Enhancing Antimicrobial Performance of Gauze via Modification by Ag-Loaded Polydopamine Submicron Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
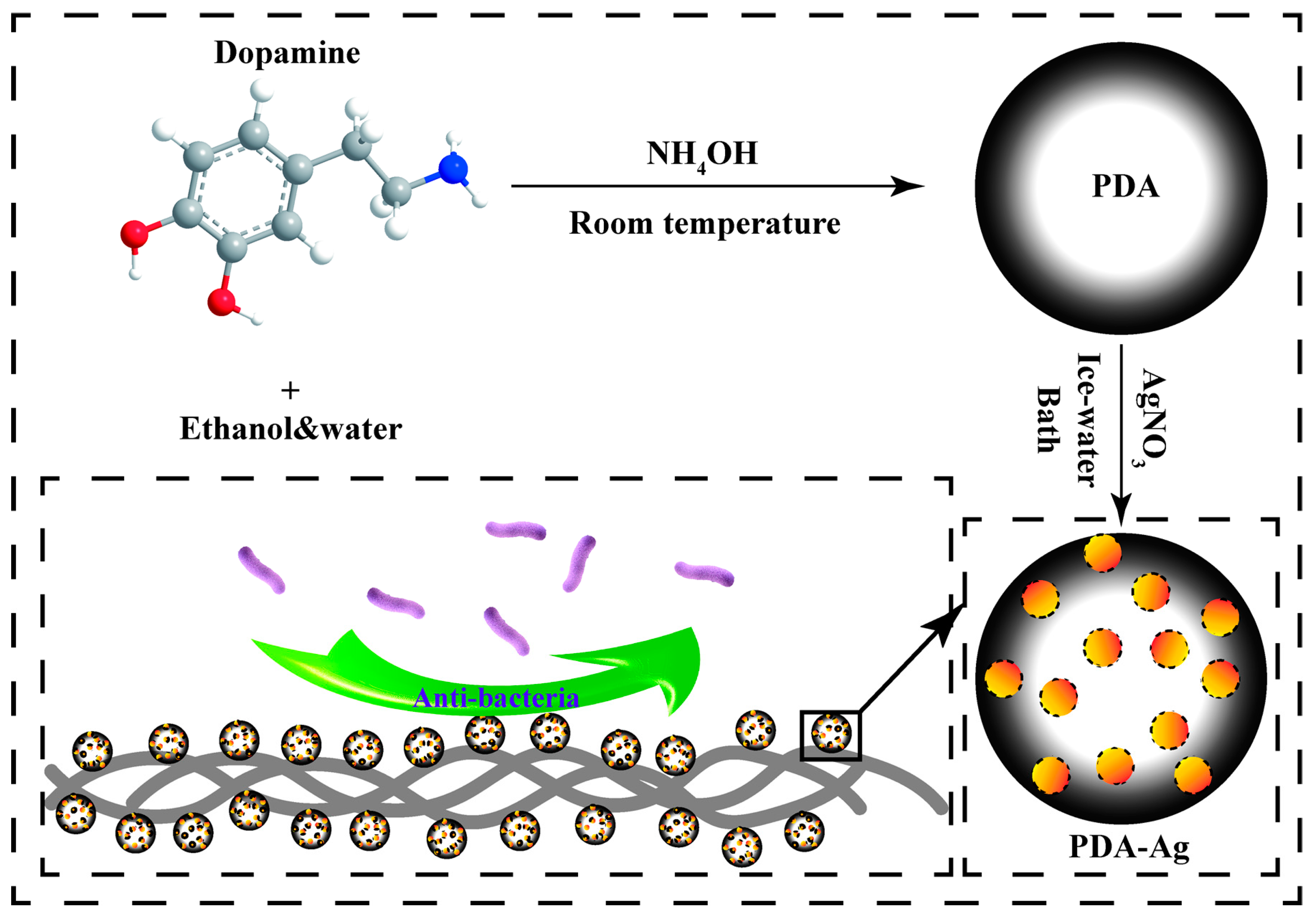
2.2. Synthesis of Polydopamine Submicron Particles
2.3. Preparation of PDA–Ag Submicron Particles
2.4. Characterization of PDA and PDA–Ag
2.5. Preparation of PDA–Ag-Loaded Gauze Surface
2.6. Bacterial Culture
2.7. Anti-Biofilm Performance Testing and Detection of Silver Ion Release
2.8. Antibacterial Performance Test Experiment
2.9. Cell Compatibility Evaluation
3. Results and Discussion
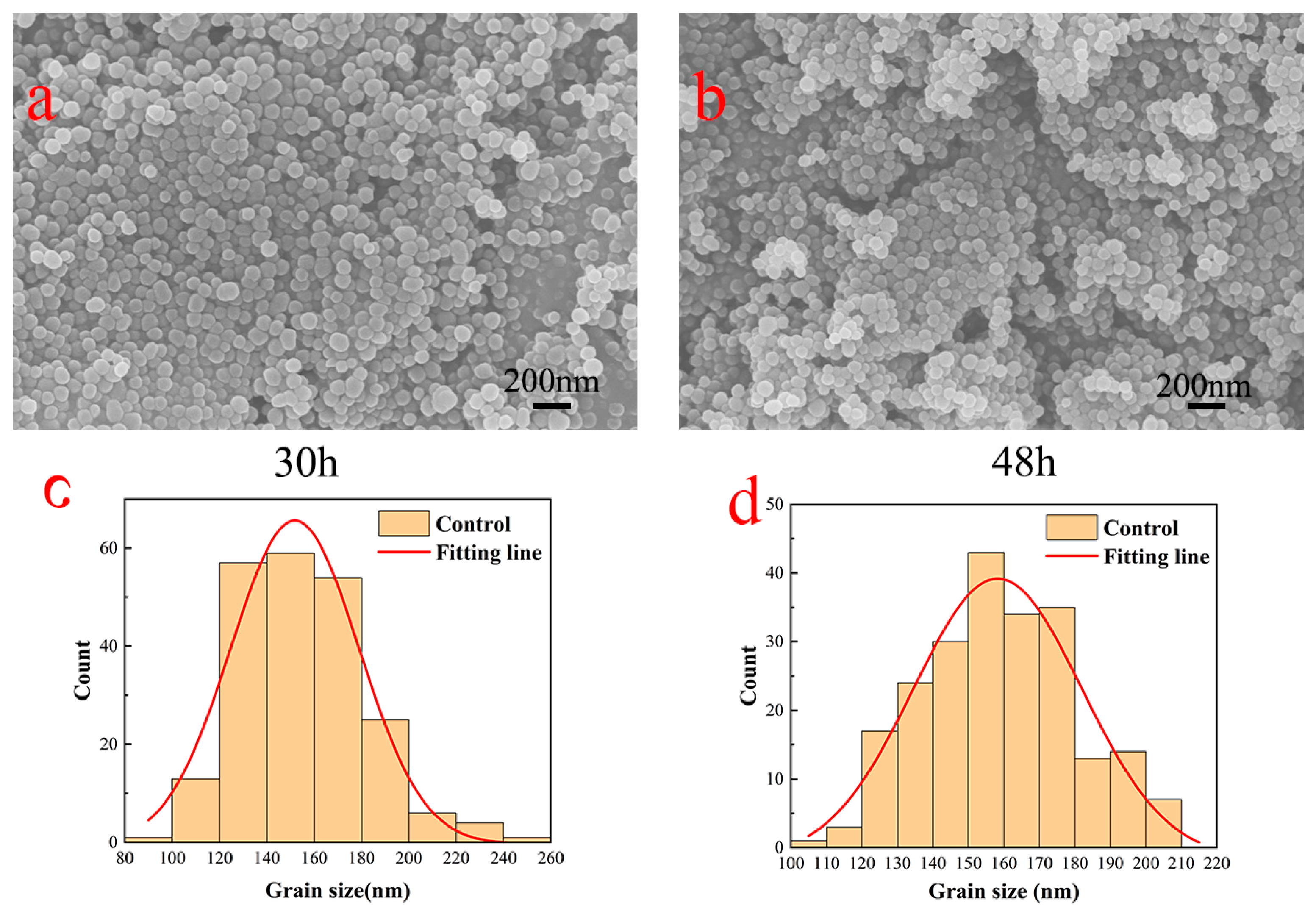
3.1. PDA Surface Topography Analysis
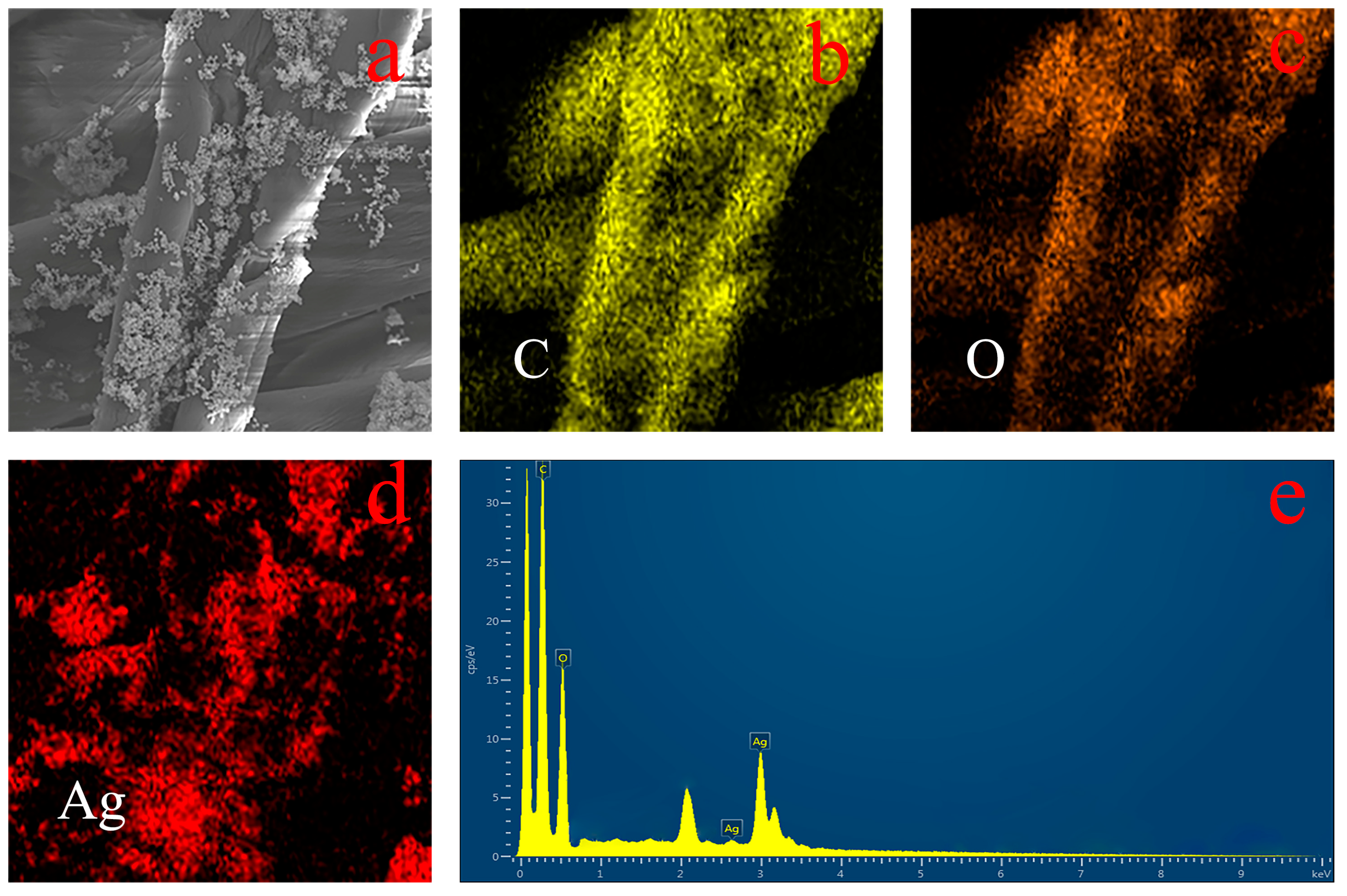
3.2. Surface Morphology Analysis of PDA–Ag-Modified Medical Gauze
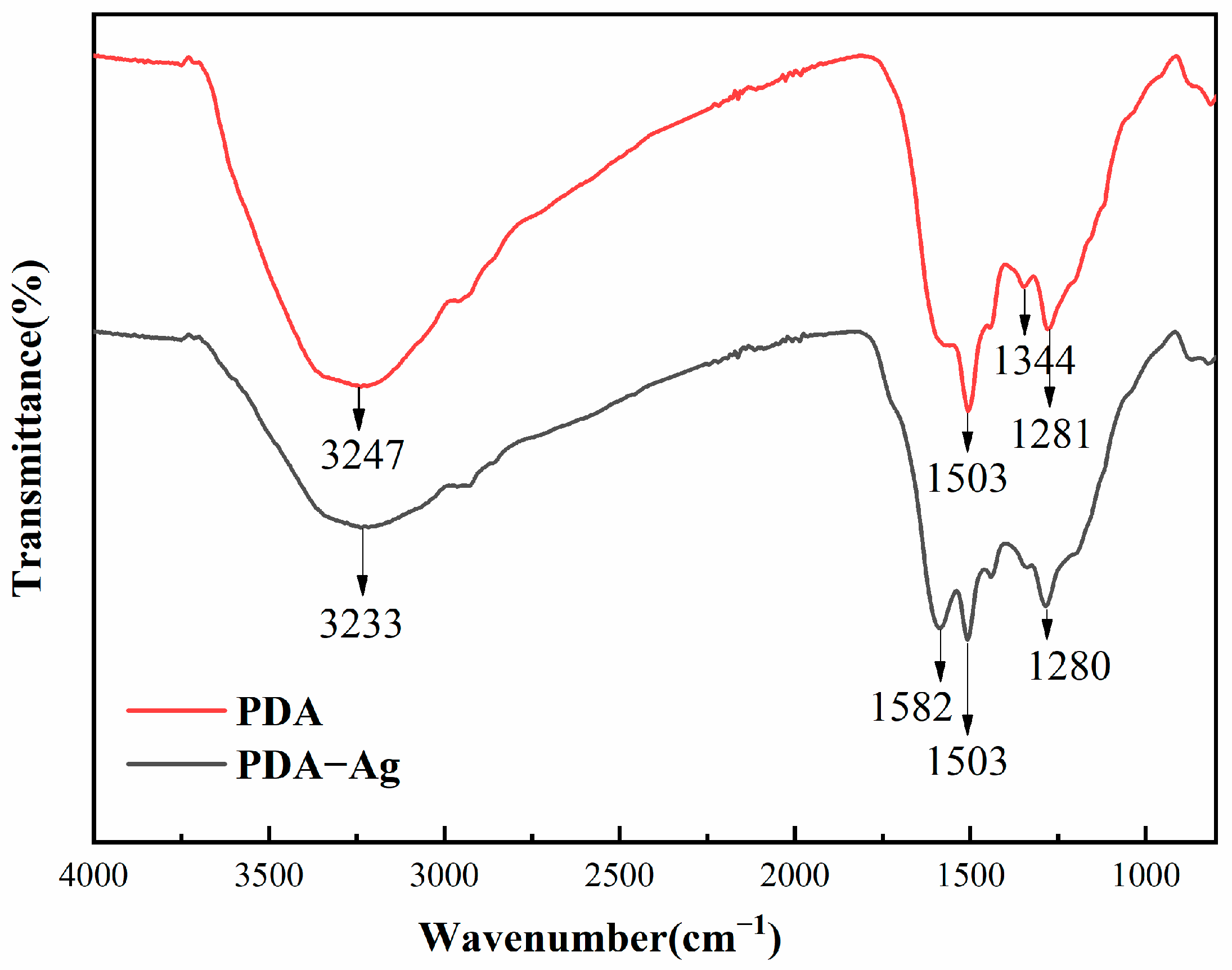
3.3. Infrared Analysis of PDA and PDA–Ag Surfaces
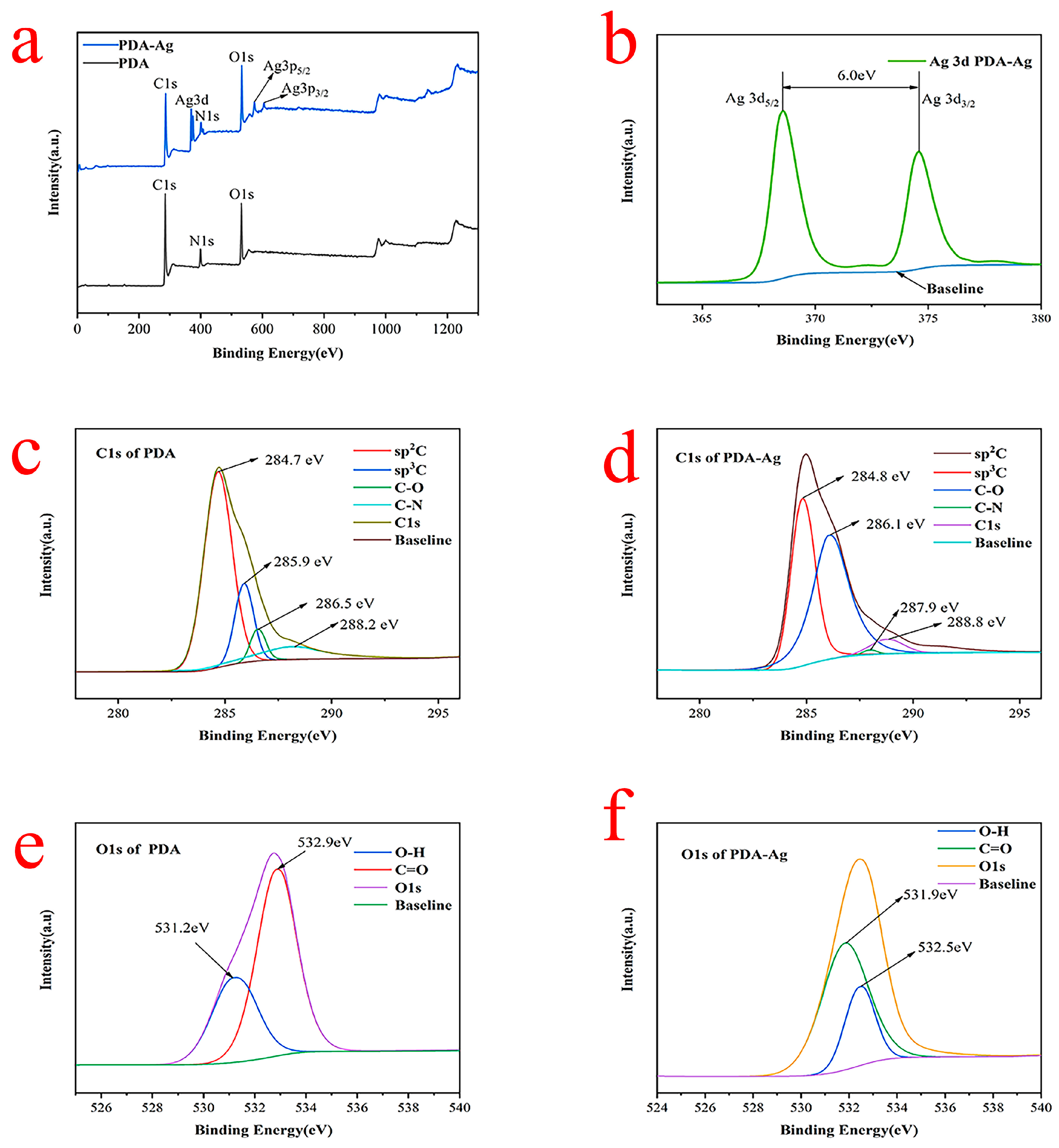
