Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models
Abstract
:1. Introduction
2. Materials and Methods
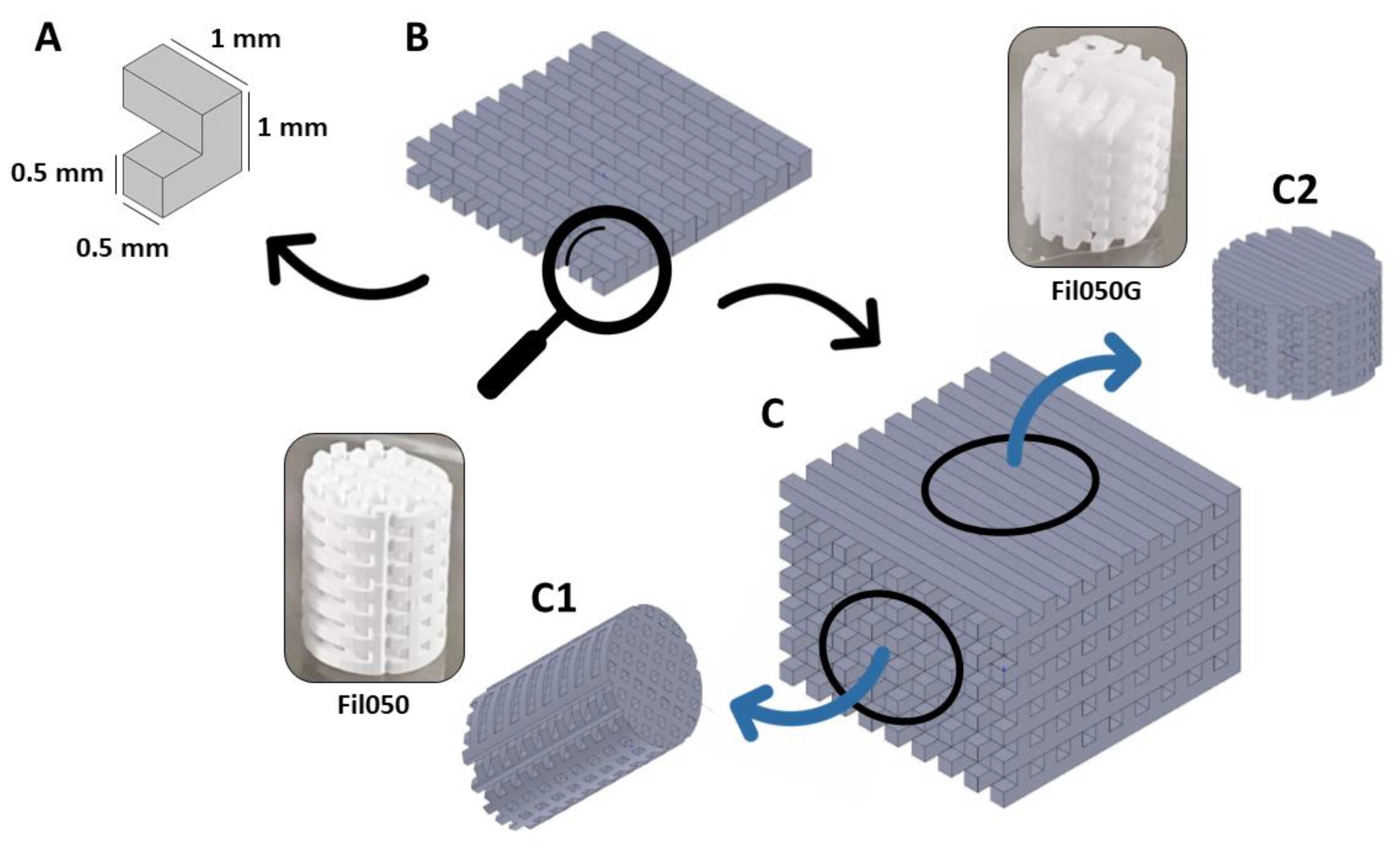
2.1. Scaffold Production
2.2. Scaffold Implantation
2.2.1. Osteoconduction
2.2.2. Bone Augmentation
2.3. Histomorphometry
2.4. Statistical Analysis
3. Results
3.1. Implantation of TCP-Based Scaffolds with TPMS Microarchitecture
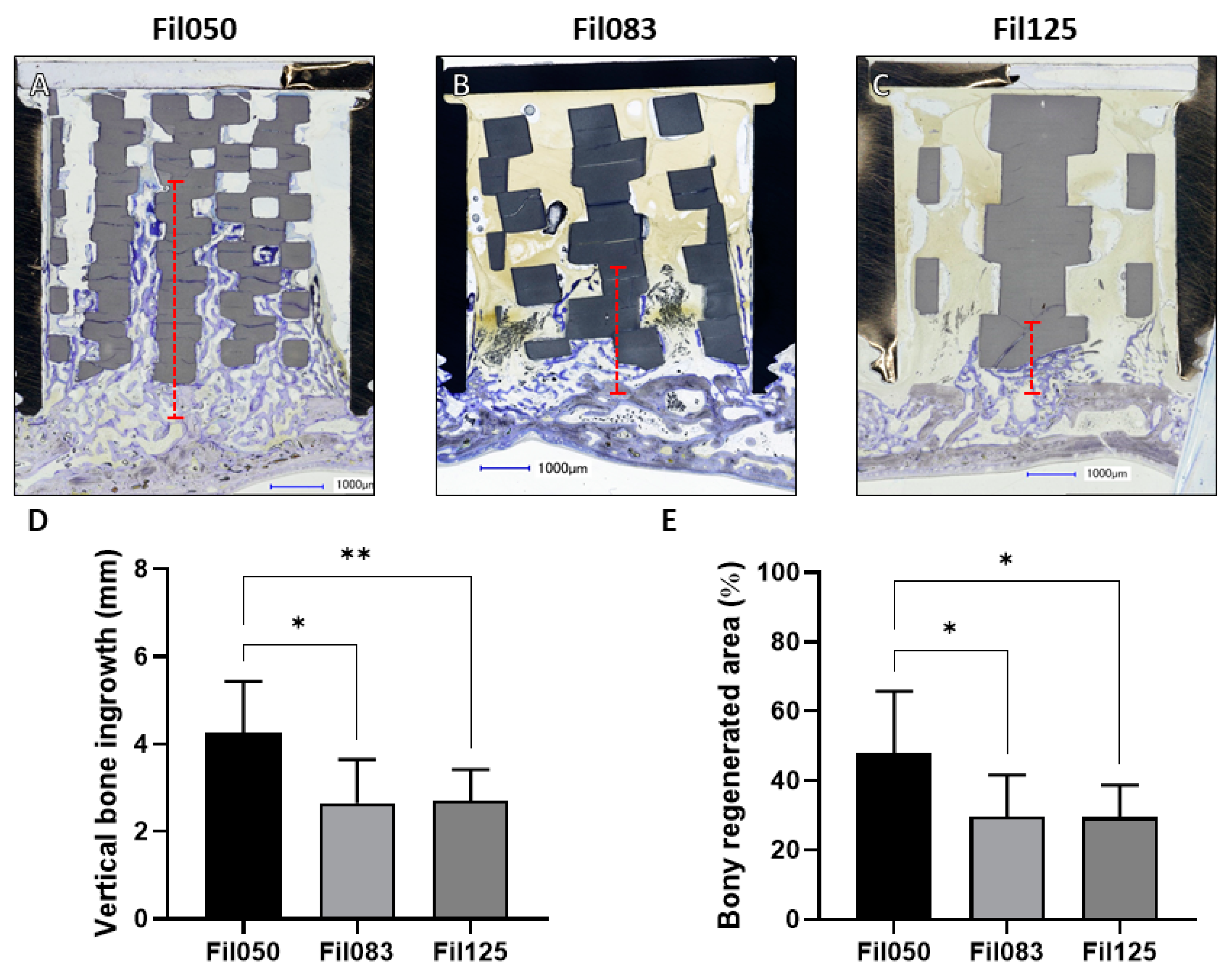
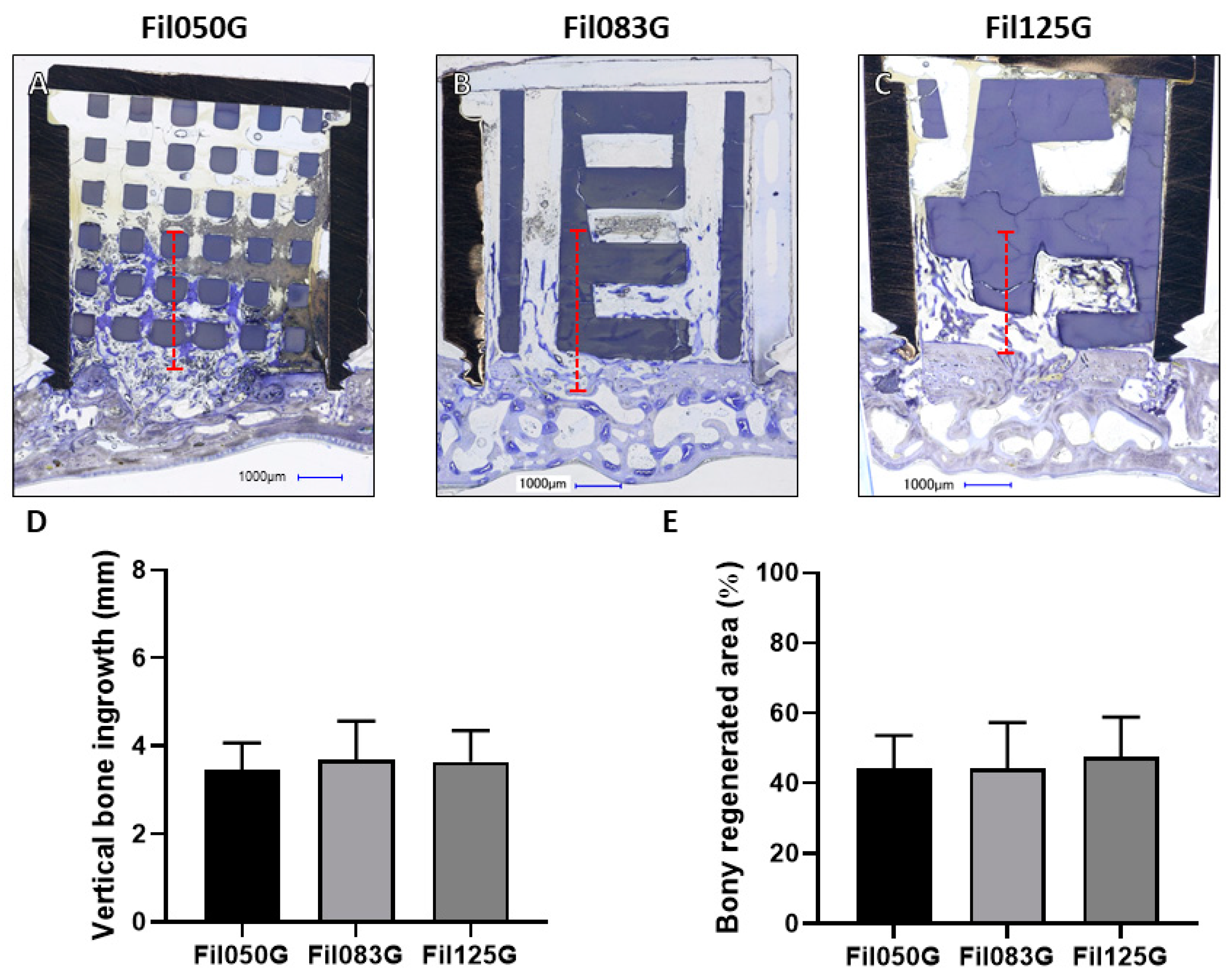
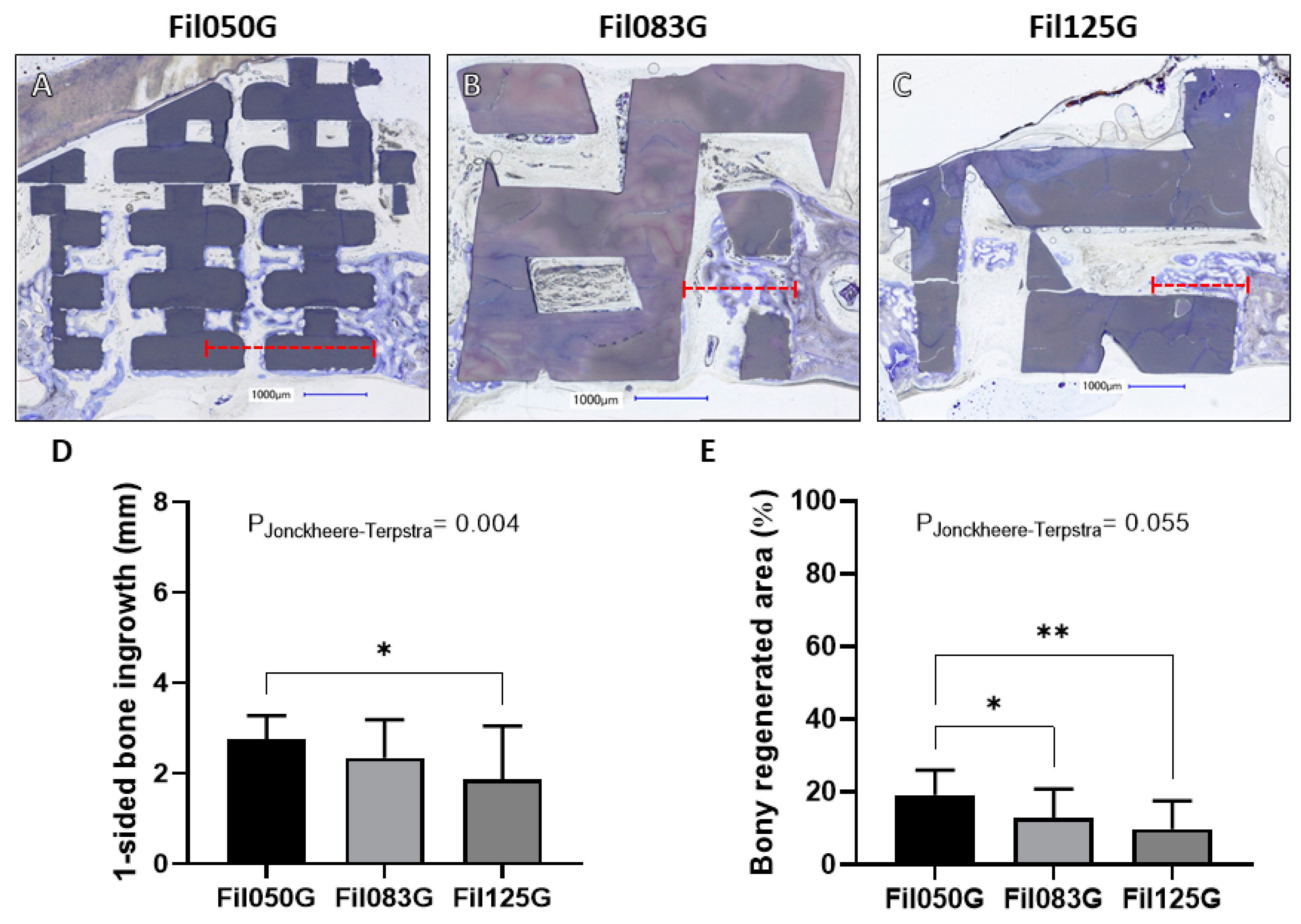
3.1.1. Performance of Filament-Based Microarchitectures in Bone Augmentation
3.1.2. Performance of Filament-Based Microarchitectures in Osteoconduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive Manufacturing of Bone Scaffolds. Int. J. Bioprint 2019, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive Manufacturing Techniques for the Production of Tissue Engineering Constructs. J. Tissue Eng. Regen. Med. 2012, 9, 174–190. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current State of Fabrication Technologies and Materials for Bone Tissue Engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhao, J.; Hussain, M.; Wang, M. Additive Manufacturing in Orthopedics: A Review. ACS Biomater. Sci. Eng. 2022, 8, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Maevskaia, E.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Influence of Scaffold Microarchitecture on Angiogenesis and Regulation of Cell Differentiation During the Early Phase of Bone Healing: A Transcriptomics and Histological Analysis. Int. J. Mol. Sci. 2023, 24, 6000. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3d Bioprinting of Tissues/Organs for Regenerative Medicine and in-Vitro Models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. Tissue Eng. Part B Rev. 2019, 25, 375–386. [Google Scholar] [CrossRef]
