFE Episcleral Buckling Implants: An Experimental and Histopathologic Study. J. Biomed. Mater. Res. 2002, 63, 686–691. [Google Scholar] [CrossRef]
- Lyall, M.G. Proplast Implant in Tenon’s Capsule after Excision of the Eye. Trans. Ophthalmol. Soc. UK 1976, 96, 79–81. [Google Scholar]
- Neuhaus, R.W.; Greider, B.; Baylis, H.I. Enucleation with Implantation of a Proplast Sphere. Ophthalmology 1984, 91, 494–496. [Google Scholar] [CrossRef]
- Whear, N.M.; Cousley, R.R.J.; Liew, C.; Henderson, D. Post-Operative Infection of Proplast Facial Implants. Br. J. Oral Maxillofac. Surg. 1993, 31, 292–295. [Google Scholar] [CrossRef]
- Girard, L.J.; Eguez, I.; Soper, J.W.; Soper, M.; Esnaola, N.; Homsy, C.A. Buried Quasi-Integrated Enucleation Implant of Proplast II. A Preliminary Report. Ophthal. Plast. Reconstr. Surg. 1990, 6, 141–143. [Google Scholar] [CrossRef]
- Shah, S.; Rhatigan, M.; Sampath, R.; Yeoman, C.; Sunderland, S.; Brammer, R.; Leatherbarrow, B. Use of Proplast II as a Subperiosteal Implant for the Correction of Anophthalmic Enophthalmos. Br. J. Ophthalmol. 1995, 79, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Christenbury, J.D. Use of Proplast II. Ophthal. Plast. Reconstr. Surg. 1991, 7, 223. [Google Scholar] [PubMed]
- Neimkin, M.G.; Reggie, S.; Holds, J.B. Proptosis and Anterior Dislocation as a Late Noninflammatory Complication of Failure of Tissue Integration in the Alphasphere Implant. Ophthal. Plast. Reconstr. Surg. 2017, 33, S173–S175. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-K.; Cho, W.-K.; Paik, J.-S.; Yang, S.-W. Long-Term Surgical Outcomes of Porous Polyethylene Orbital Implants: A Review of 314 Cases. Br. J. Ophthalmol. 2012, 96, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Liao, S.-L. Long-Term Complications of Different Porous Orbital Implants: A 21-Year Review. Br. J. Ophthalmol. 2017, 101, 681. [Google Scholar] [CrossRef] [PubMed]
- You, C.K.; Oh, S.H.; Kim, J.W.; Choi, T.H.; Lee, S.Y.; Kim, S.Y. Hydroxyapatite Coated Porous Alumina as a New Orbital Implant. Key Eng. Mater. 2002, 240, 563–566. [Google Scholar] [CrossRef]
- SEONG, Y.-S.; LEE, S.-Y.; KIM, S.-J. Morphological Study of a New Orbital Implant: Hydroxyapatite-Coated Porous Alumina in Rabbit. J. Korean Ophthalmol. Soc. 2001, 42, 1354–1361. [Google Scholar]
- Jordan, D.R.; Brownstein, S.; Gilberg, S.; Coupal, D.; Kim, S.; Mawn, L. Hydroxyapatite and Calcium Phophate Coatings on Aluminium Oxide Orbital Implants. Can. J. Ophthalmol. 2002, 37, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Song, S.-J.; Lee, S.-H.; Kim, E.-A. Fibrovascularization of Intraorbital Hydroxyapatite-Coated Alumina Sphere in Rabbits. Korean J. Ophthalmol. 2005, 19, 9. [Google Scholar] [CrossRef]
- Cui, H.; Li, H. Effect of Basic Fibroblast Growth Factor (bFGF) on the Treatment of Exposure of the Orbital Implants. J. Zhejiang Univ. Sci. B 2007, 8, 620–625. [Google Scholar] [CrossRef]
- Park, W.C.; Han, S.K.; Kim, N.J.; Chung, T.Y.; Khwarg, S.I. Effect of Basic Fibroblast Growth Factor on Fibrovascular Ingrowth into Porous Polyethylene Anophthalmic Socket Implants. Korean J. Ophthalmol. 2005, 19, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Zhu, Z.; Zhang, H.; Yi, J.; Liao, H. Effects of Basic Fibroblast Growth Factor Composite Sponge Treated Collagen on Vascularization of Orbital Implants: A Histopathologic Analysis. Zhonghua Bing Li Xue Za Zhi 2014, 43, 184–188. [Google Scholar]
- Jing, L.I.U.; Xinguang, Y.; Zhongqiao, Z.H.U.; Hua, Z.; Jinglin, Y.I.; Hongfei, L. Evaluation of bFGF Collagen Composite Sponge Promoting Vascular Ingrowth in Orbital Implantation by 99Tcm-MDP Scan. Chin. J. Exp. Ophthalmol. 2014, 12, 706–711. [Google Scholar]
- Jin, K.; Ye, X.; Li, S.; Li, B.; Zhang, C.; Gao, C.; Ye, J. A Biomimetic Collagen/Heparin Multi-Layered Porous Hydroxyapatite Orbital Implant for in Vivo Vascularization Studies on the Chicken Chorioallantoic Membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Li, B.; Lou, L.; Xu, Y.; Ye, X.; Yao, K.; Ye, J.; Gao, C. In Vivo Vascularization of MSC-Loaded Porous Hydroxyapatite Constructs Coated with VEGF-Functionalized Collagen/Heparin Multilayers. Sci. Rep. 2016, 6, 19871. [Google Scholar] [CrossRef]
