Unlocking the Future: Bioprinting Salivary Glands—From Possibility to Reality
Abstract
1. Introduction
2. Materials and Methods
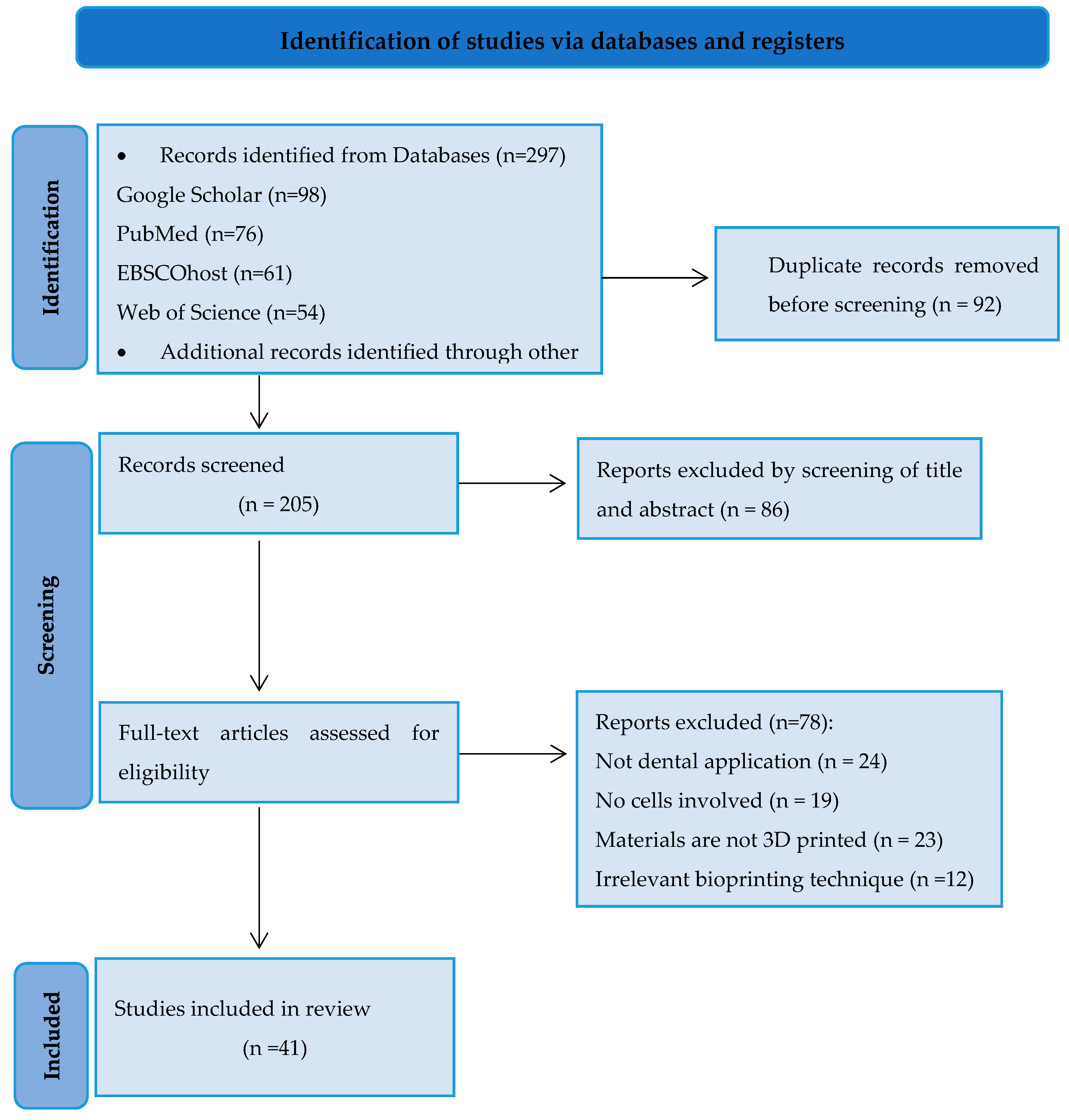
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Analysis
3. Results
3.1. Cell Source and Selection
3.2. Bioink Development
3.3. Bioprinting Technique
3.4. Structural Design
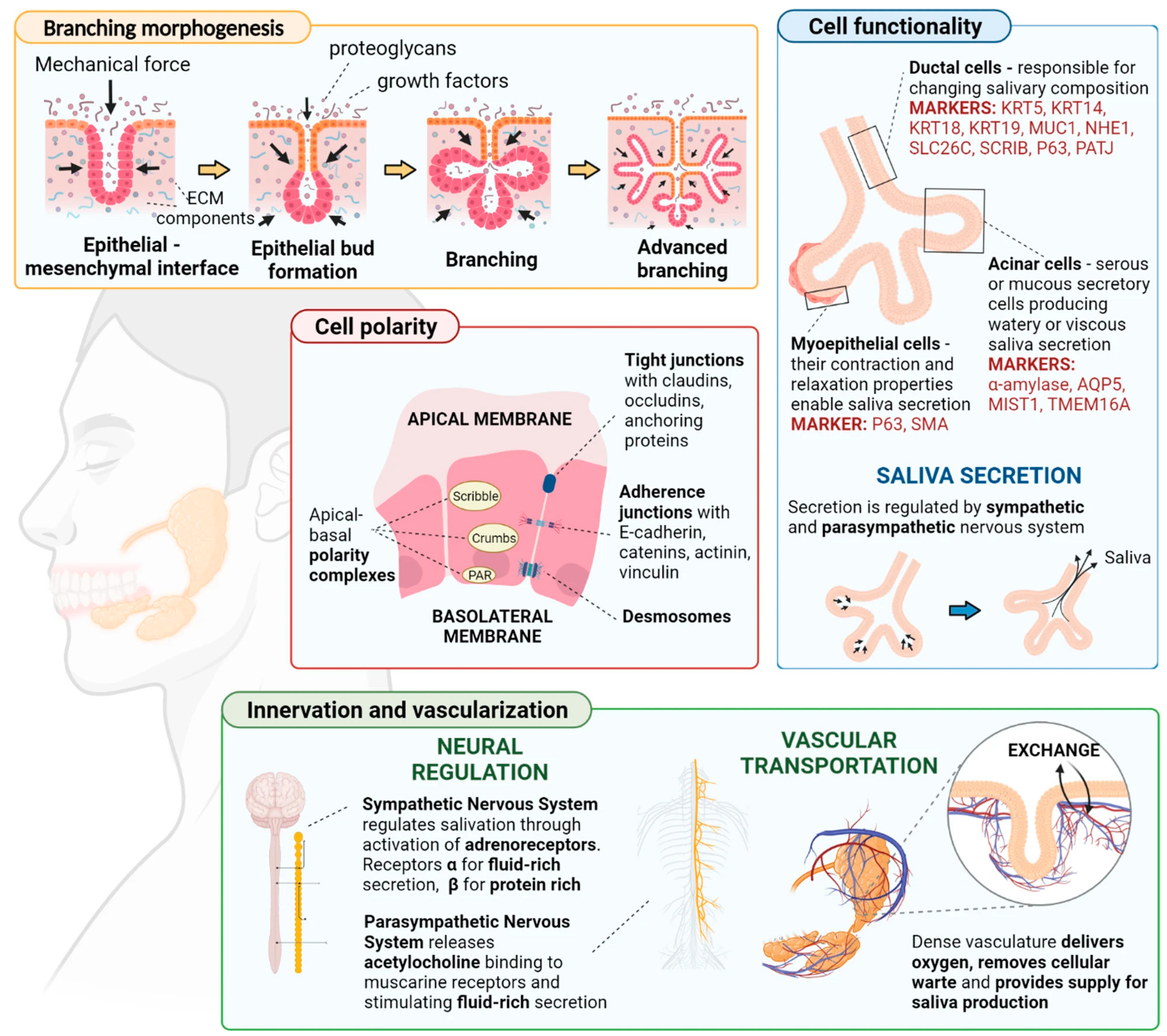