3.4. XPS Analysis of PDA–Ag and PDA
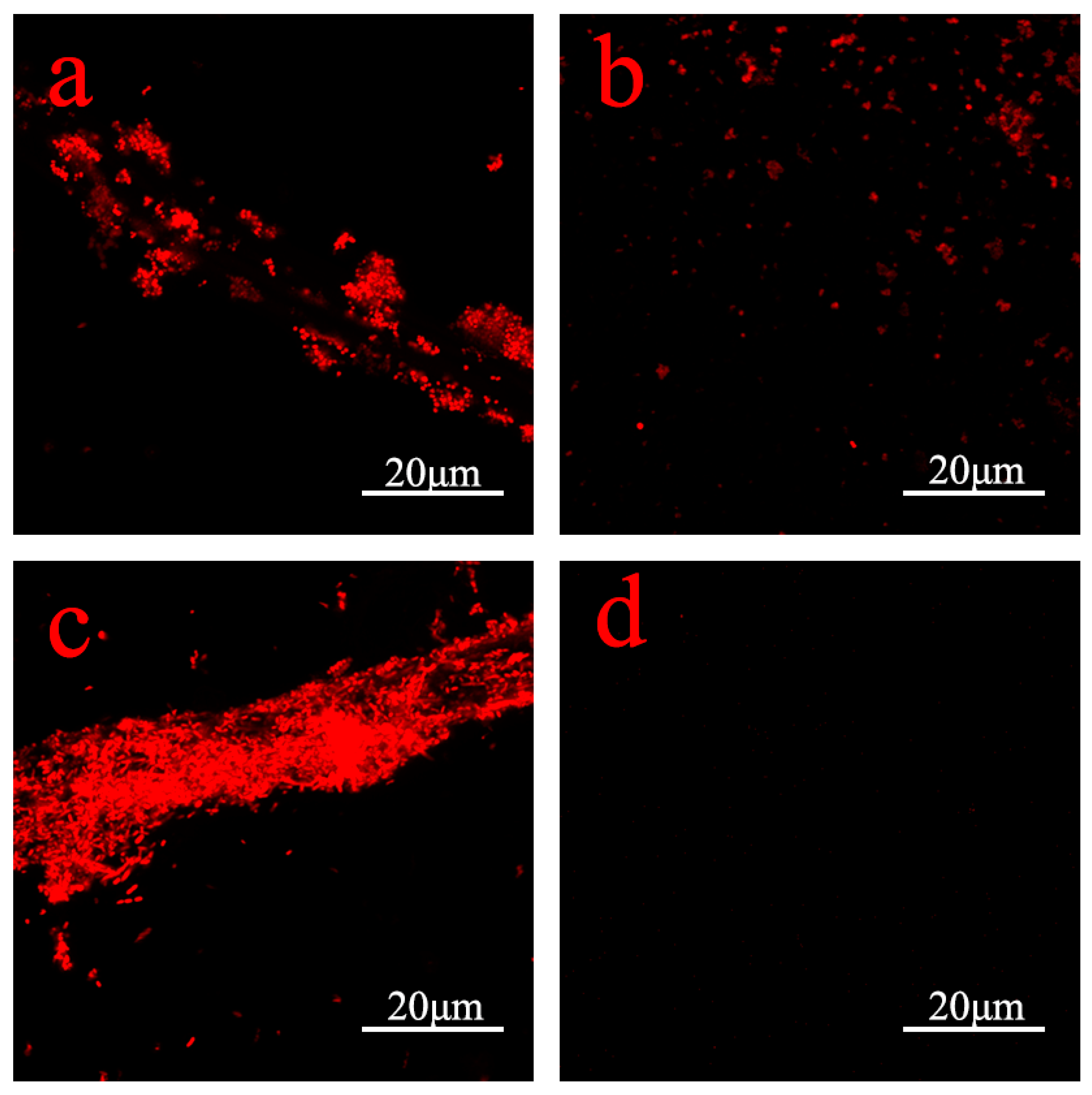
3.5. Antimicrobial Properties of PDA–Ag Dressings and Detection of Silver Ion Release
3.6. Cell Compatibility Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.P.; Li, J.W.; Zhang, S.H.; Zhang, X.Y.; Ma, J.W.; Wang, N.; Wang, S.; Wang, B.; Chen, S.J. Template-assisted magnetron sputtering of cotton nonwovens for wound healing application. ACS Appl. Bio Mater. 2020, 3, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.M.; Xu, Y.B.; Li, X.Y.; Yuan, L.; Tan, H.; Li, D.F.; Mu, C.D. Fabrication of antibacterial collagen-based composite wound dressing. ACS Sustain. Chem. Eng. 2018, 6, 9153–9166. [Google Scholar] [CrossRef]
- Li, S.Y.; Chen, A.Q.; Chen, Y.X.; Yang, Y.; Zhang, Q.W.; Luo, S.; Ye, M.Q.; Zhou, Y.J.; An, Y.; Huang, W.; et al. Lotus leaf-inspired antiadhesive and antibacterial gauze for enhanced infected dermal wound regeneration. Chem. Eng. J. 2020, 402, 126202. [Google Scholar] [CrossRef]