- Yang, J.W.; Choi, J.; Lee, S.G.; Kim, D.S. Antibacterial Properties Of Artificial Eyes Containing Nano-Sized Particle Silver. Orbit 2011, 30, 77–81. [Google Scholar] [CrossRef]
- Baino, F.; Ferraris, S.; Miola, M.; Perero, S.; Verné, E.; Coggiola, A.; Dolcino, D.; Ferraris, M. Novel Antibacterial Ocular Prostheses: Proof of Concept and Physico-Chemical Characterization. Mater. Sci. Eng. C 2016, 60, 467–474. [Google Scholar] [CrossRef]
- Ye, J.; He, J.; Wang, C.; Yao, K.; Gou, Z. Copper-Containing Mesoporous Bioactive Glass Coatings on Orbital Implants for Improving Drug Delivery Capacity and Antibacterial Activity. Biotechnol. Lett. 2014, 36, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Verné, E.; Fiume, E.; Peitl, O.; Zanotto, E.D.; Brandão, S.M.; Schellini, S.A. Bioactive Glass and Glass-ceramic Orbital Implants. Int. J. Appl. Ceram. Technol. 2019, 16, 1850–1863. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Huang, T.; Ding, L.; Huang, Z.; Zhang, X. An Experimental Study of Bioactive Glass Ceramics as Orbital Implants. Hunan Yi Ke Xue Xue Bao Hunan Yike Daxue Xuebao Bull. Hunan Med. Univ. 1997, 22, 25–28. [Google Scholar]
- Xu, X.; Huang, Z.; Wang, C. Clinical study of bioactive glass ceramics as orbital implants. Hunan Yi Ke Xue Xue Bao Hunan Yike Daxue Xuebao Bull. Hunan Med. Univ. 1997, 22, 440–442. [Google Scholar]
- Heringer, D.M.; Ng, J.D. A Novel Approach to Re-Pegging Hydroxyapatite Implants Using Bioactive Glass. Ophthal. Plast. Reconstr. Surg. 2006, 22, 45–47. [Google Scholar] [CrossRef]
- Wang, C.; Jin, K.; He, J.; Wang, J.; Yang, X.; Yao, C.; Dai, X.; Gao, C.; Gou, Z.; Ye, J. Synergistic Effect of Copper-Containing Mesoporous Bioactive Glass Coating on Stimulating Vascularization of Porous Hydroxyapatite Orbital Implants in Rabbits. J. Biomed. Nanotechnol. 2018, 14, 688–697. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, J.-E.; Park, H.J.; Oum, B.S. Effect of Synthetic Bone Glass Particulate on the Fibrovascularization of Porous Polyethylene Orbital Implants. Ophthal. Plast. Reconstr. Surg. 2006, 22, 121–125. [Google Scholar] [CrossRef]
- Naik, M.N.; Murthy, R.K.; Honavar, S.G. Comparison of Vascularization of Medpor and Medpor-Plus Orbital Implants: A Prospective, Randomized Study. Ophthal. Plast. Reconstr. Surg. 2007, 23, 463–467. [Google Scholar] [CrossRef]
- Ma, X.; Schou, K.R.; Maloney-Schou, M.; Harwin, F.M.; Ng, J.D. The Porous Polyethylene/Bioglass Spherical Orbital Implant: A Retrospective Study of 170 Cases. Ophthal. Plast. Reconstr. Surg. 2011, 27, 21–27. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A Multipurpose, Highly Bioactive Glass-Ceramic. In Vitro, in Vivo and Clinical Trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Brandão, S.M.; Schellini, S.A.; Moraes, A.D.; Padovani, C.R.; Pellizzon, C.H.; Peitl, O.; Zanotto, E.D. Biocompatibility Analysis of Bioglass® 45S5 and Biosilicate® Implants in the Rabbit Eviscerated Socket. Orbit 2012, 31, 143–149. [Google Scholar] [CrossRef]
- Brandão, S.M.; Schellini, S.A.; Padovani, C.R.; Peitl, O.; Hashimoto, E. Biocompatibility Analisys of Bioglass® 45S5 and Biosilicate® Cone in Rabbit Eviscerated Cavity. Rev. Bras. Oftalmol. 2013, 72, 21–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Fujioka, J.K.; Daigle, P.; Tran, S.D. The Use of Functional Biomaterials in Aesthetic and Functional Restoration in Orbital Surgery. J. Funct. Biomater. 2024, 15, 33. https://doi.org/10.3390/jfb15020033
Wu KY, Fujioka JK, Daigle P, Tran SD. The Use of Functional Biomaterials in Aesthetic and Functional Restoration in Orbital Surgery. Journal of Functional Biomaterials. 2024; 15(2):33. https://doi.org/10.3390/jfb15020033
Chicago/Turabian StyleWu, Kevin Y., Jamie K. Fujioka, Patrick Daigle, and Simon D. Tran. 2024. "The Use of Functional Biomaterials in Aesthetic and Functional Restoration in Orbital Surgery" Journal of Functional Biomaterials 15, no. 2: 33. https://doi.org/10.3390/jfb15020033
APA StyleWu, K. Y., Fujioka, J. K., Daigle, P., & Tran, S. D. (2024). The Use of Functional Biomaterials in Aesthetic and Functional Restoration in Orbital Surgery. Journal of Functional Biomaterials, 15(2), 33. https://doi.org/10.3390/jfb15020033







