Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Anesthesia and Surgical Procedure
2.3. Radiographic Analysis
2.4. Analysis of Bone Turnover Markers in Blood Plasma
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suresh, S.; Lee, J.; Noguchi, C.T. Effects of Erythropoietin in White Adipose Tissue and Bone Microenvironment. Front. Cell Dev. Biol. 2020, 8, 584696. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Menger, M.D.; Scheuer, C.; Meier, C.; Culemann, U.; Wirbel, R.J.; Garcia, P.; Pohlemann, T. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007, 80, 893–900. [Google Scholar] [CrossRef]
- Mihmanli, A.; Dolanmaz, D.; Avunduk, M.C.; Erdemli, E. Effects of Recombinant Human Erythropoietin on Mandibular Distraction Osteogenesis. J. Oral. Maxillofac. Surg. 2009, 67, 2337–2343. [Google Scholar] [CrossRef]
- Betsch, M.; Thelen, S.; Santak, L.; Herten, M.; Jungbluth, P.; Miersch, D.; Hakimi, M.; Wild, M. The role of erythropoietin and bone marrow concentrate in the treatment of osteochondral defects in mini-pigs. PLoS ONE 2014, 9, e92766. [Google Scholar] [CrossRef][Green Version]
- Rölfing, J.; Bendtsen, M.; Jensen, J.; Stiehler, M.; Foldager, C.B.; Hellfritzsch, M.B.; Bünger, C. Erythropoietin augments bone formation in a rabbit posterolateral spinal fusion model. J. Orthop. Res. 2012, 30, 1083–1088. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Jung, Y.; Ziegler, A.M.; Pedersen, E.A.; Wang, J.; Wang, Z.; Song, J.; Wang, J.; Lee, C.H.; Sud, S.; et al. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef] [PubMed]
- Heschl, M.; Gombotz, H.; Haslinger-Eisterer, B.; Hofmann, A.; Böhler, N.; Meier, J. The efficacy of pre-operative preparation with intravenous iron and/or erythropoietin in anaemic patients undergoing orthopaedic surgery: An observational study. Eur. J. Anaesthesiol. 2018, 35, 289–297. [Google Scholar] [CrossRef]
- Yufang, Y.; Zhishan, Y.; Mianmian, D.; Yiheng, L.; Zhenglong, T.; Yu, W. Application and prospects of erythropoietin in bone tissue engineering. Chin. J. Tissue Eng. Res. 2024, 28, 1443–1449. [Google Scholar]
- Pandya, M.; Saxon, M.; Bozanich, J.; Tillberg, C.; Luan, X.; Diekwisch, T. The Glycoprotein/Cytokine Erythropoietin Promotes Rapid Alveolar Ridge Regeneration In Vivo by Promoting New Bone Extracellular Matrix Deposition in Conjunction with Coupled Angiogenesis/Osteogenesis. Int. J. Mol. Sci. 2021, 22, 2788. [Google Scholar] [CrossRef]
- Bae, J.E.; Hwang, S.M.; Aryal, Y.P.; Kim, T.Y.; Sohn, W.J.; An, S.Y.; Kim, J.Y.; An, C.H.; Lee, Y.; Kim, Y.G.; et al. Effects of erythropoietin on osteoblast in the tooth extraction socket in mice periodontitis model. Front. Physiol. 2022, 13, 987625. [Google Scholar] [CrossRef]
