Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices
Abstract
1. Introduction
2. Materials and Methods
2.1. 2D CAD Drawing and Dimensions
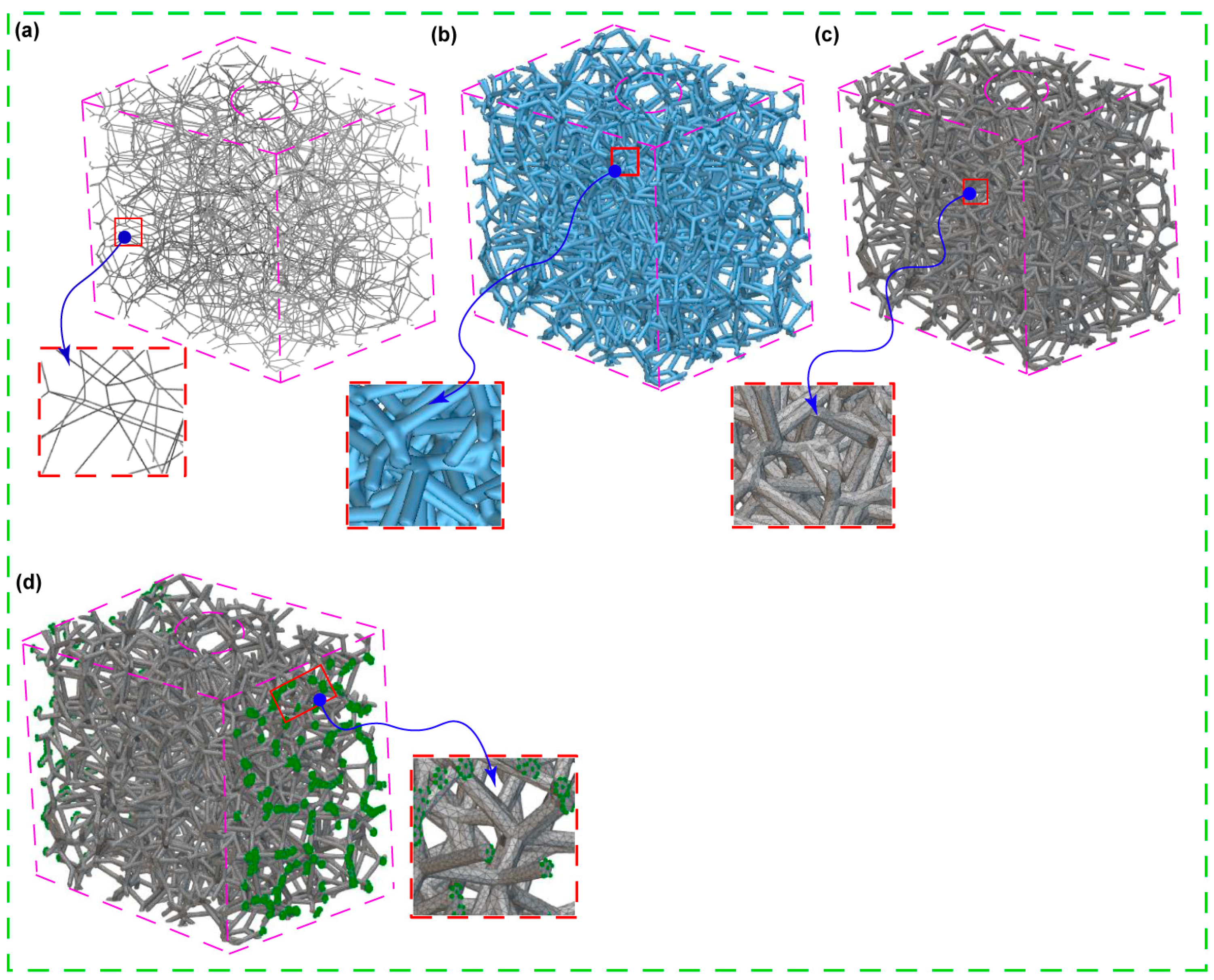
2.2. Pore Sizes and Voronoi Latticed Bone
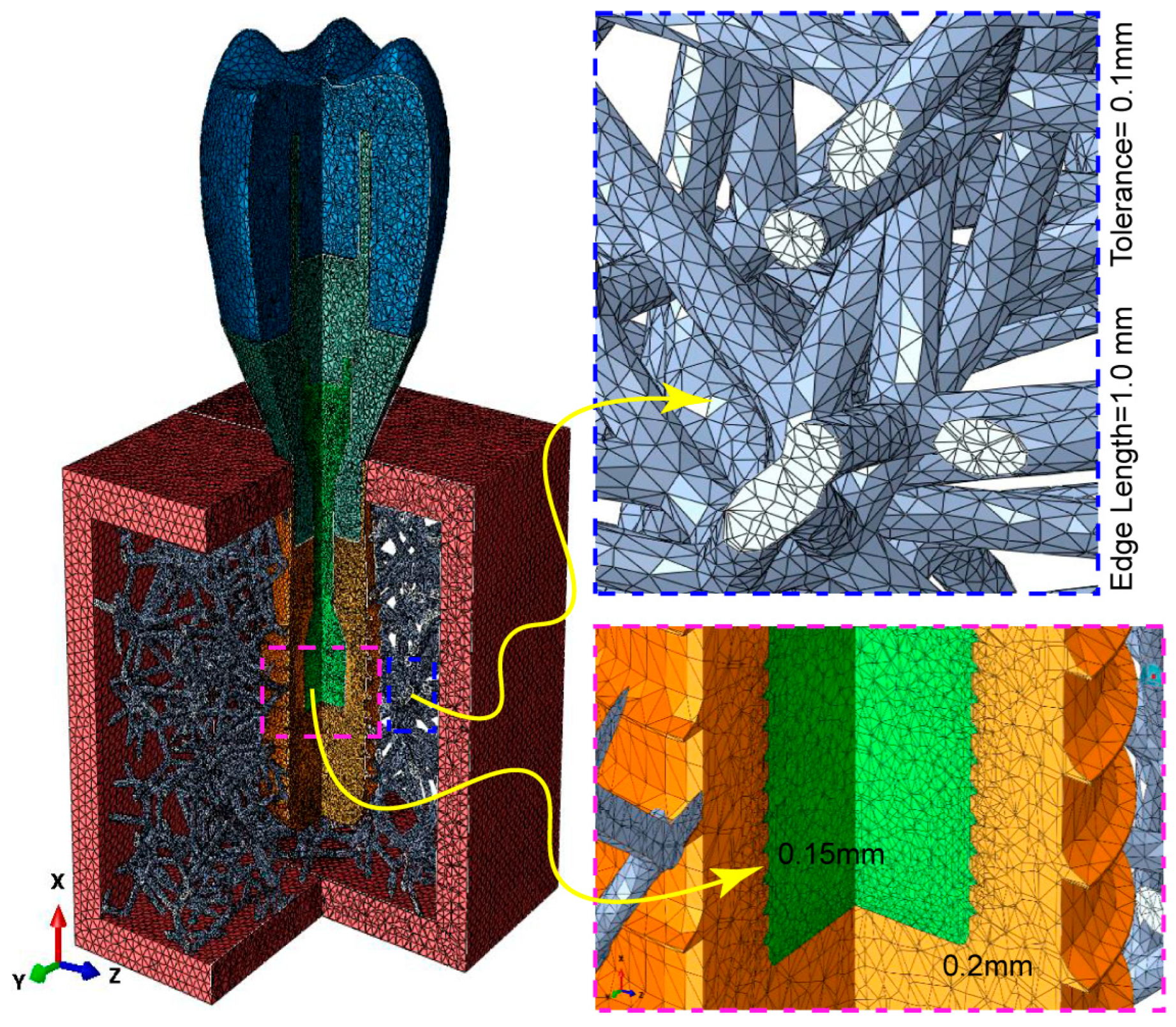
2.3. Building, FE Volume Meshing, and Boundary Condition of Voronoi Lattice
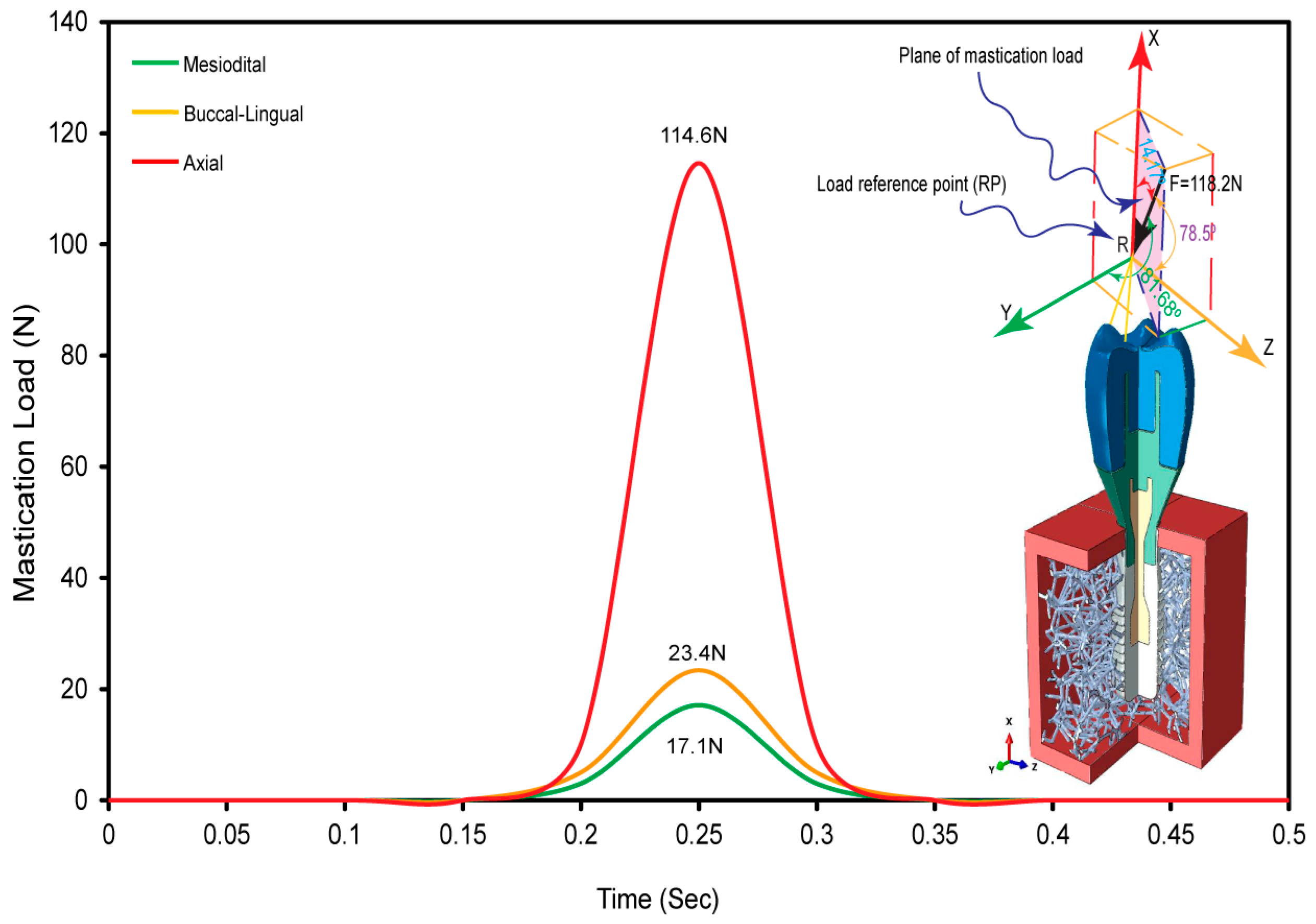