- Verykokou, S.; Ioannidis, C.; Soile, S.; Angelopoulos, C.; Theodoridis, K.; Arampatzis, A.S.; Assimopoulou, A.N.; Christofilos, D.; Kapourani, A.; Pantazos, I.; et al. The Role of Cone Beam Computed Tomography in Periodontology: From 3d Models of Periodontal Defects to 3d-Printed Scaffolds. J. Pers. Med. 2024, 14, 207. [Google Scholar] [CrossRef]
- Charbonnier, B.; Hadida, M.; Marchat, D. Additive Manufacturing Pertaining to Bone: Hopes, Reality and Future Challenges for Clinical Applications. Acta Biomater. 2020, 121, 1–28. [Google Scholar] [CrossRef]
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in Additive Manufacturing for Bone Tissue Engineering Scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, X.; Wu, J.; Zhang, X.; Su, R.; Ma, L.; Sun, Q.; He, R. Additive Manufacturing of Bioceramic Implants for Restoration Bone Engineering: Technologies, Advances, and Future Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 1164–1189. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of Pore Size and Morphology in Musculo-Skeletal Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Deering, J.; Grandfield, K. Current Interpretations on the in Vivo Response of Bone to Additively Manufactured Metallic Porous Scaffolds: A Review. Biomater. Biosyst. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.N.; Lane, J.M. Current Understanding of Osteoconduction in Bone Regeneration. Clin. Orthop. Relat. Res. 1998, 355, S267–S273. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3d Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.W.; Park, J.Y.; Lee, D.N.; Jin, X.; Cha, J.K.; Paik, J.W.; Choi, S.H. Three-Dimensionally Printed Biphasic Calcium Phosphate Blocks with Different Pore Diameters for Regeneration in Rabbit Calvarial Defects. Biomater. Res. 2022, 26, 25. [Google Scholar] [CrossRef] [PubMed]
- Seehanam, S.; Khrueaduangkham, S.; Sinthuvanich, C.; Sae-Ueng, U.; Srimaneepong, V.; Promoppatum, P. Evaluating the Effect of Pore Size for 3d-Printed Bone Scaffolds. Heliyon 2024, 10, e26005. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, Y.; Huang, Z.; Wu, T.; Xu, W.; Wu, W.; Xu, Z. Advances in Filament Structure of 3d Bioprinted Biodegradable Bone Repair Scaffolds. Int. J. Bioprint 2021, 7, 426. [Google Scholar] [CrossRef]
- Guerrero, J.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Osteoconductivity of Bone Substitutes with Filament-Based Microarchitectures: Influence of Directionality, Filament Dimension, and Distance. Int. J. Bioprint 2022, 9, 626. [Google Scholar] [CrossRef]
- Ghayor, C.; Chen, T.H.; Bhattacharya, I.; Ozcan, M.; Weber, F.E. Microporosities in 3d-Printed Tricalcium-Phosphate-Based Bone Substitutes Enhance Osteoconduction and Affect Osteoclastic Resorption. Int. J. Mol. Sci. 2020, 21, 9270. [Google Scholar] [CrossRef]
- Benito-Garzon, L.; Guadilla, Y.; Diaz-Guemes, I.; Valdivia-Gandur, I.; Manzanares, M.C.; de Castro, A.G.; Padilla, S. Nanostructured Zn-Substituted Monetite Based Material Induces Higher Bone Regeneration Than Anorganic Bovine Bone and Beta-Tricalcium Phosphate in