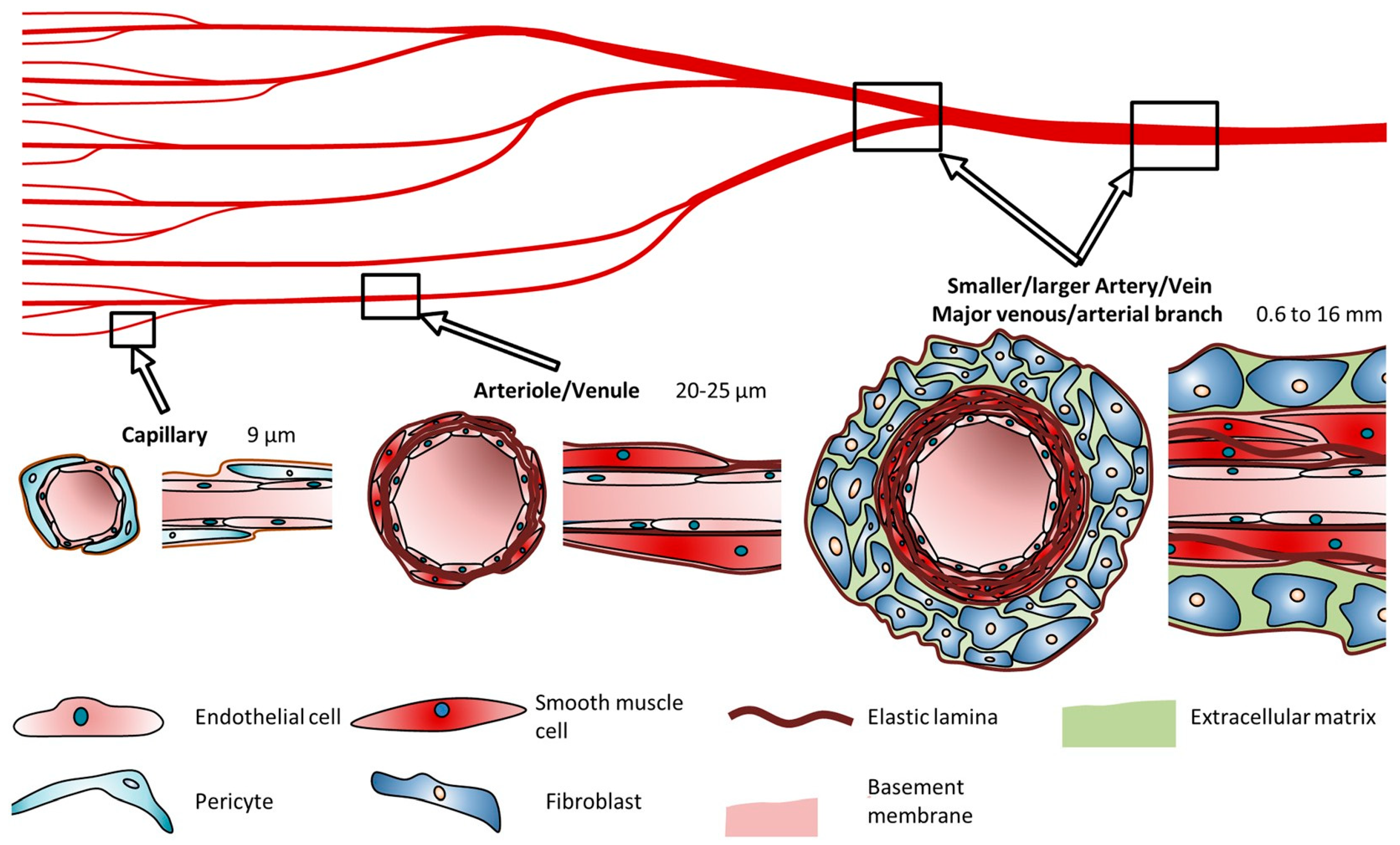
3.5. Vascularization
3.6. Maturation and Integration
3.7. Functionality Assessment
4. Discussion
5. Conclusions
6. Limitations
- Salivary organs have a complex microarchitecture, including acini (secretory units), ductal systems, and a rich vascular supply. Precisely imitating this complex structure with bioprinting innovations is highly challenging.
- Obtaining adequate numbers of reasonable cells from salivary organs for bioprinting is troublesome. Essential salivary organ cells are troublesome to culture and the utilization of stem cells requires exact separation techniques to guarantee that they create useful salivary organ cells.
- The choice of bioinks is vital for bioprinting. The improvement and optimization of reasonable bio-inks remains a noteworthy challenge.