2.4. Oblique Load and FE Boundary Conditions
2.5. Physical Properties and FE Mesh
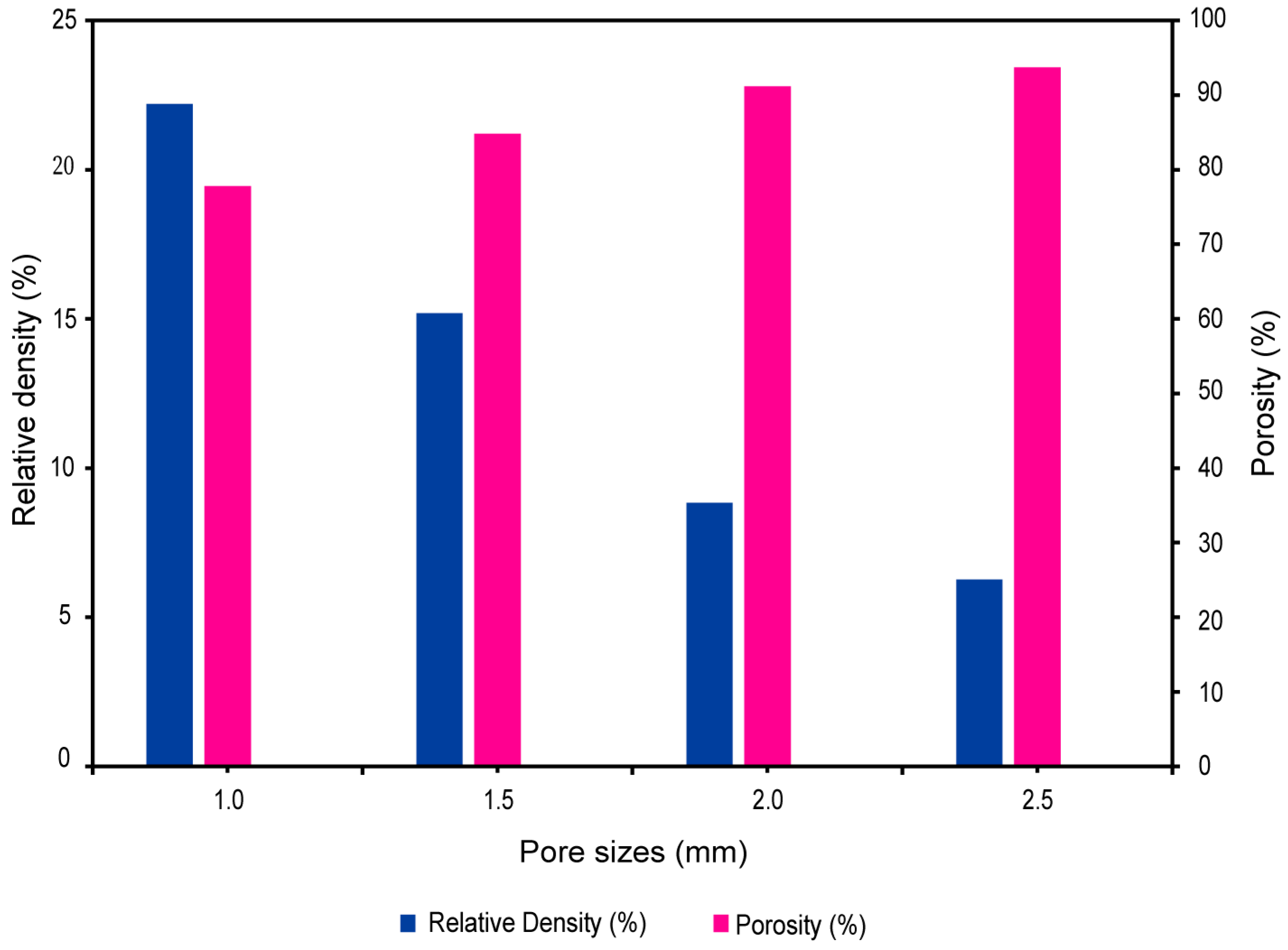
2.6. Relative Density and Porosity of Biomimetic Voronoi Lattice
3. Results
3.1. Voronoi Lattice Pore Size
3.2. Pore Size versus Relative Density and Porosity
3.3. Dynamic Oblique Loading
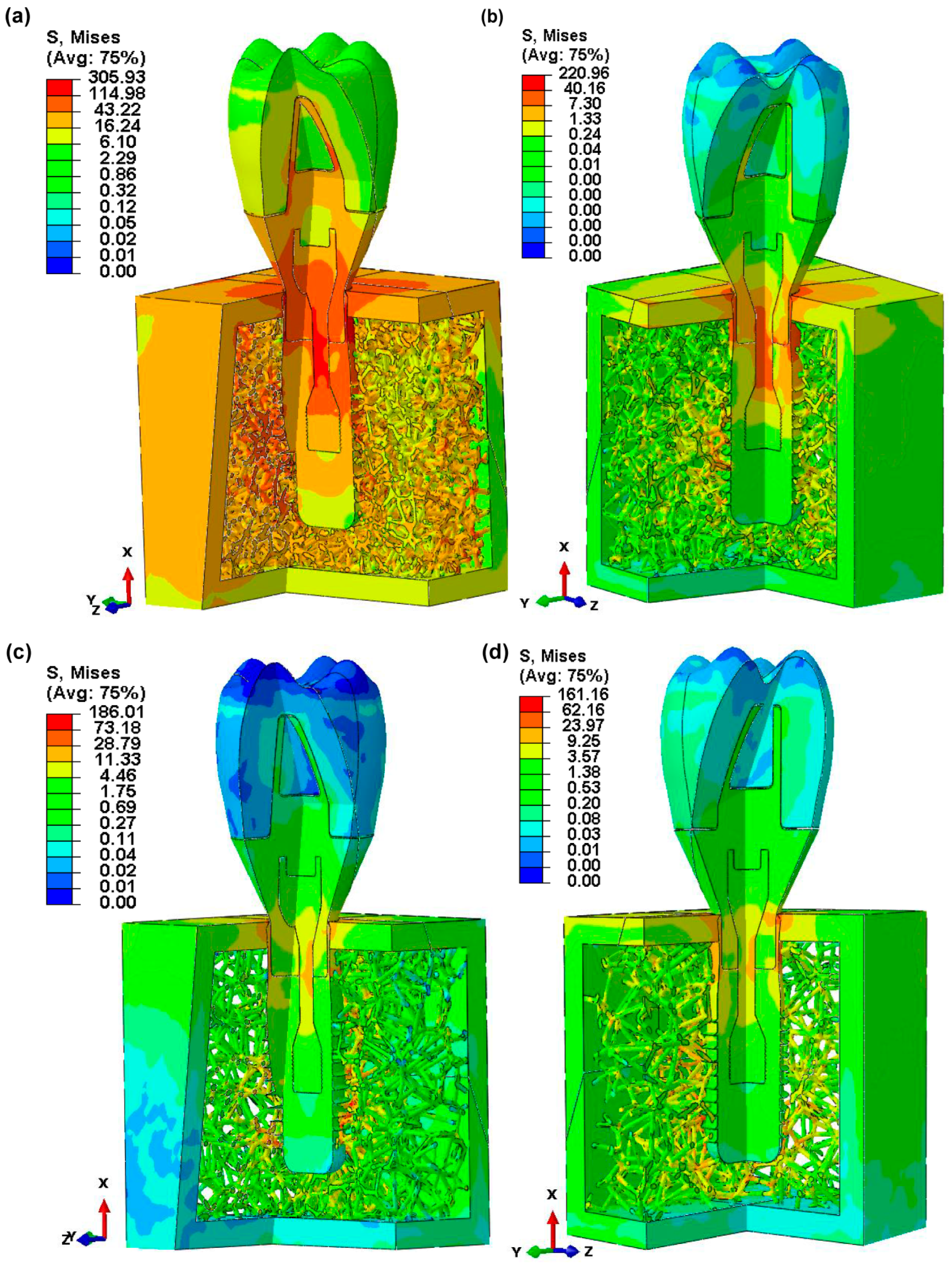