Vertical Augmentation Model in Rabbit Calvaria. Nanomaterials 2021, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.E.; Ku, H.C.; Dluzen, D.F.; Xing, C.; Zhou, Z. A Nonparametric Alternative to the Cochran-Armitage Trend Test in Genetic Case-Control Association Studies: The Jonckheere-Terpstra Trend Test. PLoS ONE 2023, 18, e0280809. [Google Scholar] [CrossRef] [PubMed]
- Tom, T.; Sreenilayam, S.P.; Brabazon, D.; Jose, J.P.; Joseph, B.; Madanan, K.; Thomas, S. Additive Manufacturing in the Biomedical Field-Recent Research Developments. Results Eng. 2022, 16, 100661. [Google Scholar] [CrossRef]
- Zeng, X.; Meng, Z.; He, J.; Mao, M.; Li, X.; Chen, P.; Fan, J.; Li, D. Embedded Bioprinting for Designer 3d Tissue Constructs with Complex Structural Organization. Acta Biomater. 2021, 140, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of Bone Scaffold Porosity Distributions. Sci. Rep. 2019, 9, 9170. [Google Scholar] [CrossRef]
- Lee, J.B.; Maeng, W.Y.; Koh, Y.H.; Kim, H.E. Porous Calcium Phosphate Ceramic Scaffolds with Tailored Pore Orientations and Mechanical Properties Using Lithography-Based Ceramic 3d Printing Technique. Materials 2018, 11, 1711. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone Defect Animal Models for Testing Efficacy of Bone Substitute Biomaterials. J. Orthop. Translat 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Tsetsenekou, E.; Papadopoulos, T.; Kalyvas, D.; Papaioannou, N.; Tangl, S.; Watzek, G. The Influence of Alendronate on Osseointegration of Nanotreated Dental Implants in New Zealand Rabbits. Clin. Oral. Implants Res. 2011, 23, 659–666. [Google Scholar] [CrossRef]
