Advanced Ti–Nb–Ta Alloys for Bone Implants with Improved Functionality
Abstract
:1. Introduction
2. Materials and Methods
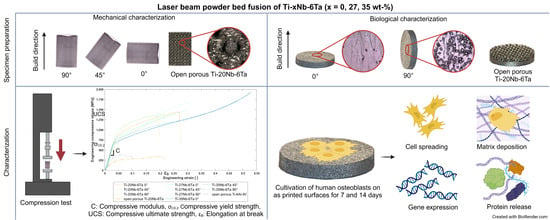
2.1. Specimen Manufacturing by Laser Beam Powder Bed Fusion
2.1.1. Specimens for Mechanical Characterization
2.1.2. Specimens for Biological Characterization
2.2. Microstructural Characterization
2.3. Mechanical Characterization
2.4. Biological Characterization
2.4.1. Cell Biological Experiments
2.4.2. Analysis of Cell Mediators Involved in Bone Formation, Remodeling, and Inflammation
2.5. Statistical Evaluation
3. Results
3.1. Microstructure Characterization
3.2. Mechanical Characterization by Compression Testing
3.3. Biological Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Pandey, A.; Awasthi, A.; Saxena, K.K. Metallic implants with properties and latest production techniques: A review. Adv. Mater. Process. Technol. 2020, 6, 405–440. [Google Scholar] [CrossRef]
- Farrahnoor, A.; Zuhailawati, H. Review on the mechanical properties and biocompatibility of titanium implant: The role of niobium alloying element. Int. J. Mater. Res. 2021, 112, 505–513. [Google Scholar] [CrossRef]
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.S.V.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Rao, S.; Ito, Y.; Tateishi, T. Corrosion resistance, mechanical properties, corrosion fatigue strength and cytocompatibility of new Ti alloys without Al and V. Biomaterials 1998, 19, 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Savio, D.; Bagno, A. When the Total Hip Replacement Fails: A Review on the Stress-Shielding Effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Schulze, C.; Weinmann, M.; Schweigel, C.; Keßler, O.; Bader, R. Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb. Materials 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Ozan, S.; Lin, J.; Li, Y.; Wen, C. New Ti-Ta-Zr-Nb alloys with ultrahigh strength for potential orthopedic implant applications. J. Mech. Behav. Biomed. Mater. 2017, 75, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Stráský, J.; Harcuba, P.; Václavová, K.; Horváth, K.; Landa, M.; Srba, O.; Janeček, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater. 2017, 71, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ozan, S.; Lin, J.; Li, Y.; Ipek, R.; Wen, C. Development of Ti-Nb-Zr alloys with high elastic admissible strain for temporary orthopedic devices. Acta Biomater. 2015, 20, 176–187. [Google Scholar] [CrossRef]
- Luo, J.P.; Sun, J.F.; Huang, Y.J.; Zhang, J.H.; Zhang, Y.D.; Zhao, D.P.; Yan, M. Low-modulus biomedical Ti-30Nb-5Ta-3Zr additively manufactured by Selective Laser Melting and its biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 275–284. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Martensitic transformations in Ti-(16–26 at%) Nb alloys. J. Mater. Sci. 1996, 31, 4267–4276. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, D.; Cheng, J.; Tsoi, J.K.H.; Chen, J. Mechanical and biological properties of Ti-(0-25 wt%)Nb alloys for biomedical implants application. Regen. Biomater. 2020, 7, 119–127. [Google Scholar] [CrossRef]