3.4. Von Mises Stress in Dental Implant Assembly
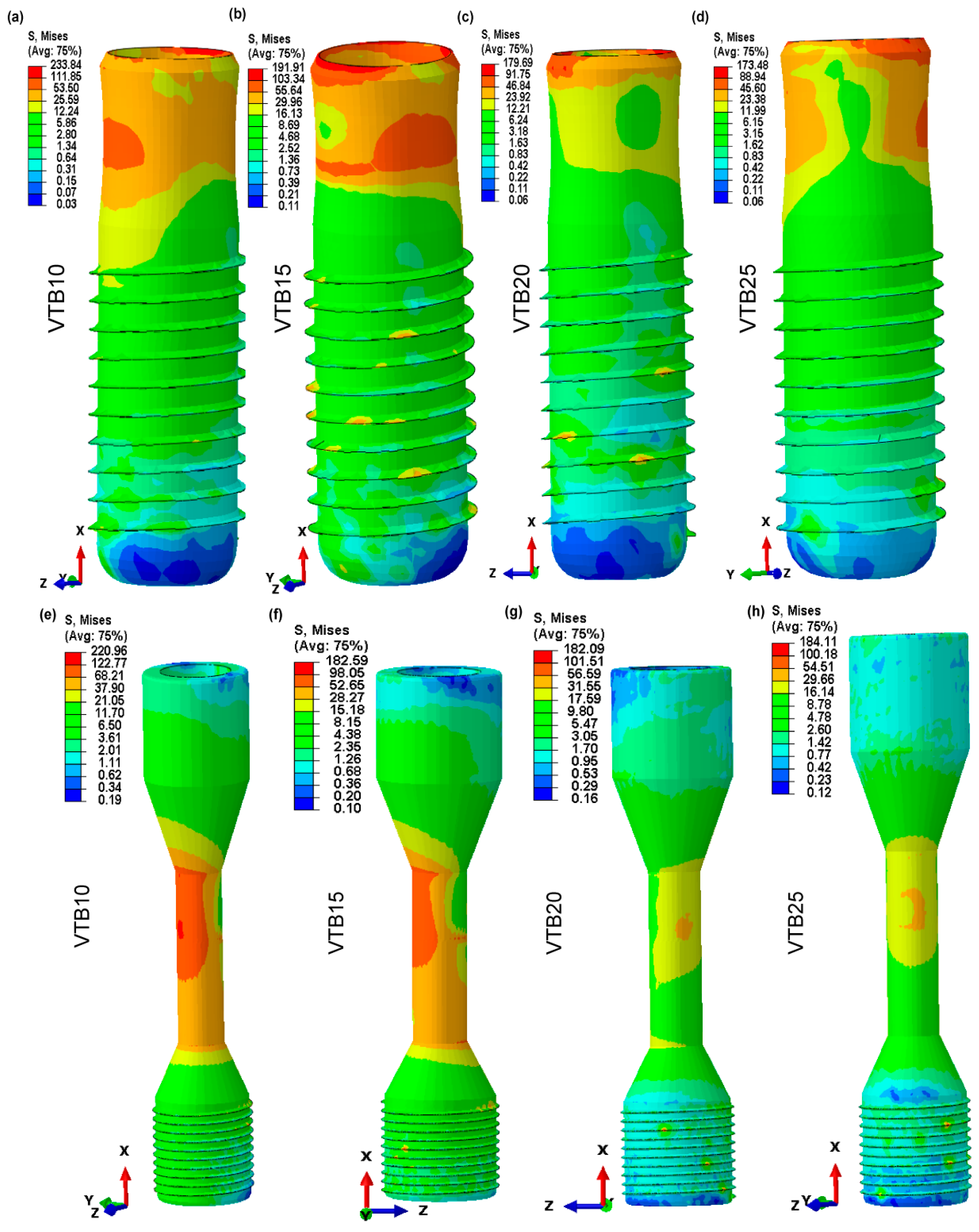
3.5. Von Mises Stress in Dental Implant and Retaining Screw
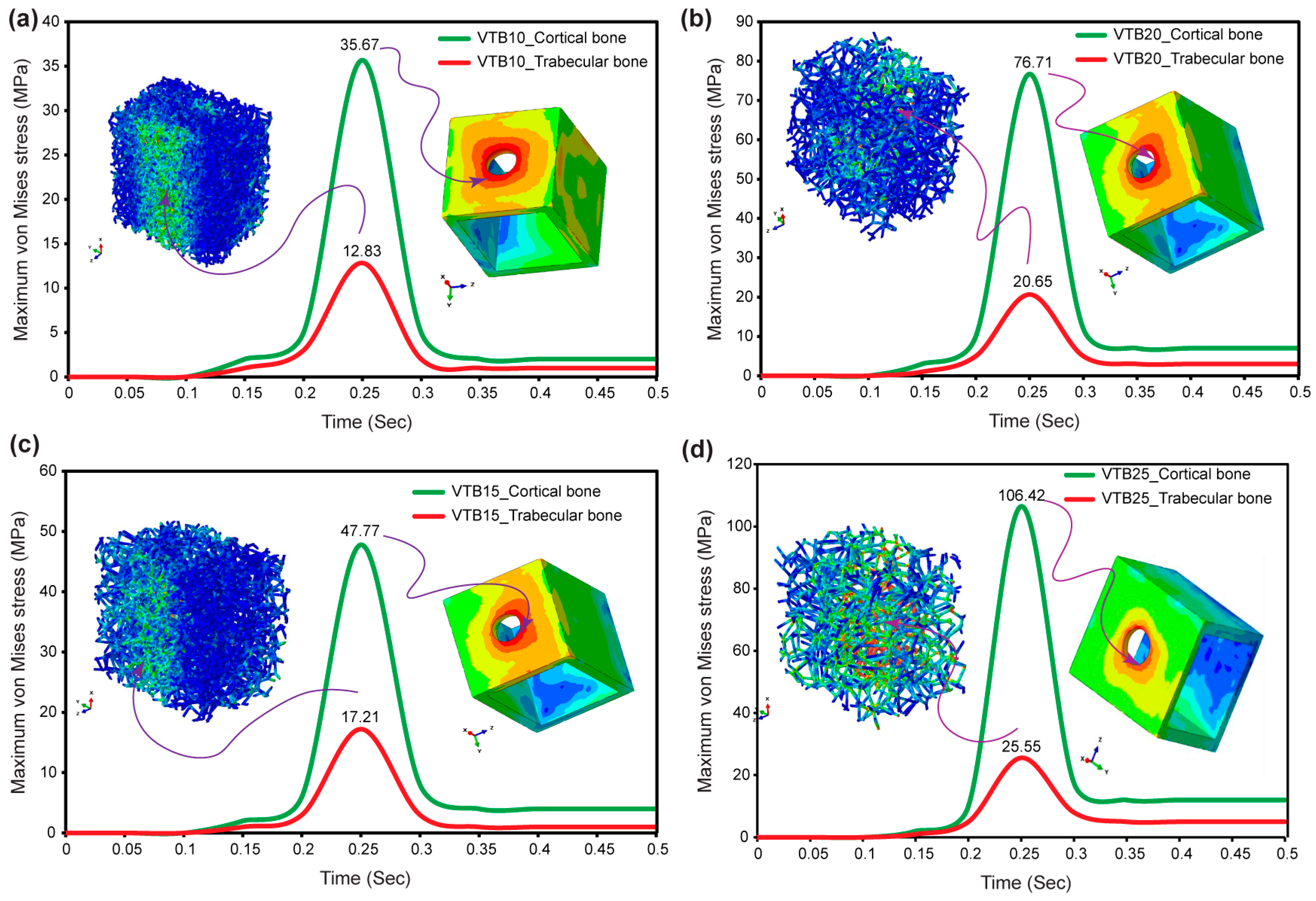
3.6. Von Mises Stress in Cortical and Voronoi Trabecular Bone
3.7. Micromotions in Voronoi Trabecular Bone
3.8. Displacement in Dental Implant Assembly
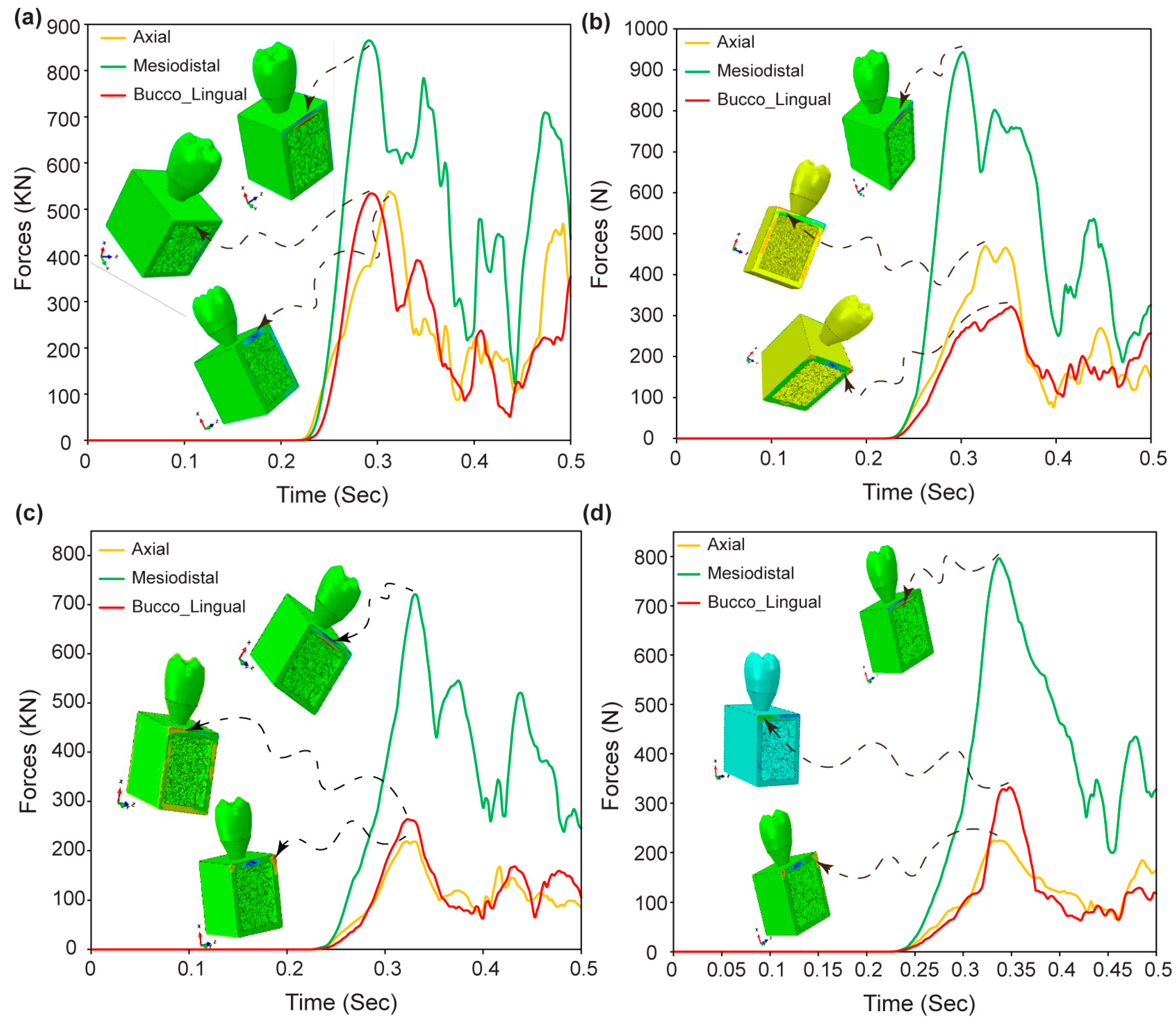
3.9. Reaction Forces in Dental Implant Assembly
4. Discussion
5. Conclusions
- A direct relationship was discovered between the pore size and micromotion magnitude. This highlights the impact of pore size on the biomechanical behavior of biomimetic Voronoi trabecular bone. This relationship has significance during dental implant designs that optimize biomechanical performance.