- Francis, A.P.; Augustus, A.R.; Chandramohan, S.; Bhat, S.A.; Priya, V.V.; Rajagooplan, R. A review on biomaterials-based scaffold: An emerging tool for bone tissue engineering. Mater. Today Commun. 2023, 34, 105124. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Rölfing, J.; Jensen, J.; Jensen, J.N.; Greve, A.; Lysdahl, H.; Chen, M.; Rejnmark, L.; Bünger, C. A single topical dose of erythropoietin applied on a collagen carrier enhances calvarial bone healing in pigs. Acta Orthop. 2014, 85, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Kim, J.S.; Shimobouji, T. Injectable hyaluronic acid microhydrogels for controlled release formulation of erythropoietin. J. Biomed. Mater. Res. Part. A 2007, 80, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Diker, N.; Sarican, H.; Cumbul, A.; Kilic, E. Effects of systemic erythropoietin treatment and heterogeneous xenograft in combination on bone regeneration of a critical-size defect in an experimental model. J. Craniomaxillofac. Surg. 2018, 46, 19191923. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, N.; Reshetov, I.; Zelianin, A.; Philippov, V.; Sergeeva, N.; Sviridova, I.; Komlev, V.; Andreeva, U.; Kuznecova, O. Three-dimensional TCP scaffolds enriched with Erythropoietin for stimulation of vascularization and bone formation. Electron. J. Gen. Med. 2019, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, R.; Chaprazov, T. Bone Healing of Critical-Sized Femoral Defects in Rats Treated with Erythropoietin Alone or in Combination with Xenograft. Vet. Sci. 2023, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Wu, Q.; Qin, Z.; Xiang, Z.; Chu, Y.; Li, J. Loading of erythropoietin on biphasic calcium phosphate bioceramics promotes osteogenesis and angiogenesis by regulating EphB4/EphrinB2 molecules. J. Mater. Sci. Mater. Med. 2022, 33, 19. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Sun, X.; Zhan, C.; Li, Z.; Qiu, L.; Luo, R.; Liu, H.; Sun, X.; Li, R.; et al. Silk Fibroin/Collagen/Hydroxyapatite Scaffolds Obtained by 3D Printing Technology and Loaded with Recombinant Human Erythropoietin in the Reconstruction of Alveolar Bone Defects. ACS Biomater. Sci. En. 2022, 8, 5245–5256. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, S.; Luo, D.; Liu, Y. Novel Inorganic Nanomaterial-Based Therapy for Bone Tissue Regeneration. Nanomaterials 2021, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Nie, P.; Yang, W.; Guo, R.; Ding, D.; Liang, R.; Wei, B.; Wei, H. An EPO-loaded multifunctional hydrogel synergizing with adipose-derived stem cells restores neurogenic erectile function via enhancing nerve regeneration and penile rehabilitation. Bioeng. Transl. Med. 2022, 7, e10319. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Kazemian, G.; Emami, M.; Nemati, A.; Karimi Yarandi, H.; Safdari, F. Local erythropoietin injection in tibiofibular fracture healing. Trauma. Mon. 2013, 17, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Aslroosta, H.; Yaghobee, S.; Akbari, S.; Kanounisabet, N. The effects of topical erythropoietin on non-surgical treatment of periodontitis: A preliminary study. BMC Oral. Health 2021, 21, 240. [Google Scholar] [CrossRef] [PubMed]
- Working, Z.M.; Morris, E.R.; Chang, J.C.; Coghlan, R.F.; Johnstone, B.; Miclau, T., 3rd; Horton, W.A.; Bahney, C.S. A quantitative serum biomarker of circulating collagen X effectively correlates with endochondral fracture healing. J. Orthop. Res. 2021, 39, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Shariyate, M.J.; Kheir, N.; Caro, D.; Abbasian, M.; Rodriguez, E.K.; Snyder, B.D.; Nazarian, A. Assessment of Bone Healing: Opportunities to Improve the Standard of Care. J. Bone Jt. Surg. Am. 2023, 105, 1193–1202. [Google Scholar] [CrossRef]
- Yoon, B.H.; Yu, W. Clinical Utility of Biochemical Marker of Bone Turnover: Fracture Risk Prediction and Bone Healing. J. Bone Metab. 2018, 25, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.P.; Dias, I.R.; Lopez-Peña, M.; Camassa, J.A.; Lourenço, P.J.; Judas, F.M.; Gomes, M.E.; Reis, R.L. Bone turnover markers for early detection of fracture healing disturbances: A review of the scientific literature. An. Acad. Bras. Cienc. 2015, 87, 1049–1061. [Google Scholar] [CrossRef]
- Sipani, A.K.; Dhar, A.; Sunge, N. Serum alkaline phosphatase: A prospective biomarker for assessment of progress of fracture healing in diaphyseal fractures of long bones in adult patients. Int. J. Orthop. Sci. 2020, 6, 248–251. [Google Scholar] [CrossRef]
- Choi, Y.J.; Sohn, Y.B.; Chung, Y.S. Updates on Paget’s Disease of Bone. Endocrinol. Metab. 2022, 37, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Sapra, A. Osteoporosis Markers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Aufderklamm, S.; Hennenlotter, J.; Rausch, S.; Bock, C.; Erne, E.; Schwentner, C.; Stenzl, A. Oncological validation of bone turnover markers c-terminal telopeptide of type I collagen (1CTP) and peptides n-terminal propeptide of type I procollagen (P1NP) in patients with prostate cancer and bone metastases. Transl. Androl. Urol. 2021, 10, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Pop, V.; Parvu, A.; Craciun, A.; Farcas, A.D.; Tomoaia, G.; Bojan, A. Modern markers for evaluating bone disease in multiple myeloma (Review). Exp. Ther. Med. 2021, 22, 1329. [Google Scholar] [CrossRef] [PubMed]