- Liu, G.Y.; Xiang, J.; Xia, Q.F.; Li, K.J.; Lan, T.X.; Yu, L. Superhydrophobic cotton gauze with durably antibacterial activity as skin wound dressing. Cellulose 2019, 26, 1383–1397. [Google Scholar] [CrossRef]
- Liu, G.Y.; Xiang, J.; Xia, Q.F.; Li, K.J.; Yan, H.; Yu, L. Fabrication of durably antibacterial cotton fabrics by robust and uniform immobilization of silver nanoparticles via mussel-inspired polydopamine/polyethyleneimine coating. Ind. Eng. Chem. Res. 2020, 59, 9666–9678. [Google Scholar] [CrossRef]
- Kim, T.; Zhang, Q.Z.; Li, J.; Zhang, L.F.; Jokerst, J.V. A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano 2018, 12, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras-Aguayo, A.; Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Alvarado-Canché, C.; Ledezma, A.; Romero-García, J.; Betancourt-Galindo, R. Poly(methacrylic acid)-modified medical cotton gauzes with antimicrobial and drug delivery properties for their use as wound dressings. Carbohydr. Polym. 2019, 205, 203–210. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.T.; He, Q.F.; Wu, J.; Liang, D.H.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Black, K.C.L.; Sileika, T.S.; Yi, J.; Zhang, R.; Rivera, J.G.; Messersmith, P.B. Bacterial killing by light-triggered release of silver from biomimetic metal nanorods. Small 2014, 10, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.S.; Yu, A.Y.H.; Hsieh, S.C.; Kung, M.L.; Huang, H.Y.; Fu, R.H.; Yeh, C.A.; Hsu, S.H. Enhanced biocompatibility and differentiation capacity of mesenchymal stem cells on poly(dimethylsiloxane) by topographically patterned dopamine. ACS Appl. Mater. Interfaces 2020, 12, 44393–44406. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kang, S.M.; Lee, K.B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ai, K.L.; Liu, J.H.; Deng, M.; He, Y.Y.; Lu, L.H. Dopamine-melanin colloidal nanospheres: An Efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; Wang, Y.L.; Li, M.G. Selecting water-alcohol mixed solvent for synthesis of polydopamine nano-spheres using solubility parameter. Sci. Rep. 2014, 4, 6070. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; del Valle, E.M.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef]
- Luo, H.Y.; Gu, C.W.; Zheng, W.H.; Dai, F.; Wang, X.L.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Gole, A.; Sainkar, S.R.; Sastry, M. Electrostatically controlled organization of carboxylic acid derivatized colloidal silver particles on amine-terminated self-assembled monolayers. Chem. Mater. 2000, 12, 1234–1239. [Google Scholar] [CrossRef]
- Outlaw, R.A.; Davidson, M.R. Small ultrahigh vacuum compatible hyperthermal oxygen atom generator. J. Vac. Sci. Technol. A Vac. Surf. Film. (USA) 1994, 12, 854–860. [Google Scholar] [CrossRef]
- Eberhart, M.E.; Donovan, M.M.; Outlaw, R.A. Ab initio calculations of oxygen diffusivity in group-IB transition metals. Phys. Rev. B Condens. Matter 1992, 46, 12744–12747. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.W.; Chen, M.; Wu, L.M. Novel method to fabricate SiO2/Ag composite spheres and their catalytic, surface-enhanced Raman scattering properties. J. Phys. Chem. C 2007, 111, 11692–11698. [Google Scholar] [CrossRef]
- Cao, P.; He, X.Y.; Xiao, J.F.; Yuan, C.Q.; Bai, X.Q. Peptide-modified stainless steel with resistance capacity of Staphylococcus aureus biofilm formation. Surf. Interface Anal. 2018, 50, 1362–1369. [Google Scholar] [CrossRef]