- The research shows that the biomimetic Voronoi-latticed trabecular bone used in FEA has properties that are similar to real human cancellous bone when subjected to the applied external dynamic mastication stresses.
- The use of anisotropic characteristics in these novel biomimetic trabecular bones, as well as the simulation of dynamic mastication stresses, greatly improved the FEA findings. This method gives a more precise description of biomechanical interactions in the oral environment, resulting in more reliable implant designs.
- The study found that a 2.0 mm (VTB20) pore size presents the optimal integration of mechanical rigidity and minimum micromotion, making it a better option to further enhance the implant lifespan and performance.
- Micromotion optimization considerably reduces the chances of implant failure, such as fractures in implant components, bone resorption, and corrosion in a biofluid environment.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beaupre, G.S.; Hayes, W.C. Finite Element Analysis of a Three-Dimensional Open-Celled Model for Trabecular Bone. J. Biomech. Eng. 1985, 107, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bregoli, C.; Fiocchi, J.; Biffi, C.A.; Tuissi, A. Additively Manufactured Medical Bone Screws: An Initial Study to Investigate the Impact of Lattice-Based Voronoi Structure on Implant Primary Stability. Rapid Prototyp. J. 2024, 30, 60–72. [Google Scholar] [CrossRef]
- Alkentar, R.; Kladovasilakis, N.; Tzetzis, D.; Mankovits, T. Effects of Pore Size Parameters of Titanium Additively Manufactured Lattice Structures on the Osseointegration Process in Orthopedic Applications: A Comprehensive Review. Crystals 2023, 13, 113. [Google Scholar] [CrossRef]
- Sivakumar, N.K.; Palaniyappan, S.; Sekar, V.; Alodhayb, A.; Braim, M. An Optimization Approach for Studying the Effect of Lattice Unit Cell’s Design-Based Factors on Additively Manufactured Poly Methyl Methacrylate Cranio-Implant. J. Mech. Behav. Biomed. Mater. 2023, 141, 105791. [Google Scholar] [CrossRef]
- Du, Y.; Liang, H.; Xie, D.; Mao, N.; Zhao, J.; Tian, Z.; Wang, C.; Shen, L. Design and Statistical Analysis of Irregular Porous Scaffolds for Orthopedic Reconstruction Based on Voronoi Tessellation and Fabricated via Selective Laser Melting (SLM). Mater. Chem. Phys. 2020, 239, 121968. [Google Scholar] [CrossRef]
- Zhou, Y.; Isaksson, P.; Persson, C. An Improved Trabecular Bone Model Based on Voronoi Tessellation. J. Mech. Behav. Biomed. Mater. 2023, 148, 106172. [Google Scholar] [CrossRef]
- Zou, S.; Gong, H.; Gao, J. Additively Manufactured Multilevel Voronoi-Lattice Scaffolds with Bonelike Mechanical Properties. ACS Biomater. Sci. Eng. 2022, 8, 3022–3037. [Google Scholar] [CrossRef]
- Kanwar, S.; Al-Ketan, O.; Vijayavenkataraman, S. A Novel Method to Design Biomimetic, 3D Printable Stochastic Scaffolds with Controlled Porosity for Bone Tissue Engineering. Mater. Des. 2022, 220, 110857. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, Z.; Peng, K.; Liu, K.; Xu, P.; Li, J.; Wei, X.; He, X. Integrated Evaluation of Biomechanical and Biological Properties of the Biomimetic Structural Bone Scaffold: Biomechanics, Simulation Analysis, and Osteogenesis. Mater. Today Bio 2024, 24, 100934. [Google Scholar] [CrossRef]
- Yang, W.; Han, Q.; Chen, H.; Li, Y.; Guo, X.; Zhang, A.; Liu, Y.; Sun, Y.; Wang, J. Additive Manufactured Trabecular-like Ti-6Al-4V Scaffolds for Promoting Bone Regeneration. J. Mater. Sci. Technol. 2024, 188, 116–130. [Google Scholar] [CrossRef]
- He, L.; Zhao, M.; Cheung, J.P.Y.; Zhang, T.; Ren, X. Gaussian Random Field-Based Characterization and Reconstruction of Cancellous Bone Microstructure Considering the Constraint of Correlation Structure. J. Mech. Behav. Biomed. Mater. 2024, 152, 106443. [Google Scholar] [CrossRef]
- Bahati, D.; Bricha, M.; El Mabrouk, K. Vat Photopolymerization Additive Manufacturing Technology for Bone Tissue Engineering Applications. Adv. Eng. Mater. 2023, 25, 2200859. [Google Scholar] [CrossRef]
- Mamidi, N.; Ijadi, F.; Norahan, M.H. Leveraging the Recent Advancements in GelMA Scaffolds for Bone Tissue Engineering: An Assessment of Challenges and Opportunities. Biomacromolecules 2023, 10, 1–39. [Google Scholar] [CrossRef]
- Rahatuzzaman, M.; Mahmud, M.; Rahman, S.; Hoque, M.E. Design, Fabrication, and Characterization of 3D-Printed ABS and PLA Scaffolds Potentially for Tissue Engineering. Results Eng. 2024, 21, 101685. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Lyu, Y.; Cheng, L. On the Various Numerical Techniques for the Optimization of Bone Scaffold. Materials 2023, 16, 974. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Chen, Y.; Xie, L.; Wang, L.; Zhang, L.-C.; Lu, W.; Chen, G. Non-Negligible Role of Gradient Porous Structure in Superelasticity Deterioration and Improvement of NiTi Shape Memory Alloys. J. Mater. Sci. Technol. 2024, 186, 48–63. [Google Scholar] [CrossRef]
- Zioupos, P.; Cook, R.B.; Hutchinson, J.R. Some Basic Relationships between Density Values in Cancellous and Cortical Bone. J. Biomech. 2008, 41, 1961–1968. [Google Scholar] [CrossRef]
- Abdullah, N.N.A.A.; Abdullah, A.H.; Ramlee, M.H. Current Trend of Lattice Structures Designed and Analysis for Porous Hip Implants: A Short Review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Ataollahi, S. A Review on Additive Manufacturing of Lattice Structures in Tissue Engineering. Bioprinting 2023, 35, e00304. [Google Scholar] [CrossRef]
- Rezapourian, M.; Hussainova, I. Optimal Mechanical Properties of Hydroxyapatite Gradient Voronoi Porous Scaffolds for Bone Applications—A Numerical Study. J. Mech. Behav. Biomed. Mater. 2023, 148, 106232. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Abdul Kadir, M.R.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef] [PubMed]
- Vaiani, L.; Uva, A.E.; Boccaccio, A. Structural and Topological Design of Conformal Bilayered Scaffolds for Bone Tissue Engineering. Thin-Walled Struct. 2023, 192, 111209. [Google Scholar] [CrossRef]
