Strontium-Doped Bioglass-Laden Gelatin Methacryloyl Hydrogels for Vital Pulp Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioglass Synthesis
2.2. Gelatin Methacryloyl Synthesis
2.3. Scanning Electron Microscopy and Energy Dispersive Spectroscopy
2.4. RAMAN Spectroscopy
2.5. Fourier Transform Infrared Spectroscopy
2.6. Swelling Capacity
2.7. Degradation Ratio
2.8. Mechanical Test
2.9. Cell Viability
2.10. Mineralized Nodule Formation
2.11. Cell Adhesion (Fluorescence)
2.12. Statistical Analysis
3. Results and Discussion
3.1. SEM and EDS
3.2. FTIR
3.3. RAMAN Spectrum
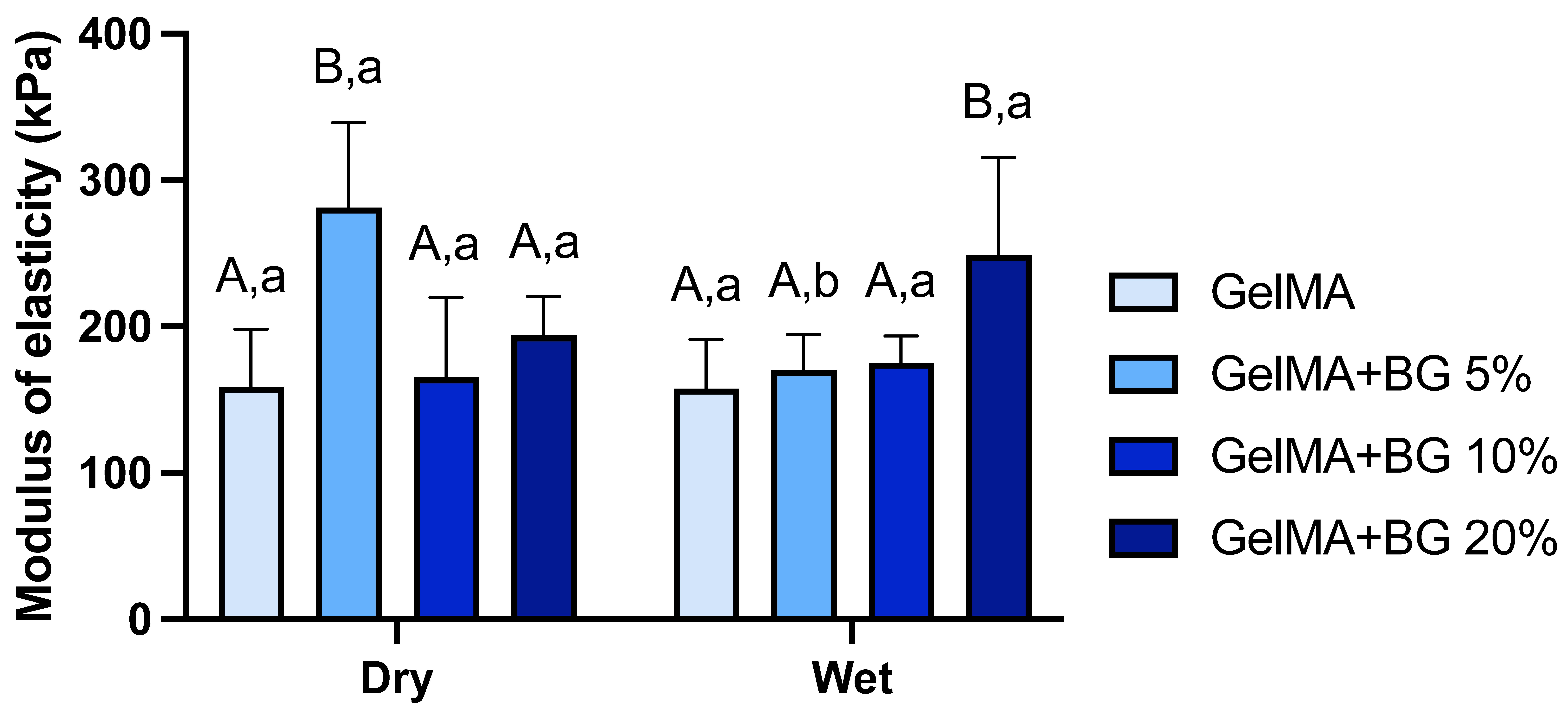
3.4. Mechanical Test
3.5. Swelling and Degradation
3.6. Cell Viability
3.7. Mineralized Matrix Formation
3.8. Cell Adhesion and Spreading
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, Y.; Tian, J.; Kong, S.; Feng, Y.; Lu, Y.; Su, L.; Cai, Y.; Li, M.; Chang, J.; Yang, C.; et al. SrCuSi4O10/GelMA Composite Hydrogel-Mediated Vital Pulp Therapy: Integrating Antibacterial Property and Enhanced Pulp Regeneration Activity. Adv. Heal. Mater. 2023, 12, e2300546. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Abbott, P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S16. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, A.; Silva, L.A.; Leonardo, M.R.; Rocha, L.B.; Rossi, M.A. Effect of rotary or manual instrumentation, with or without a calcium hydroxide/1% chlorhexidine intracanal dressing, on the healing of experimentally induced chronic periapical lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Fransson, H.; Dawson, V. Tooth survival after endodontic treatment. Int. Endod. J. 2023, 56 (Suppl. S2), 140–153. [Google Scholar] [CrossRef] [PubMed]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Iaculli, F.; Rodríguez-Lozano, F.J.; Briseño-Marroquín, B.; Wolf, T.G.; Spagnuolo, G.; Rengo, S. Vital Pulp Therapy of Permanent Teeth with Reversible or Irreversible Pulpitis: An Overview of the Literature. J. Clin. Med. 2022, 11, 4016. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp capping materials modulate the balance between inflammation and regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Fukushima, K.A.; Marques, M.M.; Tedesco, T.K.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.; Morimoto, S.; Moreira, M.S. Screening of hydrogel-based scaffolds for dental pulp regeneration—A systematic review. Arch. Oral Biol. 2019, 98, 182–194. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Mei, L.; Dubey, N.; Fenno, J.C.; Piva, E.; Lund, R.G.; Schwendeman, A.; Bottino, M.C. Injectable MMP-Responsive Nanotube-Modified Gelatin Hydrogel for Dental Infection Ablation. ACS Appl. Mater. Interfaces 2020, 12, 16006–16017. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jiang, X.; Zhou, X.; Tan, W.; Luo, H.; Lei, S.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Saino, E.; Grandi, S.; Quartarone, E.; Maliardi, V.; Galli, D.; Bloise, N.; Fassina, L.; De Angelis, M.G.; Mustarelli, P.; Imbriani, M.; et al. In vitro calcified matrix deposition by human osteoblasts onto a zinc-containing bioactive glass. Eur. Cells Mater. 2011, 21, 59–72, discussion 72. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Karpukhina, N.; Brauer, D.S.; Hill, R.G. Novel Highly Degradable Chloride Containing Bioactive Glasses. Biomed. Glas. 2015, 1, 108–118. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Panpisut, P.; Patntirapong, S. Zinc and Strontium-Substituted Bioactive Glass Nanoparticle/Alginate Composites Scaffold for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 6150. [Google Scholar] [CrossRef]