- Ren, T.T.; Yang, M.Q.; Wang, K.K.; Zhang, Y.; He, J.H. Cuo nanoparticles-containing highly transparent and superhydrophobic coatings with extremely low bacterial adhesion and excellent bactericidal property. ACS Appl. Mater. Interfaces 2018, 10, 25717–25725. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; d’Ischia, M. Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef] [PubMed]
- Panácek, A.; Kvítek, L.; Smékalová, M.; Vecerová, R.; Kolár, M.; Röderová, M.; Dycka, F.; Sebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.Q.; Cong, H.L. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.J.; Cai, C.Z.; Shi, L.; Liu, S.; Ren, L.; Wang, Y.J. Multi-biofunctionalization of a titanium surface with a mixture of peptides to achieve excellent antimicrobial activity and biocompatibility. J. Mater. Chem. B 2015, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Shu, H.; Zhu, P.; Cao, Z.; Wang, S.; Cao, P. Enhancing Antimicrobial Performance of Gauze via Modification by Ag-Loaded Polydopamine Submicron Particles. J. Funct. Biomater. 2024, 15, 152. https://doi.org/10.3390/jfb15060152
Cui J, Shu H, Zhu P, Cao Z, Wang S, Cao P. Enhancing Antimicrobial Performance of Gauze via Modification by Ag-Loaded Polydopamine Submicron Particles. Journal of Functional Biomaterials. 2024; 15(6):152. https://doi.org/10.3390/jfb15060152
Chicago/Turabian StyleCui, Junnan, Haobo Shu, Panpan Zhu, Zhimin Cao, Shuilin Wang, and Pan Cao. 2024. "Enhancing Antimicrobial Performance of Gauze via Modification by Ag-Loaded Polydopamine Submicron Particles" Journal of Functional Biomaterials 15, no. 6: 152. https://doi.org/10.3390/jfb15060152
APA StyleCui, J., Shu, H., Zhu, P., Cao, Z., Wang, S., & Cao, P. (2024). Enhancing Antimicrobial Performance of Gauze via Modification by Ag-Loaded Polydopamine Submicron Particles. Journal of Functional Biomaterials, 15(6), 152. https://doi.org/10.3390/jfb15060152