- Horas, K.; Seibel, M. Chapter 30—Bone remodeling markers and bone cancer. In Bone Sarcomas and Bone Metastases—From Bench to Bedside, 3rd ed.; Heymann, D., Ed.; Academic Press: New York, NY, USA, 2022; pp. 413–429. [Google Scholar]
- Jadon, D.R.; Nightingale, A.L.; McHugh, N.J.; Lindsay, M.A.; Korendowych, E.; Sengupta, R. Serum soluble bone turnover biomarkers in psoriatic arthritis and psoriatic spondyloarthropathy. J. Rheumatol. 2015, 42, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Janković, T.; Mikov, M.; Zvekić Svorcan, J.; Minaković, I.; Mikov, J.; Bošković, K.; Mikić, D. Changes in Bone Metabolism in Patients with Rheumatoid Arthritis during Tumor Necrosis Factor Inhibitor Therapy. J. Clin. Med. 2023, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, K.K.; McGuigan, F.E.; Malmgren, L.; Gerdhem, P.; Johansson, H.; Kanis, J.A.; Akesson, K.E. Bone Turnover Marker Profiling and Fracture Risk in Older Women: Fracture Risk from Age 75 to 90. Calcif. Tissue Int. 2022, 111, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.C.; O’Hara, N.N.; Bzovsky, S.; Bahney, C.S.; Sprague, S.; Slobogean, G.P.; Vita-Shock Investigators. Bone turnover markers as surrogates of fracture healing after intramedullary fixation of tibia and femur fractures. Bone Jt. Res. 2022, 11, 239–250. [Google Scholar] [CrossRef]
- Paris, B.L.; Leatherwood, J.L.; Arnold, C.E.; Glass, K.G.; Conrad, M.B.; George, J.M.; Martinez, R.E.; Vergara-Hernandez, F.B.; Colbath, A.C.; Nielsen, B.D.; et al. 10 Extra-label bisphosphonate effects on bone turnover biomarkers in juvenile, exercising horses. J. Equine Vet. Sci. 2023, 124, 104312. [Google Scholar] [CrossRef]
- Knych, H.K.; Finno, C.J.; Katzman, S.; Ryan, D.; McKemie, D.S.; Kass, P.H.; Arthur, R.M. Clodronate detection and effects on markers of bone resorption are prolonged following a single administration to horses. Equine Vet. J. 2023, 55, 696–706. [Google Scholar] [CrossRef]
- Seebeck, P.; Bail, H.J.; Exner, C.; Schell, H.; Michel, R.; Amthauer, H.; Bragulla, H.; Duda, G.N. Do serological tissue turnover markers represent callus formation during fracture healing? Bone 2005, 37, 669–677. [Google Scholar] [CrossRef]