- Gao, H.; Huang, J.; Wei, Q.; He, C. Advances in Animal Models for Studying Bone Fracture Healing. Bioengineering 2023, 10, 201. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of Animal Models in Biomedical Research: A Review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.P.; Wiskott, A.; Scherrer, S.; Durual, S. Large Bone Vertical Augmentation Using a Three-Dimensional Printed Tcp/Ha Bone Graft: A Pilot Study in Dog Mandible. Clin. Implant. Dent. Relat. Res. 2016, 18, 1183–1192. [Google Scholar] [CrossRef]
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone Regeneration Capability of 3d Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3d Printed Tcp/Ha Structure as a New Osteoconductive Scaffold for Vertical Bone Augmentation. Clin. Oral. Implants Res. 2014, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. Effects of Space Dimensionality within Scaffold for Bone Regeneration with Large and Oriented Blood Vessels. Materials 2023, 16, 7518. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of Macropore Size in Carbonate Apatite Honeycomb Scaffolds on Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110848. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Weber, F.E. The Optimal Microarchitecture of 3d-Printed Β-Tcp Bone Substitutes for Vertical Bone Augmentation Differs from That for Osteoconduction. Mater. Des. 2021, 204, 109650. [Google Scholar] [CrossRef]
- Dias, M.R.; Guedes, J.M.; Flanagan, C.L.; Hollister, S.J.; Fernandes, P.R. Optimization of Scaffold Design for Bone Tissue Engineering: A Computational and Experimental Study. Med. Eng. Phys. 2014, 36, 448–457. [Google Scholar] [CrossRef]
- Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.H.; Yoo, W.Y.; Kim, Y.Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Fan, J.J.; Li, Z.Q.; Liu, Y.W.; Wu, Y.P.; Liu, J. Effects of Pore Size on the Osteoconductivity and Mechanical Properties of Calcium Phosphate Cement in a Rabbit Model. Artif. Organs 2016, 41, 199–204. [Google Scholar] [CrossRef]
- Ntousi, O.; Roumpi, M.; Siogkas, P.; Deligianni, D.; Fotiadis, D.I. Computational Fluid Dynamic Analysis of Customised 3d-Printed Bone Scaffolds with Different Architectures. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2023, 2023, 1–4. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, J.; Maevskaia, E.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models. J. Funct. Biomater. 2024, 15, 174. https://doi.org/10.3390/jfb15070174
Guerrero J, Maevskaia E, Ghayor C, Bhattacharya I, Weber FE. Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models. Journal of Functional Biomaterials. 2024; 15(7):174. https://doi.org/10.3390/jfb15070174
Chicago/Turabian StyleGuerrero, Julien, Ekaterina Maevskaia, Chafik Ghayor, Indranil Bhattacharya, and Franz E. Weber. 2024. "Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models" Journal of Functional Biomaterials 15, no. 7: 174. https://doi.org/10.3390/jfb15070174
APA StyleGuerrero, J., Maevskaia, E., Ghayor, C., Bhattacharya, I., & Weber, F. E. (2024). Optimizing Filament-Based TCP Scaffold Design for Osteoconduction and Bone Augmentation: Insights from In Vivo Rabbit Models. Journal of Functional Biomaterials, 15(7), 174. https://doi.org/10.3390/jfb15070174