- Atashipour, S.R.; Baqersad, J. Noninvasive Identification of Directionally-Dependent Elastic Properties of Soft Tissues Using Full-Field Optical Data. J. Mech. Behav. Biomed. Mater. 2024, 151, 106266. [Google Scholar] [CrossRef]
- Bachmann, S.; Pahr, D.H.; Synek, A. A Density-Dependent Target Stimulus for Inverse Bone (Re) Modeling with Homogenized Finite Element Models. Ann. Biomed. Eng. 2023, 51, 925–937. [Google Scholar] [CrossRef]
- Daynes, S. Isotropic Cellular Structure Design Strategies Based on Triply Periodic Minimal Surfaces. Addit. Manuf. 2024, 81, 104010. [Google Scholar] [CrossRef]
- Hussain, Z.; Mehmood, S.; Liu, X.; Liu, Y.; Wang, G.; Pei, R. Decoding Bone-Inspired and Cell-Instructive Cues of Scaffolds for Bone Tissue Engineering. Eng. Regen. 2023, 5, 21–44. [Google Scholar] [CrossRef]
- Jiang, H.; Bednarcyk, B.A.; Le Barbenchon, L.; Chen, Y. Elastically Anisotropic Architected Metamaterials with Enhanced Energy Absorption. Thin-Walled Struct. 2023, 192, 111115. [Google Scholar] [CrossRef]
- Ritschl, L.; Schilling, P.; Wittmer, A.; Bohner, M.; Bernstein, A.; Schmal, H.; Seidenstuecker, M. Composite Material Consisting of Microporous Beta-TCP Ceramic and Alginate-Dialdehyde-Gelatin for Controlled Dual Release of Clindamycin and Bone Morphogenetic Protein 2. J. Mater. Sci. Mater. Med. 2023, 34, 39. [Google Scholar] [CrossRef] [PubMed]
- AboElhassan, R.G.; Watts, D.C.; Alamoush, R.A.; Elraggal, A. Biomechanical Behavior and Weibull Survival of CAD-CAM Endocrowns with Different Marginal Designs: A 3D Finite Element Analysis. Dent. Mater. 2024, 40, 227–235. [Google Scholar] [CrossRef]
- Kirmali, Ö.; Icen, G.; Celik, H.K.; Rennie, A.E.W. Evaluation of Stress Distribution on an Endodontically Treated Maxillary Central Tooth with Lesion Restored with Different Crown Materials: A Finite Element Analysis. Heliyon 2024, 10, e25829. [Google Scholar] [CrossRef]
- Robau-Porrua, A.; González, J.E.; Rodríguez-Guerra, J.; González-Mederos, P.; Navarro, P.; de la Rosa, J.E.; Carbonell-González, M.; Araneda-Hernández, E.; Torres, Y. Biomechanical Behavior of a New Design of Dental Implant: Influence of the Porosity and Location in the Maxilla. J. Mater. Res. Technol. 2024, 29, 3255–3267. [Google Scholar] [CrossRef]
- Alemayehu, D.-B.; Jeng, Y.-R. Three-Dimensional Finite Element Investigation into Effects of Implant Thread Design and Loading Rate on Stress Distribution in Dental Implants and Anisotropic Bone. Materials 2021, 14, 6974. [Google Scholar] [CrossRef] [PubMed]
- Pandoleon, P.; Bakopoulou, A.; Papadopoulou, L.; Koidis, P. Evaluation of the Biological Behaviour of Various Dental Implant Abutment Materials on Attachment and Viability of Human Gingival Fibroblasts. Dent. Mater. 2019, 35, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Bulaqi, H.A.; Mashhadi, M.M.; Safari, H.; Samandari, M.M.; Geramipanah, F. Effect of Increased Crown Height on Stress Distribution in Short Dental Implant Components and Their Surrounding Bone: A Finite Element Analysis. J. Prosthet. Dent. 2015, 113, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kayabaşı, O.; Yüzbasıoğlu, E.; Erzincanlı, F. Static, Dynamic and Fatigue Behaviors of Dental Implant Using Finite Element Method. Adv. Eng. Softw. 2006, 37, 649–658. [Google Scholar] [CrossRef]
- Geramizadeh, M.; Katoozian, H.; Amid, R.; Kadkhodazadeh, M. Finite Element Analysis of Dental Implants with and without Microthreads under Static and Dynamic Loading. J. Long. Term. Eff. Med. Implants 2017, 27, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hao, H.E.; Li, Y.-M.; Ting, L.I.; Guo, X.-P.; Wang, R.-F. Finite Element Analysis of Stress at Implant–Bone Interface of Dental Implants with Different Structures. Trans. Nonferrous Met. Soc. China 2011, 21, 1602–1610. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Wu, T.-H.; Tseng, Y.-S.; Chen, W.-F. Development of Hybrid 3D Printing Approach for Fabrication of High-Strength Hydroxyapatite Bioscaffold Using FDM and DLP Techniques. Biofabrication 2024, 16, 25003. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhu, J.; Jing, Y.; Long, S.; Tang, L.; Cheng, L.; Shi, Z. Effect of 3D-Printed Porous Titanium Alloy Pore Structure on Bone Regeneration: A Review. Coatings 2024, 14, 253. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, B.; Liu, Y.; Liao, Y.; Zhou, Y.; Li, W. Influence of Structural Parameters of 3D-printed Triply Periodic Minimal Surface Gyroid Porous Scaffolds on Compression Performance, Cell Response, and Bone Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35337. [Google Scholar] [CrossRef]
- Ashby, M.F.; Gibson, L.J. Cellular Solids: Structure and Properties; Press Syndicate of the University of Cambridge: Cambridge, UK, 1997; pp. 175–231. [Google Scholar]
- Distefano, F.; Pasta, S.; Epasto, G. Titanium Lattice Structures Produced via Additive Manufacturing for a Bone Scaffold: A Review. J. Funct. Biomater. 2023, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, C.M.; Sagar, S.; Bhallamudi, R.; Mishra, S. Effective Design and Mechanical Response of Gyroid Lattice Scaffold for Orthopedic Implants. Manuf. Lett. 2023, 35, 493–501. [Google Scholar] [CrossRef]
- Verma, V.; Pal, K. A Finite Element Investigation on the Design of Mechanically Compatible Functionally Graded Orthopaedic Plate for Diaphyseal Tibia Transverse Fracture. Compos. Part C Open Access 2022, 7, 100228. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Liu, T.; Zou, C.; Zhou, C. Mechanical Property of Ti6Al4V Cylindrical Porous Structure for Dental Implants Fabricated by Selective Laser Melting. Comput. Methods Biomech. Biomed. Engin. 2023, 10, 1–19. [Google Scholar] [CrossRef]
- Fan, Z.; Huang, G.; Lu, Y.; Chen, Y.; Zeng, F.; Lin, J. Full Compression Response of FG-Based Scaffolds with Varying Porosity via an Effective Numerical Scheme. Int. J. Mech. Sci. 2022, 223, 107294. [Google Scholar] [CrossRef]
- Rezapourian, M.; Kamboj, N.; Jasiuk, I.; Hussainova, I. Journal of the Mechanical Behavior of Biomedical Materials Biomimetic Design of Implants for Long Bone Critical-Sized Defects. J. Mech. Behav. Biomed. Mater. 2022, 134, 105370. [Google Scholar] [CrossRef]