- Brankovič, J.; Leskovec, J.; Šturm, S.; Cerkvenik-Flajs, V.; Šterpin, S.; Osredkar, J.; Pogorevc, E.; Antolinc, D.; Vrecl, M. Experimental Exposure to Bisphenol A Has Minimal Effects on Bone Tissue in Growing Rams—A Preliminary Study. Animals 2022, 12, 2179. [Google Scholar] [CrossRef]
- Breur, G.J.; Allen, M.J.; Carlson, S.J.; Richardson, D.C. Markers of bone metabolism in dog breeds of different sizes. Res. Vet. Sci. 2004, 76, 53–55. [Google Scholar] [CrossRef]
- DeLaurier, A.; Jackson, B.; Ingham, K.; Pfeiffer, D.; Horton, M.A.; Price, J.S. Biochemical markers of bone turnover in the domestic cat: Relationships with age and feline osteoclastic resorptive lesions. J. Nut. 2002, 132, 1742S–1744S. [Google Scholar] [CrossRef] [PubMed]
- DeLaurier, A.; Jackson, B.; Pfeiffer, D.; Ingham, K.; Horton, M.A.; Price, J.S. A comparison of methods for measuring serum and urinary markers of bone metabolism in cats. Res. Vet. Sci. 2004, 77, 29–39. [Google Scholar] [CrossRef]
- Yamka, R.; Friesen, K.; Lowry, S.; Coffman, L. Measurement of arthritic and bone serum metabolites in arthritic, noon-arthritic and geriatric cats fed wellness food. Int. J. Appl. Res. Vet. Med. 2006, 4, 265–273. [Google Scholar]
- Ekici, M.; Koçkaya, M.; Baş-Ekici, H. The influence of sex and age on bone turnover markers in the adult to geriatric Kangal shepherd dogs. Vet. Clin. Pathol. 2023, 52, 353–359. [Google Scholar] [CrossRef]
- Matthaei, M.O.; Kononov, S.U.; Rehage, J.; Szura, G.; Leiter, I.; Hansen, K.; Daenicke, S.; von Soosten, D.; Kersten, S.; Meyer, U.; et al. Does bone mobilization interfere with energy metabolism in transition cows? JDS Commun. 2022, 3, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Paskalev, M. Comparative investigation on blood bone markers in normally healing and infected bone fracture models in dogs. Bul. J. Vet. Med. 2010, 13, 235–244. [Google Scholar]
- Barger, A.; Baker, K.; Driskell, E.; Sander, W.; Roady, P.; Berry, M.; Schnelle, A.; Fan, T.M. The use of alkaline phosphatase and runx2 to distinguish osteosarcoma from other common malignant primary bone tumors in dogs. Vet. Pathol. 2022, 59, 427–432. [Google Scholar] [CrossRef]
- Paskalev, M.; Krastev, S.; Filipov, J. Changes in some serum bone markers after experimental fracture and intramedullary osteosynthesis in dogs. Trakia J. Sci. 2005, 3, 46–50. [Google Scholar]