- Guaranteeing the satisfactory vascularization of the bioprinted salivary organ tissue is fundamental for its survival and usefulness after implantation. Current bioprinting strategies struggle to create the complex vascular systems required for an adequate blood supply.
- Effectively joining bioprinted salivary organs with existing tissues and providing suitable neural associations for useful discharge and control is another major challenge.
- The host’s immune reaction to the embedded bioprinted tissue can lead to aggravation or dismissal. The immunocompatibility of the bioprinted tissue must be carefully considered.
- Bioprinting for clinical use must go through complex administrative pathways to guarantee security and adequacy. Moral contemplations with respect to the source of cells, particularly in the event that stem cells are utilized, and the long-term impacts of embedded bioprinted tissues require careful consideration.
- Tending to these limitations requires the collaboration between scholars, materials researchers, engineers, and clinicians. Future studies and mechanical investigations are fundamental to overcoming these challenges and realizing the full potential of bioprinting useful salivary organs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Salvador, M.; Ruiz-Cantu, L. Revealing emerging science and technology research for dentistry applications of 3D bioprinting. Int. J. Bioprinting 2019, 5, 170. [Google Scholar] [CrossRef] [PubMed]
- von Bültzingslöwen, I.; Sollecito, T.P.; Fox, P.C.; Daniels, T.; Jonsson, R.; Lockhart, P.B.; Wray, D.; Brennan, M.T.; Carrozzo, M.; Gandera, B.; et al. Salivary dysfunction associated with systemic diseases: Systematic review and clinical management recommendations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2007, 103, S57.e1–S57.e15. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef] [PubMed]
- Kruszka, P.; O’BRIAN, R.J. Diagnosis and management of Sjögren syndrome. Am. Fam. Physician 2009, 79, 465–470. [Google Scholar] [PubMed]
- Yoo, C.; Vines, J.B.; Alexander, G.; Murdock, K.; Hwang, P.; Jun, H.W. Adult stem cells and tissue engineering strategies for salivary gland regeneration: A review. Biomater. Res. 2014, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Rasoolzade, B.; Namanloo, R.A.; Azarpira, N.; Dortaj, H. Stem cells and common biomaterials in dentistry: A review study. J. Mater. Sci. Mater. Med. 2022, 33, 55. [Google Scholar] [CrossRef]
- de Almeida, P.D.V.; Gregio, A.M.; Machado, M.A.; De Lima, A.A.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pr. 2008, 9, 72–80. [Google Scholar]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Edgar, W.M. Saliva: Its secretion, composition and functions. Br. Dent. J. 1992, 172, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Rehman, A.; Shah, K.; Kamran, M.; Mashal, S.; Rustam, S.; Sabir, M.W.; Muzammal, M. Composition and function of saliva: A review. World J. Pharm. Pharm. Sci 2020, 9, 1552–1567. [Google Scholar]
- Dodds, M.W.; Johnson, D.A.; Yeh, C.K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kashyap, N.; Avinash, A.; Chevvuri, R.; Sagar, M.K.; Shrikant, K. The composition, function and role of saliva in maintaining oral health: A review. Proteins 2017, 220, 140–640. [Google Scholar]
- Adine, C.; Ferreira, J. Bioprinting strategies to engineer functional salivary gland organoids. In Organ Tissue Engineering; Springer: Cham, Switzerland, 2020; pp. 173–197. [Google Scholar]
- Urkasemsin, G.; Ferreira, J.N. Unveiling stem cell heterogeneity toward the development of salivary gland regenerative strategies. Stem Cells Heterog. Nov. Concepts 2019, 1123, 151–164. [Google Scholar]
- Hajiabbas, M.; D’Agostino, C.; Simińska-Stanny, J.; Tran, S.D.; Shavandi, A.; Delporte, C. Bioengineering in salivary gland regeneration. J. Biomed. Sci. 2022, 29, 35. [Google Scholar] [CrossRef] [PubMed]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Munguia-Lopez, J.G.; Tran, S.D. Bioengineered Salivary Gland Microtissues─ A Review of 3D Cellular Models and their Applications. ACS Appl. Bio Mater. 2024, 7, 2620–2636. [Google Scholar] [CrossRef]