- Munir, K.; Lin, J.; Wright, P.F.A.; Ozan, S.; Li, Y.; Wen, C. Mechanical, corrosion, nanotribological, and biocompatibility properties of equal channel angular pressed Ti-28Nb-35.4Zr alloys for biomedical applications. Acta Biomater. 2022, 149, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gustmann, T.; Günther, F.; Zimmermann, M.; Kühn, U.; Gebert, A. Controlling the Young’s modulus of a ß-type Ti-Nb alloy via strong texturing by LPBF. Mater. Des. 2022, 216, 110516. [Google Scholar] [CrossRef]
- Hussein, A.H.; Gepreel, M.A.-H.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti-Nb-Ta base alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Huang, J.C.; Chao, C.-Y.; Wei, L.L.; Tsai, M.T.; Chen, Y.H. Microstructure and elastic modulus evolution of TiTaNb alloys. J. Mech. Behav. Biomed. Mater. 2018, 86, 224–231. [Google Scholar] [CrossRef]
- Johannsen, J.; Lauhoff, C.; Stenzel, M.; Schnitter, C.; Niendorf, T.; Weinmann, M. Laser beam powder bed fusion of novel biomedical Titanium/Niobium/Tantalum alloys: Powder synthesis, microstructure evolution and mechanical properties. Mater. Des. 2023, 233, 112265. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; de Freitas Quadros, F.; Sousa, K.D.S.J.; Donato, T.A.G.; de Araújo, R.O.; Grandini, C.R. Preparation, structural, microstructural, mechanical and cytotoxic characterization of as-cast Ti-25Ta-Zr alloys. J. Mater. Sci. Mater. Med. 2020, 31, 19. [Google Scholar] [CrossRef]
- Kong, W.; Cox, S.C.; Lu, Y.; Villapun, V.; Xiao, X.; Ma, W.; Liu, M.; Attallah, M.M. Microstructural Evolution, Mechanical Properties, and Preosteoblast Cell Response of a Post-Processing-Treated TNT5Zr β Ti Alloy Manufactured via Selective Laser Melting. ACS. Biomater. Sci. Eng. 2022, 8, 2336–2348. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Zheng, C.Y.; Hu, Q.M.; Yang, R. Super-elastic titanium alloy with unstable plastic deformation. Appl. Phys. Lett. 2005, 87, 91906. [Google Scholar] [CrossRef]
- Challis, V.J.; Xu, X.; Halfpenny, A.; Cramer, A.D.; Saunders, M.; Roberts, A.P.; Sercombe, T.B. Understanding the effect of microstructural texture on the anisotropic elastic properties of Selective Laser Melted Ti-24Nb-4Zr-8Sn. Acta Mater. 2023, 254, 119021. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Wu, W.T.; Guo, Z.X. Theoretical study of the effects of alloying elements on the strength and modulus of β-type bio-titanium alloys. Mater. Sci. Eng. A 1999, 260, 269–274. [Google Scholar] [CrossRef]
- Soni, R.; Pande, S.; Salunkhe, S.; Natu, H.; Abouel Nasr, E.; Shanmugam, R.; Hussein, H.M.A.M. In Vitro and Electrochemical Characterization of Laser-Cladded Ti-Nb-Ta Alloy for Biomedical Applications. Crystals 2022, 12, 954. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio. 2019, 3, 100024. [Google Scholar] [CrossRef]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive manufacturing of titanium alloys in the biomedical field: Processes, properties and applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 57–67. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S. Biomedical applications of additive manufacturing: Present and future. Curr. Opin. Biomed. Eng. 2017, 2, 105–115. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y.; Munir, K.; Lin, J.; Brandt, M.; Atrens, A.; Xiao, Y.; Kanwar, J.R.; Wen, C. Novel β-Ti35Zr28Nb alloy scaffolds manufactured using selective laser melting for bone implant applications. Acta Biomater. 2019, 87, 273–284. [Google Scholar] [CrossRef]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical behaviours of the bone-implant interface: A review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef]
- Günther, F.; Pilz, S.; Hirsch, F.; Wagner, M.; Kästner, M.; Gebert, A.; Zimmermann, M. Shape optimization of additively manufactured lattices based on triply periodic minimal surfaces. Addit. Manuf. 2023, 73, 103659. [Google Scholar] [CrossRef]
- Song, C.; Liu, L.; Deng, Z.; Lei, H.; Yuan, F.; Yang, Y.; Li, Y.; Yu, J. Research progress on the design and performance of porous titanium alloy bone implants. J. Mater. Res. Technol. 2023, 23, 2626–2641. [Google Scholar] [CrossRef]
- Antonysamy, A.A.; Meyer, J.; Prangnell, P.B. Effect of build geometry on the β-grain structure and texture in additive manufacture of Ti6Al4V by selective electron beam melting. Mater. Charact. 2013, 84, 153–168. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti–6Al–4V. Mater. Sci. Eng. A 2014, 616, 1–11. [Google Scholar] [CrossRef]
- Sui, Q.; Li, P.; Wang, K.; Yin, X.; Liu, L.; Zhang, Y.; Zhang, Q.; Wang, S.; Wang, L. Effect of Build Orientation on the Corrosion Behavior and Mechanical Properties of Selective Laser Melted Ti-6Al-4V. Metals 2019, 9, 976. [Google Scholar] [CrossRef]
- Luo, X.; Song, T.; Gebert, A.; Neufeld, K.; Kaban, I.; Ma, H.; Cai, W.; Lu, H.; Li, D.; Li, N.; et al. Programming Crystallographic Orientation in Additive-Manufactured Beta-Type Titanium Alloy. Adv. Sci. (Weinh) 2023, 10, e2302884. [Google Scholar] [CrossRef]
- Ginestra, P.; Ferraro, R.M.; Zohar-Hauber, K.; Abeni, A.; Giliani, S.; Ceretti, E. Selective Laser Melting and Electron Beam Melting of Ti6Al4V for Orthopedic Applications: A Comparative Study on the Applied Building Direction. Materials 2020, 13, 5584. [Google Scholar] [CrossRef]
- Avila, J.D.; Bose, S.; Bandyopadhyay, A. Additive manufacturing of titanium and titanium alloys for biomedical applications. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–343. ISBN 9780128124567. [Google Scholar]
- Harun, W.; Manam, N.S.; Kamariah, M.; Sharif, S.; Zulkifly, A.H.; Ahmad, I.; Miura, H. A review of powdered additive manufacturing techniques for Ti-6al-4v biomedical applications. Powder Technol. 2018, 331, 74–97. [Google Scholar] [CrossRef]
- Aufa, A.N.; Hassan, M.Z.; Ismail, Z. Recent advances in Ti-6Al-4V additively manufactured by selective laser melting for biomedical implants: Prospect development. J. Alloys Compd. 2022, 896, 163072. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, K.; Święszkowski, W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Pramanik, A.; Basak, A.K.; Dong, Y.; Prakash, C.; Debnath, S.; Shankar, S.; Jawahir, I.S.; Dixit, S.; Buddhi, D. A critical review on additive manufacturing of Ti-6Al-4V alloy: Microstructure and mechanical properties. J. Mater. Res. Technol. 2022, 18, 4641–4661. [Google Scholar] [CrossRef]