- Dhillon, M.; Dha, S. Introduction to fractures and dislocation. In First Aid and Emergency Management in Orthopedic Injuries, 1st ed.; Dhillon, M., Dha, S., De Boer, P., Eds.; JP Medical Ltd.: New Delhi, India, 2012; pp. 2–4. [Google Scholar]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Qiu, C.; Chen, L.; Wang, X. Injectable thermosensitive hydrogel loading erythropoietin and FK506 alleviates gingival inflammation and promotes periodontal tissue regeneration. Front. Bioeng. Biotechnol. 2024, 11, 1323554. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.R.; Mabrouk, A.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Leung, K.S.; Fung, K.P.; Sher, A.H.; Li, C.K.; Lee, K.M. Plasma bone-specific alkaline phosphatase as an indicator of osteoblastic activity. J. Bone Jt. Surf. 1993, 75, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, M.J.; Mahjoub, S. Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget’s specimens. Caspian J. Int. Med. 2012, 3, 478–483. [Google Scholar]
- Paskalev, M.; Krastev, S.; Sotirov, L. Variations of serum bone alkaline phosphatase activities and osteocalcin concentrations in dogs with experimental osteotomy fixed by two different osteosynthesis techniques. Rev. Méd. Vét 2008, 159, 444–449. [Google Scholar]
- Meller, Y.; Kestenbaum, R.S.; Shany, S.; Galinsky, D.; Zuili, I.; Yankowitz, N.; Giat, J.; Konforti, A.; Torok, G. Parathyroid hormone, calcitonin and vitamin D metabolites during normal fracture healing in humans a preliminary report. Clin. Orthop. Relat. Res. 1984, 183, 238–245. [Google Scholar] [CrossRef]
- Rathwa, H.S.; Verma, T.; Chavali, V.H. Assessment of union in fractures: Role of Serum Alkaline Phosphatase and Ultrasonography. J. Clin. Orthop. Trauma. 2020, 14, 94–100. [Google Scholar] [CrossRef]
- Li, C.; Shi, C.; Kim, J.; Chen, Y.; Ni, S.; Jiang, L.; Zhang, C.; Li, D.; Hou, J.; Taichman, R.S.; et al. Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J. Dent. Res. 2015, 94, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y.; Sun, H.; Joseph, J.; Mishra, A.; Shiozawa, Y.; Wang, J.; Krebsbach, P.H.; Taichman, R.S. Erythropoietin mediated bone formation is regulated by mTOR signaling. J. Cell Biochem. 2012, 113, 220–228. [Google Scholar] [CrossRef]
- Rolfing, J.H.; Baatrup, A.; Stiehler, M.; Jensen, J.; Lysdahl, H.; Bunger, C. The osteogenic effect of erythropoietin on human mesenchymal stromal cells is dose-dependent and involves non-hematopoietic receptors and multiple intracellular signaling pathways. Stem Cell Rev. 2014, 10, 69–78. [Google Scholar] [CrossRef]