- Muallah, D.; Matschke, J.; Kappler, M.; Kroschwald, L.M.; Lauer, G.; Eckert, A.W. Dental Pulp Stem Cells for Salivary Gland Regeneration—Where Are We Today? Int. J. Mol. Sci. 2023, 24, 8664. [Google Scholar] [CrossRef]
- Rose, S.C.; Larsen, M.; Xie, Y.; Sharfstein, S.T. Salivary Gland Bioengineering. Bioengineering 2024, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, C.; Barazzuol, L.; Coppes, R.P. The evolving definition of salivary gland stem cells. NPJ Regen. Med. 2021, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Almansoori, A.A.; Kim, B.; Lee, J.H.; Tran, S.D. Tissue engineering of oral mucosa and salivary gland: Disease modeling and clinical applications. Micromachines 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, Y.; Suhito, I.R.; Choi, Y.; Kwon, M.; Son, H.; Kim, H.-R.; Kim, T.-H. Raman spectroscopy-based 3D analysis of odontogenic differentiation of human dental pulp stem cell spheroids. Anal. Chem. 2021, 93, 9995–10004. [Google Scholar] [CrossRef] [PubMed]
- y Baena, A.R.; Casasco, A.; Monti, M. Hypes and hopes of stem cell therapies in dentistry: A review. Stem Cell Rev. Rep. 2022, 18, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- TaTanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 4216. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhang, S.; Li, Y.; Zhang, X.; Hu, W.; Fen, Y.; Xiong, J.; Zhang, Y.; Wei, S. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res. Ther. 2020, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.; Kazi, G.A.; Taketa, H.; Hara, E.S.; Oshima, M.; Kuboki, T.; Matsumoto, T. Fibronectin-induced ductal formation in salivary gland self-organization model. Dev. Dyn. 2019, 248, 813–825. [Google Scholar] [CrossRef]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef]
- Tanaka, J.; Senpuku, H.; Ogawa, M.; Yasuhara, R.; Ohnuma, S.; Takamatsu, K.; Watanabe, T.; Mabuchi, Y.; Nakamura, S.; Ishida, S.; et al. Human induced pluripotent stem cell-derived salivary gland organoids model SARS-CoV-2 infection and replication. Nat. Cell Biol. 2022, 24, 1595–1605. [Google Scholar] [CrossRef]
- Marinkovic, M.; Tran, O.N.; Wang, H.; Abdul-Azees, P.; Dean, D.D.; Chen, X.-D.; Yeh, C.-K. Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration. Int. J. Oral. Sci. 2023, 15, 18. [Google Scholar] [CrossRef]
- Pillai, S.; Munguia-Lopez, J.G.; Tran, S.D. Hydrogels for salivary gland tissue engineering. Gels 2022, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Dos Santos, H.; Nam, K.; Brown, C.; Dean, S.; Lewis, S.; Pfeifer, C.; Lei, P.; Petris, M.; Andreadis, S.; Baker, O. Trimers conjugated to fibrin hydrogels promote salivary gland function. J. Dent. Res. 2021, 100, 268–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Pham, H.M.; Munguia-Lopez, J.G.; Kinsella, J.M.; Tran, S.D. The optimization of a novel hydrogel—Egg white-alginate for 2.5 D tissue engineering of salivary spheroid-like structure. Molecules 2020, 25, 5751. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable Polymers: Advanced Materials and 4D Printing for Tissue Engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, C.; Peng, S.; Lin, Y.; Ye, Z. Hydrogels in dental medicine. Adv. Ther. 2024, 7, 2300128. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Andia, I.; Perez-Valle, A.; Del Amo, C.; Maffulli, N. Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization. Int. J. Mol. Sci. 2020, 21, 6904. [Google Scholar] [CrossRef]
- Rahman, S.U.; Nagrath, M.; Ponnusamy, S.; Arany, P.R. Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering. Materials 2018, 11, 1478. [Google Scholar] [CrossRef]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Kinsella, J.M.; Tran, S.D. The applications of 3D printing for craniofacial tissue engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Jiang, L.; Zeng, Y.; Han, Y.; Sha, C.; Xie, X.; Li, H.; Zhou, J.; Lin, W. 3D bioprinting of collagen-based materials for oral medicine. Collagen Leather. 2023, 5, 23. [Google Scholar] [CrossRef]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative medicine technologies to treat dental, oral, and craniofacial defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Rademakers, T.; Horvath, J.M.; van Blitterswijk, C.A.; LaPointe, V.L. Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization. J. Tissue Eng. Regen. Med. 2019, 13, 1815–1829. [Google Scholar] [CrossRef]