- Ponader, S.; Vairaktaris, E.; Heinl, P.; Wilmowsky, C.V.; Rottmair, A.; Körner, C.; Singer, R.F.; Holst, S.; Schlegel, K.A.; Neukam, F.W.; et al. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J. Biomed. Mater. Res. A 2008, 84, 1111–1119. [Google Scholar] [CrossRef]
- Ivanov, E.; Del Rio, E.; Kapchemnko, I.; Nyström, M.; Kotila, J. Development of Bio-Compatible Beta Ti Alloy Powders for Additive Manufacturing for Application in Patient-Specific Orthopedic Implants. KEM 2018, 770, 9–17. [Google Scholar] [CrossRef]
- Kim, H.Y.; Fu, J.; Tobe, H.; Kim, J.I.; Miyazaki, S. Crystal Structure, Transformation Strain, and Superelastic Property of Ti–Nb–Zr and Ti–Nb–Ta Alloys. Shap. Mem. Superelast. 2015, 1, 107–116. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kim, H.Y.; Hosoda, H. Development and characterization of Ni-free Ti-base shape memory and superelastic alloys. Mater. Sci. Eng. A 2006, 438–440, 18–24. [Google Scholar] [CrossRef]
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-Hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 28, 1055–1063. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus-density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Sui, Q.; Meng, L.; Wang, S.; Li, P.; Yin, X.; Wang, L. Effect of Nb addition on mechanical properties and corrosion behavior of Ti6Al4V alloy produced by selective laser melting. J. Mater. Res. 2020, 35, 571–579. [Google Scholar] [CrossRef]
- Ishimoto, T.; Hagihara, K.; Hisamoto, K.; Sun, S.-H.; Nakano, T. Crystallographic texture control of beta-type Ti–15Mo–5Zr–3Al alloy by selective laser melting for the development of novel implants with a biocompatible low Young’s modulus. Scr. Mater. 2017, 132, 34–38. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhang, Y.; Zhang, L.-C.; Wang, L. Deformation mechanisms of additively manufactured TiNbTaZrMo refractory high-entropy alloy: The role of cellular structure. Int. J. Plast. 2024, 173, 103884. [Google Scholar] [CrossRef]
- Reilly, D.T.; Burstein, A.H. The elastic and ultimate properties of compact bone tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef]
- do Prado, R.F.; Esteves, G.C.; Santos, E.L.D.S.; Bueno, D.A.G.; Cairo, C.A.A.; de Vasconcellos, L.G.O.; Sagnori, R.S.; Tessarin, F.B.P.; Oliveira, F.E.; de Oliveira, L.D.; et al. In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS ONE 2018, 13, e0196169. [Google Scholar] [CrossRef]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109908. [Google Scholar] [CrossRef]
- Markhoff, J.; Weinmann, M.; Schulze, C.; Bader, R. Influence of different grained powders and pellets made of Niobium and Ti-42Nb on human cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 756–766. [Google Scholar] [CrossRef]