- Balaian, E.; Wobus, M.; Weidner, H.; Baschant, U.; Stiehler, M.; Ehninger, G.; Bornhäuser, M.; Hofbauer, L.C.; Rauner, M.; Platzbecker, U. Erythropoietin inhibits osteoblast function in myelodysplastic syndromes via the canonical Wnt pathway. Haematologica 2018, 103, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Baudach, J.; Scheuer, C.; Osche, D.; Veith, N.T.; Braun, B.J.; Rollmann, M.F.; Herath, S.C.; Pohlemann, T.; Menger, M.D.; et al. Erythropoietin does not improve fracture healing in aged mice. Exp. Gerontol. 2019, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, M.O.; Sietsema, D.L.; Burgers, T.A.; Mason, J.; Williams, B.O.; Jones, C.B. Recent advances in the use of serological bone formation markers to monitor callus development and fracture healing. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Hitz, M.F.; Jensen, J.E.; Eskildsen, R.C. Bone mineral density and bone markers in patients with a recent low-energy fracture: Effect of 1 y of treatment with calcium and vitamin D. Am. J. Clin. Nutr. 2007, 86, 251–259. [Google Scholar] [CrossRef]
- Ivaska, K.K.; Gerdhem, P.; Akesson, K.; Garnero, P.; Obrant, K.J. Effect of fracture on bone turnover markers: A longitudinal study comparing marker levels before and after injury in 113 elderly women. J. Bone Miner. Res. 2007, 22, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Macías, I.; Alcorta-Sevillano, N.; Rodríguez, C.I.; Infante, A. Osteoporosis and the Potential of Cell-Based Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1653. [Google Scholar] [CrossRef] [PubMed]
- Paskalev, M.; Krastev, S. Alterations in serum tartrate-resistant acid phosphatase and C-terminal telopeptide of type I collagen in experimental canine osteotomies fixed using 2 different techniques. Turk. J. Vet. Anim. Sci. 2010, 4, A2. [Google Scholar] [CrossRef]
- Theyse, L.F.H.; Mol, J.A.; Voorhout, G.; Terlou, M.; Hazewinkel, H.A.W. The efficacy of the bone markers osteocalcin and the carboxyterminal cross-linked telopeptide of type-I collagen in evaluating osteogenesis in a canine crural lengthening model. Vet. J. 2006, 171, 525–531. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Gassmann, M.; Rauner, M.; Franke, K.; Wielockx, B.; Neumann, D.; Gabet, Y. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015, 29, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; de Castro, L.F.; Dey, S.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Kolomansky, A.; Hiram-Bab, S.; Ben-Califa, N.; Liron, T.; Deshet-Unger, N.; Mittelman, M.; Oster, H.S.; Rauner, M.; Wielockx, B.; Neumann, D.; et al. Erythropoietin Mediated Bone Loss in Mice Is Dose-Dependent and Mostly Irreversible. Int. J. Mol. Sci. 2020, 21, 3817. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E.; Howe, C.J.; Nguyen, T.V.; Seibel, M.J.; Baxter, R.C.; Handelsman, D.J.; Kazlauskas, R.; Ho, K.K. Erythropoietin administration does not influence the GH–IGF axis or makers of bone turnover in recreational athletes. Clin. Endocrinol. 2005, 63, 305–309. [Google Scholar] [CrossRef]