- Nesic, D.; Durual, S.; Marger, L.; Mekki, M.; Sailer, I.; Scherrer, S.S. Could 3D printing be the future for oral soft tissue regeneration? Bioprinting 2020, 20, e00100. [Google Scholar] [CrossRef]
- Tomasina, C.; Bodet, T.; Mota, C.; Moroni, L.; Camarero-Espinosa, S. Bioprinting Vasculature: Materials, Cells and Emergent Techniques. Materials 2019, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ross, D.; Wang, G.; Jia, W.; Kirkpatrick, S.J.; Zhao, F. Upgrading prevascularization in tissue engineering: A review of strategies for promoting highly organized microvascular network formation. Acta Biomater. 2019, 95, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Schoeneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.C.; Kiessling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef]
- Khalafalla, M.G.; Woods, L.T.; Jasmer, K.J.; Forti, K.M.; Camden, J.M.; Jensen, J.L.; Limesand, K.H.; Galtung, H.K.; Weisman, G.A. P2 receptors as therapeutic targets in the salivary gland: From physiology to dysfunction. Front. Pharmacol. 2020, 11, 222. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Barrows, C.M.; Wu, D.; Farach-Carson, M.C.; Young, S. Building a functional salivary gland for cell-based therapy: More than secretory epithelial acini. Tissue Eng. Part A 2020, 26, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, M. Organoids: The body builders. Nat. Methods 2018, 15, 19–23. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Liao, L.; Tian, W. Oral organoids: Progress and challenges. J. Dent. Res. 2021, 100, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, B.; Xu, F. Recent advances in 4D bioprinting. Biotechnol. J. 2020, 15, 1900086. [Google Scholar] [CrossRef]
- Ionov, L. 4D biofabrication: Materials, methods, and applications. Adv. Healthc. Mater. 2018, 7, 1800412. [Google Scholar] [CrossRef]
- Xu, X.; Liao, L.; Tian, W. Strategies of prevascularization in tissue engineering and regeneration of craniofacial tissues. Tissue Eng. Part B Rev. 2022, 28, 464–475. [Google Scholar] [CrossRef]
- Sasmal, P.; Datta, P.; Wu, Y.; Ozbolat, I.T. 3D bioprinting for modelling vasculature. Microphysiol. Syst. 2018, 2. [Google Scholar] [CrossRef]
- Li, Y.; Fraser, D.; Mereness, J.; Van Hove, A.; Basu, S.; Newman, M.; Benoit, D.S. Tissue engineered neurovascularization strategies for craniofacial tissue regeneration. ACS Appl. Bio Mater. 2021, 5, 20–39. [Google Scholar] [CrossRef]
- Chansaenroj, A.; Yodmuang, S.; Ferreira, J.N. Trends in salivary gland tissue engineering: From stem cells to secretome and organoid bioprinting. Tissue Eng. Part B Rev. 2021, 27, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yue, M.; Liu, Y.; Zhang, P.; Qing, J.; Liu, H.; Zhou, Y. Advances of engineered hydrogel organoids within the stem cell field: A systematic review. Gels 2022, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Meng, C.; Cui, N.; Sha, J.; Sun, L.; Zhu, D. Organoid models for salivary gland biology and regenerative medicine. Stem Cells Int. 2021, 2021, 9922597. [Google Scholar] [CrossRef] [PubMed]
- Calà, G.; Sina, B.; De Coppi, P.; Giobbe, G.G.; Gerli, M.F.M. Primary human organoids models: Current progress and key milestones. Front. Bioeng. Biotechnol. 2023, 11, 1058970. [Google Scholar] [CrossRef] [PubMed]

| Property | 3D Printing | 4D Printing |
|---|---|---|
| Manufacturing process | 2D sections of a 3D structure (with respect to the z-axis) are built layer-by-layer from top to bottom or from bottom to top | Produced in the same way as 3D printed products, but changes shape or function after manufacturing, upon exposure to a specific stimulus |