- Afshar, M.; Anaraki, A.P.; Montazerian, H.; Kadkhodapour, J. Additive Manufacturing and Mechanical Characterization of Graded Porosity Scaffolds Designed Based on Triply Periodic Minimal Surface Architectures. J. Mech. Behav. Biomed. Mater. 2016, 62, 481–494. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef]
- Cantó-Navés, O.; Medina-Galvez, R.; Marimon, X.; Ferrer, M.; Figueras-Álvarez, Ó.; Cabratosa-Termes, J. A 3D Finite Element Analysis Model of Single Implant-Supported Prosthesis under Dynamic Impact Loading for Evaluation of Stress in the Crown, Abutment and Cortical Bone Using Different Rehabilitation Materials. Materials 2021, 14, 3519. [Google Scholar] [CrossRef]
- Adachi, T.; Tsubota, K.; Tomita, Y.; Hollister, S.J. Trabecular Surface Remodeling Simulation for Cancellous Bone Using Microstructural Voxel Finite Element Models. J. Biomech. Eng. 2001, 123, 403–409. [Google Scholar] [CrossRef]
- Juneja, S.; Miranda, G.; Eram, A.; Shetty, N.; KN, C.; Keni, L.G. Investigating the Influence of All-Ceramic Prosthetic Materials on Implants and Their Effect on the Surrounding Bone: A Finite Element Analysis. Prosthesis 2024, 6, 74–88. [Google Scholar] [CrossRef]
- Kowalczyk, P. Elastic Properties of Cancellous Bone Derived from Finite Element Models of Parameterized Microstructure Cells. J. Biomech. 2003, 36, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-W.; Huang, H.-L.; Hsu, J.-T.; Fuh, L.-J. Effects of Diameters of Implant and Abutment Screw on Stress Distribution within Dental Implant and Alveolar Bone: A Three-Dimensional Finite Element Analysis. J. Dent. Sci. 2024, 19, 1126–1134. [Google Scholar] [CrossRef]
- Ulrich, D.; van Rietbergen, B.; Weinans, H.; Rüegsegger, P. Finite Element Analysis of Trabecular Bone Structure: A Comparison of Image-Based Meshing Techniques. J. Biomech. 1998, 31, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Vafaeian, B.; El-Rich, M.; El-Bialy, T.; Adeeb, S. The Finite Element Method for Micro-Scale Modeling of Ultrasound Propagation in Cancellous Bone. Ultrasonics 2014, 54, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ye, H.; Wu, Y.; Jiang, Z.; Yao, H.; Xu, X.; Zhang, Y.; Du, W.; Li, W.; Zheng, Y. Effect of Bone Mass Density and Alveolar Bone Resorption on Stress in Implant Restoration of Free-End Edentulous Posterior Mandible: Finite Element Analysis of Double-Factor Sensitivity. Ann. Anat. Anat. Anz. 2024, 253, 152210. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, A.; Aktas, S.; Celik, T.; Kisioglu, Y. Effects of the Internal Contact Surfaces of Dental Implants on Screw Loosening: A 3-Dimensional Finite Element Analysis. J. Prosthet. Dent. 2023, 130, 603-e1. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, C.; Valente, F.; Vasta, M.; Traini, T. Finite Element Analysis in Implant Dentistry: State of the Art and Future Directions. Dent. Mater. 2023, 39, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Basu, B. Biomechanical Analysis of Peri-Prosthetic Bone Response to Hybrid Threaded Zirconia Dental Implants: An in Silico Model. J. Mech. Behav. Biomed. Mater. 2024, 150, 106310. [Google Scholar] [CrossRef]
- Młynarek-Żak, K.; Żmudzki, J. The Effect of Porous Compliance Bushings in a Dental Implant on the Distribution of Occlusal Loads. Sci. Rep. 2024, 14, 1607. [Google Scholar] [CrossRef]
- Qiu, P.; Cao, R.; Li, Z.; Fan, Z. A Comprehensive Biomechanical Evaluation of Length and Diameter of Dental Implants Using Finite Element Analyses: A Systematic Review. Heliyon 2024, 10, e26876. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-S.; Tsai, M.-H.; Wu, Y.-L.; Chen, H.-S.; Lei, Y.-N.; Wu, A.Y.-J. Biomechanical Analysis of Titanium Dental Implants in the All-on-4 Treatment with Different Implant–Abutment Connections: A Three-Dimensional Finite Element Study. J. Funct. Biomater. 2023, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, J.; Wang, Y.; Luo, T.; Zhang, X.; Wang, X.; Bian, X.; Xie, H. Multicomponent Functional Scaffolds with Multiscale Porosity to Improve Biomimetic and Mechanical Properties. Preprint Article, Available SSRN 4725552. Available online: https://ssrn.com/abstract=4725552 (accessed on 28 February 2024). [CrossRef]
- Karimi, M.; Asadi-Eydivand, M.; Abolfathi, N.; Chehrehsaz, Y.; Solati-Hashjin, M. The Effect of Pore Size and Layout on Mechanical and Biological Properties of 3D-printed Bone Scaffolds with Gradient Porosity. Polym. Compos. 2023, 44, 1343–1359. [Google Scholar] [CrossRef]
- Mao, Y.; Yuan, J.; Heng, Y.; Feng, K.; Cai, D.; Wei, Q. Effect of Hot Isostatic Pressing Treatment on Porosity Reduction and Mechanical Properties Enhancement of 316L Stainless Steel Fabricated by Binder Jetting. Virtual Phys. Prototyp. 2023, 18, e2174703. [Google Scholar] [CrossRef]
- Zheng, G.; Wei, K.; Kang, X.; Fan, W.; Ma, N.L.; Verma, M.; Ng, H.S.; Ge, S. A New Attempt to Control Volatile Organic Compounds (VOCs) Pollution-Modification Technology of Biomass for Adsorption of VOCs Gas. Environ. Pollut. 2023, 336, 122451. [Google Scholar] [CrossRef] [PubMed]
- Araya, M.; Jaskari, M.; Rautio, T.; Guillén, T.; Järvenpää, A. Assessing the Compressive and Tensile Properties of TPMS-Gyroid and Stochastic Ti64 Lattice Structures: A Study on Laser Powder Bed Fusion Manufacturing for Biomedical Implants. J. Sci. Adv. Mater. Devices 2024, 9, 100663. [Google Scholar] [CrossRef]
- Seehanam, S.; Khrueaduangkham, S.; Sinthuvanich, C.; Sae-Ueng, U.; Srimaneepong, V.; Promoppatum, P. Evaluating the Effect of Pore Size for 3d-Printed Bone Scaffolds. Heliyon 2024, 10, e26005. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, J.; Min, S.; Liu, Y.; Liu, B.; Hu, Y.; Wang, Z.; Mao, F.; Wang, C.; Ma, X. The Effect of Pore Size on the Mechanical Properties, Biodegradation and Osteogenic Effects of Additively Manufactured Magnesium Scaffolds after High Temperature Oxidation: An in Vitro and in Vivo Study. Bioact. Mater. 2023, 28, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, J.; Kim, N.; Min, K. Artificial Intelligence in the Design of Innovative Metamaterials: A Comprehensive Review. Int. J. Precis. Eng. Manuf. 2024, 25, 225–244. [Google Scholar] [CrossRef]