- Jonitz, A.; Lochner, K.; Lindner, T.; Hansmann, D.; Marrot, A.; Bader, R. Oxygen consumption, acidification and migration capacity of human primary osteoblasts within a three-dimensional tantalum scaffold. J. Mater. Sci. Mater. Med. 2011, 22, 2089–2095. [Google Scholar] [CrossRef]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Li, Z.; Gao, F.; Zhang, Q.; Bianco, A.; Liu, H.; Ge, S.; Ma, B. Materials-Mediated In Situ Physical Cues for Bone Regeneration. Adv. Funct. Mater. 2023, 34, 2306534. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Wang, R.; Han, Y.; Zhou, H. Effect of Controlled Microtopography on Osteogenic Differentiation of Mesenchymal Stem Cells. J. Healthc. Eng. 2022, 2022, 7179723. [Google Scholar] [CrossRef]
- Lauria, I.; Kutz, T.N.; Böke, F.; Rütten, S.; Zander, D.; Fischer, H. Influence of nanoporous titanium niobium alloy surfaces produced via hydrogen peroxide oxidative etching on the osteogenic differentiation of human mesenchymal stromal cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Weißmann, V.; Drescher, P.; Seitz, H.; Hansmann, H.; Bader, R.; Seyfarth, A.; Klinder, A.; Jonitz-Heincke, A. Effects of Build Orientation on Surface Morphology and Bone Cell Activity of Additively Manufactured Ti6Al4V Specimens. Materials 2018, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Jenkins, B.J.; Quinn, J.M.W.; Nakamura, A.; Glatt, M.; Gillespie, M.T.; Ernst, M.; Martin, T.J. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J. Clin. Investig. 2004, 113, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wan, Y.; Wang, H.; Yu, M.; Zhao, Z.; Wang, T.; Ma, G.; Fan, S.; Liu, Z. Effects of surface morphology and composition of titanium implants on osteogenesis and inflammatory responses: A review. Biomed. Mater. 2023, 18, 042002. [Google Scholar] [CrossRef]








| Material | Scanning Speed [mm/s] | Laser Power [W] | Layer Thickness [mm] | Resulting Density [%] | |
|---|---|---|---|---|---|
| Ti–20Nb–6Ta | 1250 | 170 | 0.30 | 99.96 | (0.01) a |
| Ti–27Nb–6Ta | 1350 | 170 | 0.30 | 99.97 | (0.01) a |
| Ti–35Nb–6Ta | 1500 | 170 | 0.30 | 99.97 | (0.01) a |
| Ti–6Al–4V | 1200 | 240 | 0.60 | >99.7 b | |
| Material | Strut Thickness [mm] | Pore Size [mm] | Porosity [%] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Front | Top | Front | Top | |||||||
| Ti–20Nb–6Ta | 0.47 | (0.04) | 0.43 | (0.05) | 0.47 | (0.06) | 0.54 | (0.05) | 49.47 | (0.49) |
| Ti–6Al–4V | 0.44 | (0.02) | 0.46 | (0.02) | 0.58 | (0.02) | 0.55 | (0.02) | 47.36 | (1.30) |
| Material | Ti–20Nb–6Ta | Ti–27Nb–6Ta | Ti–35Nb–6Ta | Ti–6Al–4V | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BO [°] | 0 | 90 | 0 | 90 | 0 | 90 | 0 | 90 | ||||||||
| Ra [µm] | 4.0 | (0.7) | 5.7 | (0.8) | 6.6 | (1.3) | 8.0 | (2.0) | 9.0 | (1.9) | 8.6 | (1.6) | 5.9 | (0.9) | 11.6 | (1.0) |
| Rz [µm] | 34.7 | (8.1) | 47.9 | (5.2) | 56.2 | (13.7) | 63.5 | (9.7) | 77.1 | (16.3) | 68.9 | (9.8) | 68.1 | (11.7) | 103.5 | (18.3) |
| Primer | Sequences (5′–3′) |
|---|---|
| Actin Beta (ACTB) | fwd: CTTCCTGGGCATGGAGTC rev: AGCACTGTGTTGGCGTACAG |
| Alkaline Phosphatase, Biomineralization Associated (ALPL) | fwd: CATTGTGACCACCACGAGAG rev: CCATGATCACGTCAATGTCC |
| Bone Gamma-Carboxyglutamate Protein (BGLAP) | fwd: TCAGCCAACTCGTCACAGTC rev: GGTGCAGCCTTTGTGTCC |