| Materials used | Thermoplastic polymers, ceramics, metals, biomaterials, and their composites | Smart materials (polymers, ceramics, metals, biomaterials, and composites) that undergo a change in property or function over time in response to a specific stimulus |
| Material programmability | Not possible | Material properties and function are programmable with a specific exposure sequence and time of stimulus, and the spatial organization of material in desired final product |
| Object shape/ function | Stable over time | Object shape or function changes over time when structure is exposed to a specific external stimulus |
| Application area | Field including but not limited to medical, engineering, dentistry, automotive, jewelry etc. | All 3D print application areas where a dynamic change in configuration is required or beneficial |
| Printing Techniques | Resolution | Pros | Cons |
|---|---|---|---|
| Inkjet-based bioprinting | About 100 µm | Low cost; high print speed; High cell survival rate (80–90%) | Low cell viscosity and density; Easily clogged nozzles; Unreliable cell encapsulation |
| Extrusion-based bioprinting | > 100 µm | Ability to print high cell densities models | Limited resolution; Low print speed; Low probability cell viability |
| Light-assisted bioprinting | 10–50 µm | High resolution, good cell viability (> 95%) | High cost, less efficient |
| Key Benefit/Topic | Area of Application/Significance | References |
|---|---|---|
| Cell Source and Selection | Adult and embryonic SGs—in vitro SG 3D models | (Pillai et al., 2024) [20] |
| SG spheroids and organoids—to study SG pathophysiology | (Pillai et al., 2024) [20] | |
| The salivary gland-like organoids—stimulated epithelial and neuronal growth | (Adine et al., 2018) [30] | |
| Autologous MSCs—restoration of SG function | (Marinkovic et al., 2023) [32] | |
| Bioink Development | Ideal biomaterial—cell proliferation and migration, selective differentiation of SG stem/progenitor cells, reorganization, support matrix remodeling, and allow duct expansion | (Pillai et al., 2022) [33] |
| Fibrin- and laminin-based hydrogels | (Wu et al., 2021) [34] | |
| Laminin-I II trimers conjugated with fibrin hydrogels | (Dos Santos et al., 2021) [35] | |
| Fibronectin and placenta basement membrane | (Zhang et al., 2020) [36] | |
| Self-assembled hydrogels—biocompatibility, targeting ability, and biomedical safety, | (Chen et al., 2024) [38] | |
| Bioprinting Technique | Freezing | (Andia et al., 2020) [40] |
| Structural Design | Computational design features—highly tailored mechanical, structural, and biochemical properties | (Latimer et al., 2021) [44] |
| Vascularization | Co-printing endothelial cells or incorporating bioactive factors—to stimulate angiogenesis | (Nesic et al.) [46] (Tomasina et al.) [47] |
| Maturation and Integration | Maturation process—fully functional and well-differentiated | (Hajiabbas et al., 2022) [18] (Porcheri et al., 2019) [50] |
| Functionality Assessment | Neurotransmitter’s signaling—saliva flow and protein secretion under normal reflex conditions | (Hajiabbas et al., 2022) [18] (Khalafalla et al., 2020) [51] (Pedersen et al., 2018) [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shopova, D.; Yaneva, A.; Mihaylova, A.; Dinkova, A.; Bakova, D. Unlocking the Future: Bioprinting Salivary Glands—From Possibility to Reality. J. Funct. Biomater. 2024, 15, 151. https://doi.org/10.3390/jfb15060151
Shopova D, Yaneva A, Mihaylova A, Dinkova A, Bakova D. Unlocking the Future: Bioprinting Salivary Glands—From Possibility to Reality. Journal of Functional Biomaterials. 2024; 15(6):151. https://doi.org/10.3390/jfb15060151
Chicago/Turabian StyleShopova, Dobromira, Antoniya Yaneva, Anna Mihaylova, Atanaska Dinkova, and Desislava Bakova. 2024. "Unlocking the Future: Bioprinting Salivary Glands—From Possibility to Reality" Journal of Functional Biomaterials 15, no. 6: 151. https://doi.org/10.3390/jfb15060151
APA StyleShopova, D., Yaneva, A., Mihaylova, A., Dinkova, A., & Bakova, D. (2024). Unlocking the Future: Bioprinting Salivary Glands—From Possibility to Reality. Journal of Functional Biomaterials, 15(6), 151. https://doi.org/10.3390/jfb15060151