- de Souza, J.R.; Kukulka, E.C.; Araújo, J.C.R.; Campos, T.M.B.; do Prado, R.F.; de Vasconcellos, L.M.R.; Thin, G.P.; Borges, A.L.S. Electrospun polylactic acid scaffolds with strontium- and cobalt-doped bioglass for potential use in bone tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 151–160. [Google Scholar] [CrossRef]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hayami, R.; Gunji, T. Characterization of NMR, IR, and Raman spectra for siloxanes and silsesquioxanes: A mini review. J. Sol-Gel Sci. Technol. 2022, 104, 36–52. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Voytik-Harbin, S.L.; Nör, J.E.; Bottino, M.C. Injectable Highly Tunable Oligomeric Collagen Matrices for Dental Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel–derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.C.L.; de Souza, J.R.; Campos, T.M.B.; Marques, T.O.; Kito, L.T.; Kukulka, E.C.; de Vasconcellos, L.M.R.; Borges, A.L.S.; Thim, G.P. Chlorinated-based bioceramics incorporated in polycaprolactone membranes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singh, A.; Dey, N.; Chattopadhyay, S.; Joseph, J.P.; Gupta, D.; Ganguli, M.; Pal, A. Pathway-Driven Peptide–Bioglass Nanocomposites as the Dynamic and Self-Healable Matrix. Chem. Mater. 2021, 33, 589–599. [Google Scholar] [CrossRef]
- Siqueira, R.L.; Costa, L.C.; Schiavon, M.A.; de Castro, D.T.; dos Reis, A.C.; Peitl, O.; Zanotto, E.D. Bioglass® and resulting crystalline materials synthesized via an acetic acid-assisted sol–gel route. J. Sol-Gel Sci. Technol. 2017, 83, 165–173. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Sadeghian, A.; Kharaziha, M.; Khoroushi, M. Dentin extracellular matrix loaded bioactive glass/GelMA support rapid bone mineralization for potential pulp regeneration. Int. J. Biol. Macromol. 2023, 234, 123771. [Google Scholar] [CrossRef]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.-H.; Kim, H.-W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin–Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminmansour, S.; Gomes de Carvalho, A.B.; Medeiros Cardoso, L.; Anselmi, C.; Rahimnejad, M.; Dal-Fabbro, R.; Benavides, E.; Campos, T.M.B.; Borges, A.L.S.; Bottino, M.C. Strontium-Doped Bioglass-Laden Gelatin Methacryloyl Hydrogels for Vital Pulp Therapy. J. Funct. Biomater. 2024, 15, 105. https://doi.org/10.3390/jfb15040105
Aminmansour S, Gomes de Carvalho AB, Medeiros Cardoso L, Anselmi C, Rahimnejad M, Dal-Fabbro R, Benavides E, Campos TMB, Borges ALS, Bottino MC. Strontium-Doped Bioglass-Laden Gelatin Methacryloyl Hydrogels for Vital Pulp Therapy. Journal of Functional Biomaterials. 2024; 15(4):105. https://doi.org/10.3390/jfb15040105
Chicago/Turabian StyleAminmansour, Sepideh, Ana Beatriz Gomes de Carvalho, Lais Medeiros Cardoso, Caroline Anselmi, Maedeh Rahimnejad, Renan Dal-Fabbro, Erika Benavides, Tiago Moreira Bastos Campos, Alexandre Luiz Souto Borges, and Marco C. Bottino. 2024. "Strontium-Doped Bioglass-Laden Gelatin Methacryloyl Hydrogels for Vital Pulp Therapy" Journal of Functional Biomaterials 15, no. 4: 105. https://doi.org/10.3390/jfb15040105
APA StyleAminmansour, S., Gomes de Carvalho, A. B., Medeiros Cardoso, L., Anselmi, C., Rahimnejad, M., Dal-Fabbro, R., Benavides, E., Campos, T. M. B., Borges, A. L. S., & Bottino, M. C. (2024). Strontium-Doped Bioglass-Laden Gelatin Methacryloyl Hydrogels for Vital Pulp Therapy. Journal of Functional Biomaterials, 15(4), 105. https://doi.org/10.3390/jfb15040105