- Carter, D.R.; Hayes, W.C. The Compressiv Berhaviour of Bone as a Two-Phase Porous Material. J. Bone Joint. Surg. 1977, 59, 954–962. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Burr, D.B. Stiffness of Compact Bone: Effects of Porosity and Density. J. Biomech. 1988, 21, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. The Mechanical Behaviour of Cancellous Bone. J. Biomech. 1985, 18, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Yagihara, A.; Kawasaki, R.; Mita, A.; Takakuda, K. Impact of Dynamic and Static Load on Bone Around Implants: An Experimental Study in a Rat Model. Int. J. Oral Maxillofac. Implants 2016, 31, e49–e56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Po, J.M.C.; Kieser, J.A.; Gallo, L.M.; Tésenyi, A.J.; Herbison, P.; Farella, M. Time-Frequency Analysis of Chewing Activity in the Natural Environment. J. Dent. Res. 2011, 90, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, X.; Li, Y.; Li, T. Finite Element Analysis for Interfacial Stress and Fatigue Behaviors of Biomimetic Titanium Implant under Static and Dynamic Loading Conditions. Zhong Nan Da Xue Xue Bao. Yi Xue Ban 2010, 35, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, S.; Xu, Z.; Liu, X.; Liu, G.; Cao, F.; Ma, Y. Structural Mechanical Properties of 3D Printing Biomimetic Bone Replacement Materials. Biomimetics 2023, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.I.; Alemayehu, D.B.; Schuster, L.S.; Raab, G.I.; Chertovskikh, S.V.; Astanin, V.V.; Huang, S.-J.; Chernyak, I.N. Tribotechnical Characteristics of Commercially Pure Titanium with Different Grain Sizes and TiC and TiO2 Coatings. J. Frict. Wear 2019, 40, 349–354. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Todoh, M.; Hsieh, J.-H.; Li, C.; Huang, S.-J. Improving Pure Titanium’s Biological and Mechanical Characteristics through ECAP and Micro-Arc Oxidation Processes. Micromachines 2023, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.; Chaussé, J.; Vermette, P. Bacterial Cellulose: A Comprehensive Review. J. Appl. Polym. Sci. 2024, 141, e55163. [Google Scholar] [CrossRef]
- Mudigonda, J. 3D Bioprinting of Tissues and Organs: A Comprehensive Review of the Techniques, Recent Advances, and Their Applications in Organ Engineering and Regenerative Medicine. In Advances in 3D Bioprinting; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–54. [Google Scholar]
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Lim, K. Manufacturing 3D Biomimetic Tissue: A Strategy Involving the Integration of Electrospun Nanofibers with a 3D-Printed Framework for Enhanced Tissue Regeneration. Small 2024, 2309269. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Innovative Hybrid Nanomaterials for Precision Biomedical Solutions: Hybrid Nanomaterials for Biomedical Solutions. In Innovations and Applications of Hybrid Nanomaterials; IGI Global: Hershey, PA, USA, 2024; pp. 144–182. [Google Scholar]





| Crown | Abutment | Screw | Implant | Cortical Bone | Trabecular Bone | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone Models | No. Element | No. Node | No. Element | No. Node | No. Element | No. Node | No. Element | No. Node | No. Element | No. Node | No. Element | No. Node | No. Element | No. Node |
| VTB10 | 107,954 | 20,670 | 85,243 | 17,463 | 627,098 | 121,624 | 160,676 | 31,737 | 146,767 | 31,180 | 1,936,663 | 584,049 | 3,064,401 | 806,723 |
| VTB15 | 107,954 | 20,670 | 85,243 | 17,463 | 627,098 | 121,624 | 160,676 | 31,737 | 146,767 | 31,180 | 1,187,637 | 363,867 | 2,315,375 | 586,541 |
| VTB20 | 107,954 | 20,670 | 85,243 | 17,463 | 627,098 | 121,624 | 160,676 | 31,737 | 146,767 | 31,180 | 791,391 | 248,917 | 1,919,129 | 471,591 |
| VTB25 | 107,954 | 20,670 | 85,243 | 17,463 | 627,098 | 121,624 | 160,676 | 31,737 | 146,767 | 31,180 | 591,166 | 186,214 | 1,718,904 | 408,888 |
| Materials | Young’s Modulus E (MPa) | Poisson’s Ratio ν | Density (g/cm3) | Strength (MPa) | ||
|---|---|---|---|---|---|---|
| Cortical bone * | Ex | 12,600 | νxy | 0.3 | 1.79 | 190 |
| Ey | 12,600 | νyz | 0.253 | |||
| Ez | 19,400 | νxz | 0.253 | |||
| νyx | 0.3 | |||||
| νzy | 0.39 | |||||
| νzx | 0.39 | |||||
| Cancellous bone * | Ex | 1148 | νxy | 0.055 | 0.45 | 10 |
| Ey | 210 | νyz | 0.01 | |||
| Ez | 1148 | νxz | 0.322 | |||
| νyx | 0.01 | |||||
| νzy | 0.055 | |||||
| νzx | 0.322 | |||||
| Gold abutment * | 136,000 | 0.37 | 17.5 | 765 | ||
| Porcelain * | 68,900 | 0.28 | 2.44 | 145 | ||
| Titanium grade 4 * | 110,000 | 0.34 | 4.5 | 550 | ||
| Symbolic Name | Pore Size (mm) | Beam Thickness (mm) | Randomization Seed | Average Beam Length (mm) | Lattice Beam Account | Lattice Node Count | Relative Density (%) | Surface-Area-to-Volume Ratio (%) |
|---|---|---|---|---|---|---|---|---|
| VTB10 | 1.0 | 0.4 | 250 | 0.4614 | 25,809 | 15,186 | 22.21 | 10.49 |
| VTB15 | 1.5 | 0.4 | 250 | 0.5681 | 13,286 | 8182 | 15.19 | 11.58 |
| VTB20 | 2.0 | 0.4 | 250 | 0.7532 | 5438 | 3535 | 8.84 | 12.21 |
| VTB25 | 2.5 | 0.4 | 250 | 0.9203 | 3024 | 2058 | 6.27 | 12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemayehu, D.B.; Todoh, M.; Huang, S.-J. Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices. J. Funct. Biomater. 2024, 15, 94. https://doi.org/10.3390/jfb15040094
Alemayehu DB, Todoh M, Huang S-J. Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices. Journal of Functional Biomaterials. 2024; 15(4):94. https://doi.org/10.3390/jfb15040094
Chicago/Turabian StyleAlemayehu, Dawit Bogale, Masahiro Todoh, and Song-Jeng Huang. 2024. "Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices" Journal of Functional Biomaterials 15, no. 4: 94. https://doi.org/10.3390/jfb15040094
APA StyleAlemayehu, D. B., Todoh, M., & Huang, S.-J. (2024). Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices. Journal of Functional Biomaterials, 15(4), 94. https://doi.org/10.3390/jfb15040094