| Collagen Type I Alpha 1 Chain (COL1A1) | fwd: ACGAAGACATCCCACCAATC rev: AGATCACGTCATCGCACAAC |
| Interleukin 6 (IL6) | fwd: TGGATTCAATGAGGAGACTTGCC rev: CTGGCATTTGTGGTTGGGTC |
| Matrix Metallopeptidase 1 (MMP1) | fwd: AGAGCAGATGTGGACCATGC rev: TCCCGATGATCTCCCCTGAC |
| Runt-related Transcription Factor 2 (RUNX2) | fwd: CGCCTCACAAACAACCACAG rev: ACTGCTTGCAGCCTTAAATGAC |
| Secreted Phosphoprotein 1, Osteopontin (SPP1) | fwd: AACGCCGACCAAGGAAAACT rev: GCACAGGTGATGCCTAGGAG |
| Secreted protein acidic and rich in cysteine, Osteonectin (SPARC) | fwd: CTGGACTACATCGGGCCTTG rev: ATGGATCTTCTTCACCCGCAG |
| Tissue inhibitor of metalloproteinase-1 (TIMP1) | fwd: ATTGCTGGAAAACTGCAGGATG rev: GTCCACAAGCAATGAGTGCC |
| Nb [wt. %] | BO [°] | C [GPa] | σC0.2 [MPa] | UCS [MPa] | εB [%] | EAS [ ] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Densely manufactured | |||||||||||
| 20 | 0 | 69.0 | (2.7) | 670.1 | (6.3) | 1467.8 | (37.1) | 29.9 | (1.9) | 0.97 | (0.03) |
| 45 | 73.2 | (10.6) | 751.8 | (15.6) | 1374.6 | (30.2) | 25.3 | (1.7) | 1.05 | (0.17) | |
| 90 | 43.1 | (2.6) | 619.1 | (2.0) | 1453.3 | (21.0) | 30.2 | (1.7) | 1.44 | (0.09) | |
| 27 | 0 | 74.9 | (2.5) | 787.3 | (76.1) | 1629.1 | (191.8) | 34.9 | (5.5) | 1.05 | (0.11) |
| 45 | 87.9 | (5.4) | 954.4 | (13.6) | 1389.6 | (23.9) | 18.8 | 0.3) | 1.09 | (0.07) | |
| 90 | 51.7 | (2.1) | 695.1 | (49.1) | 1673.6 | (55.7) | 30.8 | (5.1) | 1.35 | (0.11) | |
| 35 a | 0 | 64.5 | (1.1) | 636.7 | (8.2) | - | 48.1 | (0.3) | 0.99 | (0.02) | |
| 45 | 72.6 | (2.5) | 693.4 | (3.1) | - | 48.1 | (0.1) | 0.96 | (0.03) | ||
| 90 | 54.2 | (2.9) | 597.2 | (17.0) | - | 48.1 | (0.3) | 1.12 | (0.02) | ||
| Open porous lattice-structured | |||||||||||
| Ti–20Nb–6Ta | 8.7 | (1.0) | 179.2 | (4.5) | 351.7 | (7.5) | 22.5 | (1.0) | 0.48 | (0.07) | |
| Ti–6Al–4V | 16.7 | (3.2) | 312.0 | (20.0) | 381.7 | (2.0) | 7.63 | (1.9) | 0.53 | (0.08) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sass, J.-O.; Sellin, M.-L.; Kauertz, E.; Johannsen, J.; Weinmann, M.; Stenzel, M.; Frank, M.; Vogel, D.; Bader, R.; Jonitz-Heincke, A. Advanced Ti–Nb–Ta Alloys for Bone Implants with Improved Functionality. J. Funct. Biomater. 2024, 15, 46. https://doi.org/10.3390/jfb15020046
Sass J-O, Sellin M-L, Kauertz E, Johannsen J, Weinmann M, Stenzel M, Frank M, Vogel D, Bader R, Jonitz-Heincke A. Advanced Ti–Nb–Ta Alloys for Bone Implants with Improved Functionality. Journal of Functional Biomaterials. 2024; 15(2):46. https://doi.org/10.3390/jfb15020046
Chicago/Turabian StyleSass, Jan-Oliver, Marie-Luise Sellin, Elisa Kauertz, Jan Johannsen, Markus Weinmann, Melanie Stenzel, Marcus Frank, Danny Vogel, Rainer Bader, and Anika Jonitz-Heincke. 2024. "Advanced Ti–Nb–Ta Alloys for Bone Implants with Improved Functionality" Journal of Functional Biomaterials 15, no. 2: 46. https://doi.org/10.3390/jfb15020046
APA StyleSass, J. -O., Sellin, M. -L., Kauertz, E., Johannsen, J., Weinmann, M., Stenzel, M., Frank, M., Vogel, D., Bader, R., & Jonitz-Heincke, A. (2024). Advanced Ti–Nb–Ta Alloys for Bone Implants with Improved Functionality. Journal of Functional Biomaterials, 15(2), 46. https://doi.org/10.3390/jfb15020046