- Ikegami, S.; Kamimura, M.; Nakagawa, H.; Takahara, K.; Hashidate, H.; Uchiyama, S.; Kato, H. Comparison in bone turnover markers during early healing of femoral neck fracture and trochanteric fracture in elderly patients. Orthop. Rev. 2009, 1, e21. [Google Scholar]
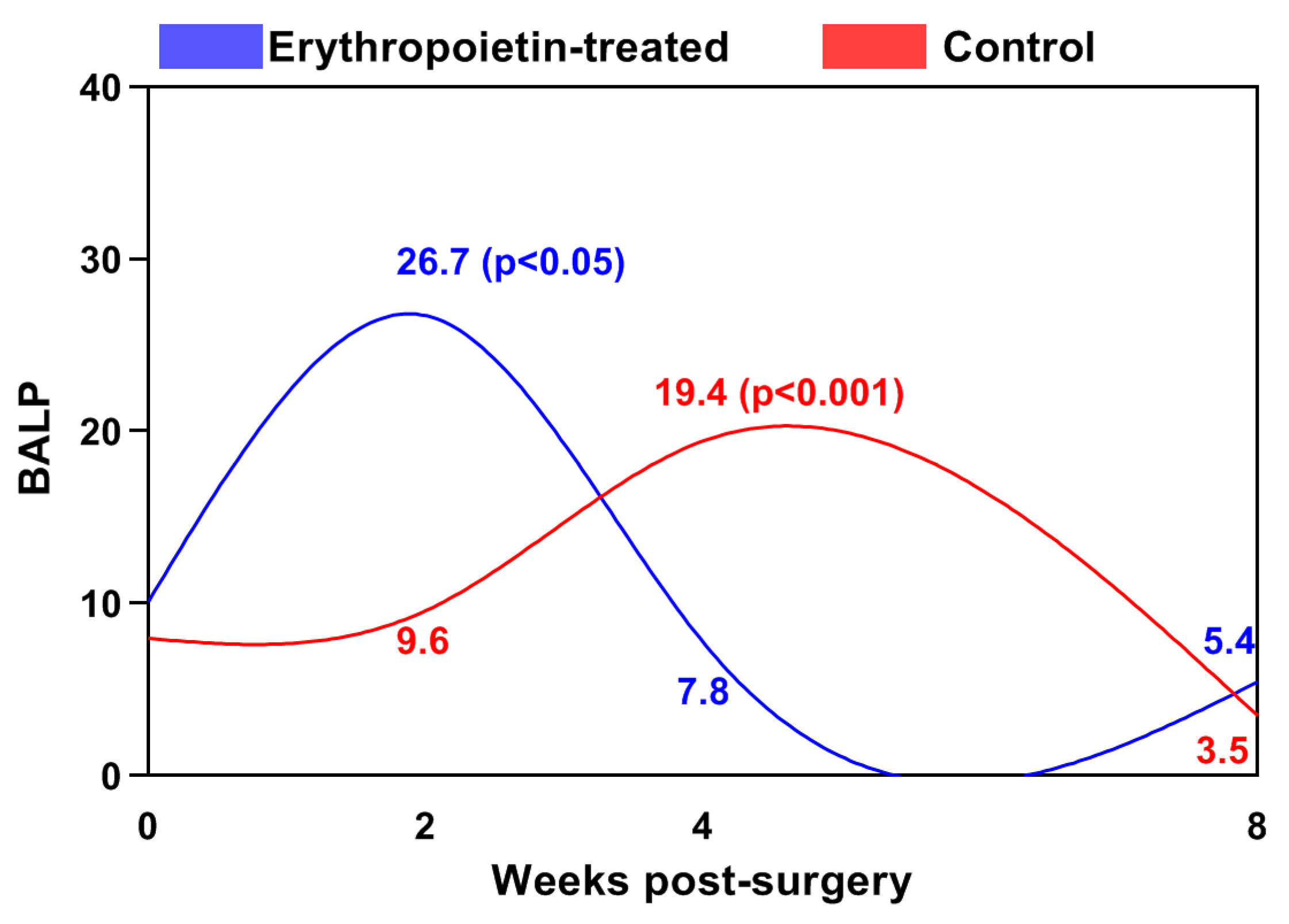

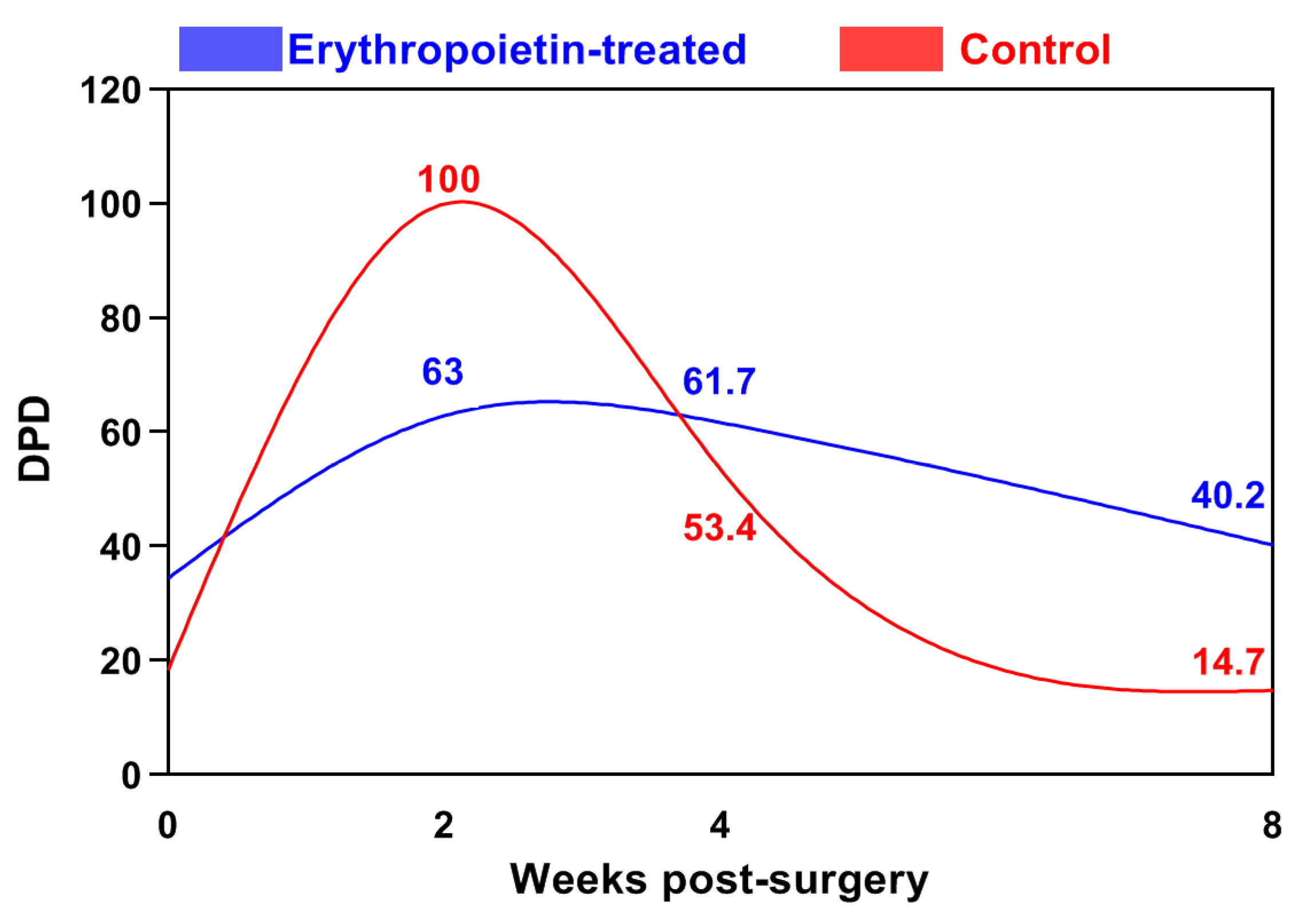
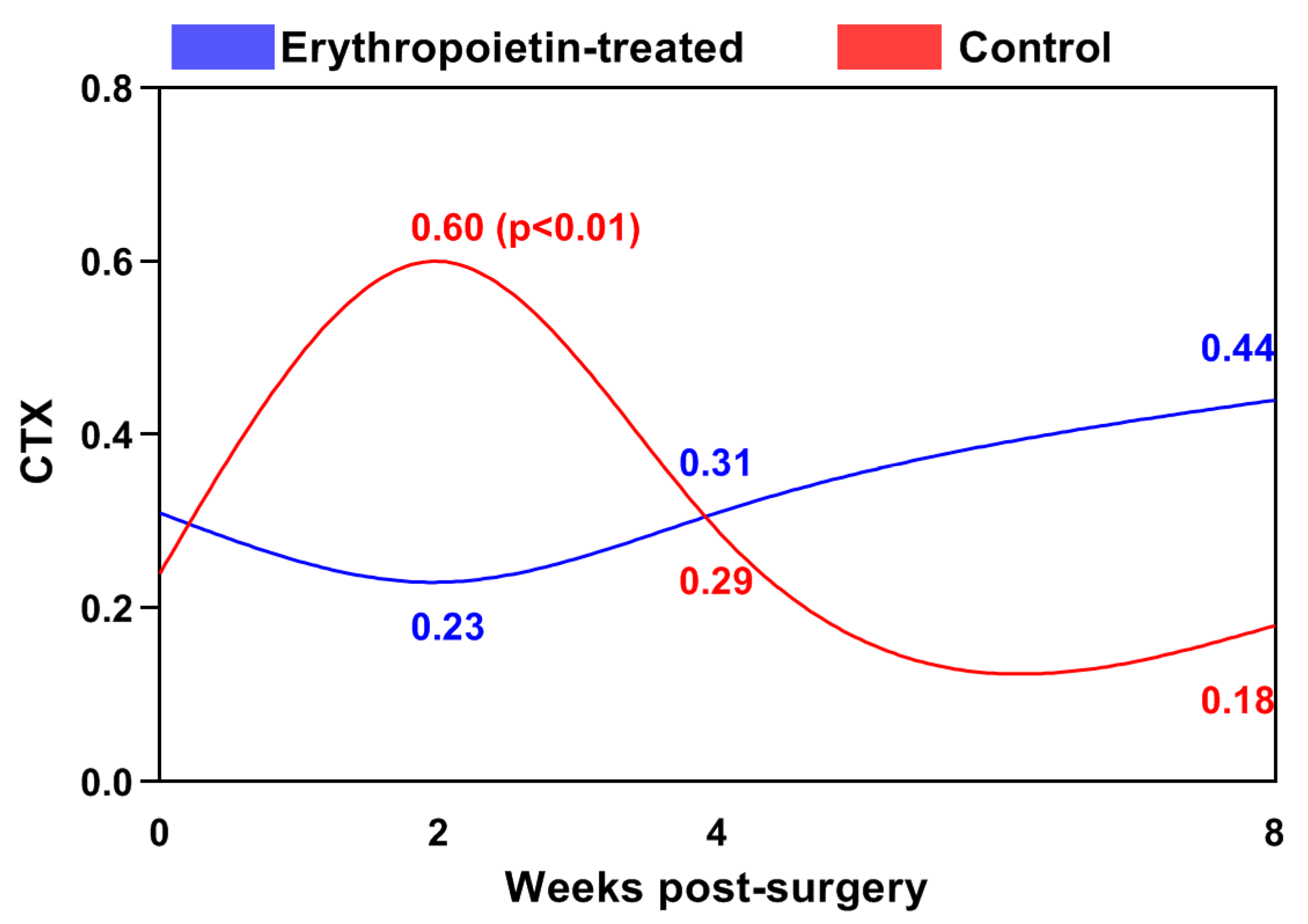
| Post-Operative Week | Control Group | EPO Group |
|---|---|---|
| 2 w | 2 (2–3) | 3 (2–30) * |
| 4 w | 3.5 (3–4) | 4.5 (4–5) * |
| 8 w | 6 (5–6) | 6 (6–6) |
| BALP (2 w) | BALP (4 w) | PINP (2 w) | PINP (4 w) | RO (2 w) | RO (4 w) | ||
|---|---|---|---|---|---|---|---|
| BALP (2 w) | Correlation coefficient | ||||||
| Significance level p | |||||||
| n | |||||||
| BALP (4 w) | Correlation coefficient | −0.336 | |||||
| Significance level p | 0.3118 | ||||||
| n | 12 | ||||||
| PINP (2 w) | Correlation coefficient | 0.918 | −0.355 | ||||
| Significance level p | 0.0001 | 0.2847 | |||||
| n | 12 | 12 | |||||
| PINP (4 w) | Correlation coefficient | 0.282 | 0.591 | 0.382 | |||
| Significance level p | 0.4011 | 0.0556 | 0.2466 | ||||
| n | 12 | 12 | 12 | ||||
| RO (2 w) | Correlation coefficient | 0.231 | −0.751 | 0.115 | −0.577 | ||
| Significance level p | 0.4945 | 0.0078 | 0.7353 | 0.0629 | |||
| n | 12 | 12 | 12 | 12 | |||
| RO (4 w) | Correlation coefficient | 0.662 | −0.584 | 0.740 | 0.039 | 0.236 | |
| Significance level p | 0.0266 | 0.0593 | 0.0093 | 0.9095 | 0.4608 | ||
| n | 12 | 12 | 12 | 12 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, R.; Chaprazov, T.; Milanova, A. Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats. J. Funct. Biomater. 2024, 15, 106. https://doi.org/10.3390/jfb15040106
Vasileva R, Chaprazov T, Milanova A. Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats. Journal of Functional Biomaterials. 2024; 15(4):106. https://doi.org/10.3390/jfb15040106
Chicago/Turabian StyleVasileva, Radina, Tsvetan Chaprazov, and Aneliya Milanova. 2024. "Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats" Journal of Functional Biomaterials 15, no. 4: 106. https://doi.org/10.3390/jfb15040106
APA StyleVasileva, R., Chaprazov, T., & Milanova, A. (2024). Effects of Erythropoietin-Promoted Fracture Healing on Bone Turnover Markers in Cats. Journal of Functional Biomaterials, 15(4), 106. https://doi.org/10.3390/jfb15040106






